The Association between Plant-Based Diets and Dietary Patterns with Cardiometabolic Risk in a Sample of Commercial Taxi Drivers in South Africa
Abstract
:1. Introduction
2. Materials and Methods
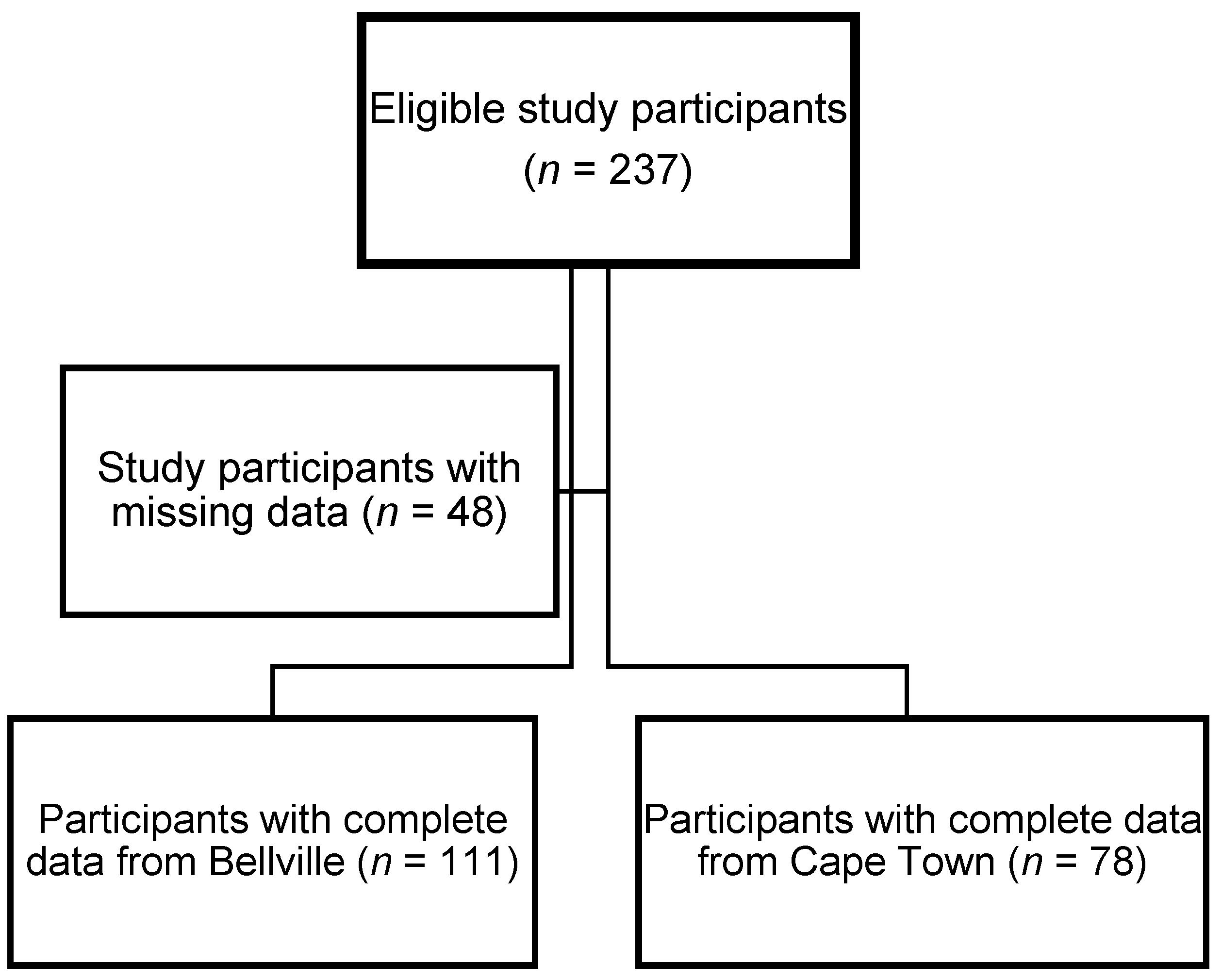
2.1. Study Design and Population
2.2. Data Collection
2.3. Dietary Assessment
2.3.1. A Priori Dietary Analysis
2.3.2. A Posteriori Dietary Analysis
2.4. Definitions and Calculations
2.5. Statistical Analysis
3. Results
3.1. Characteristics of Study Participants
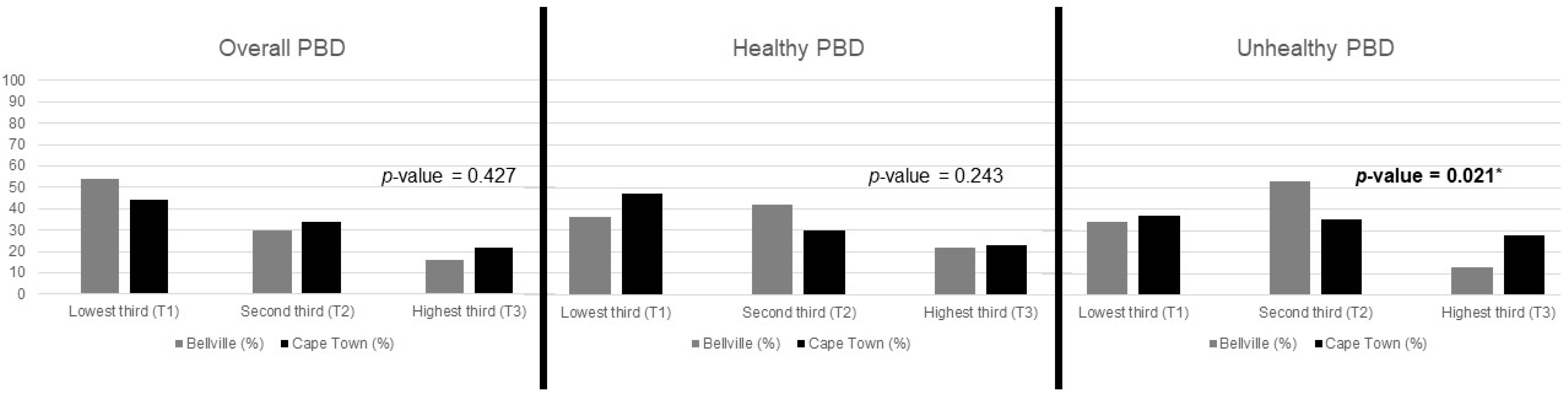
3.2. A Priori Analysis: PBD Indices
3.3. Association between PBD Scores and Cardiometabolic Risk Profiles
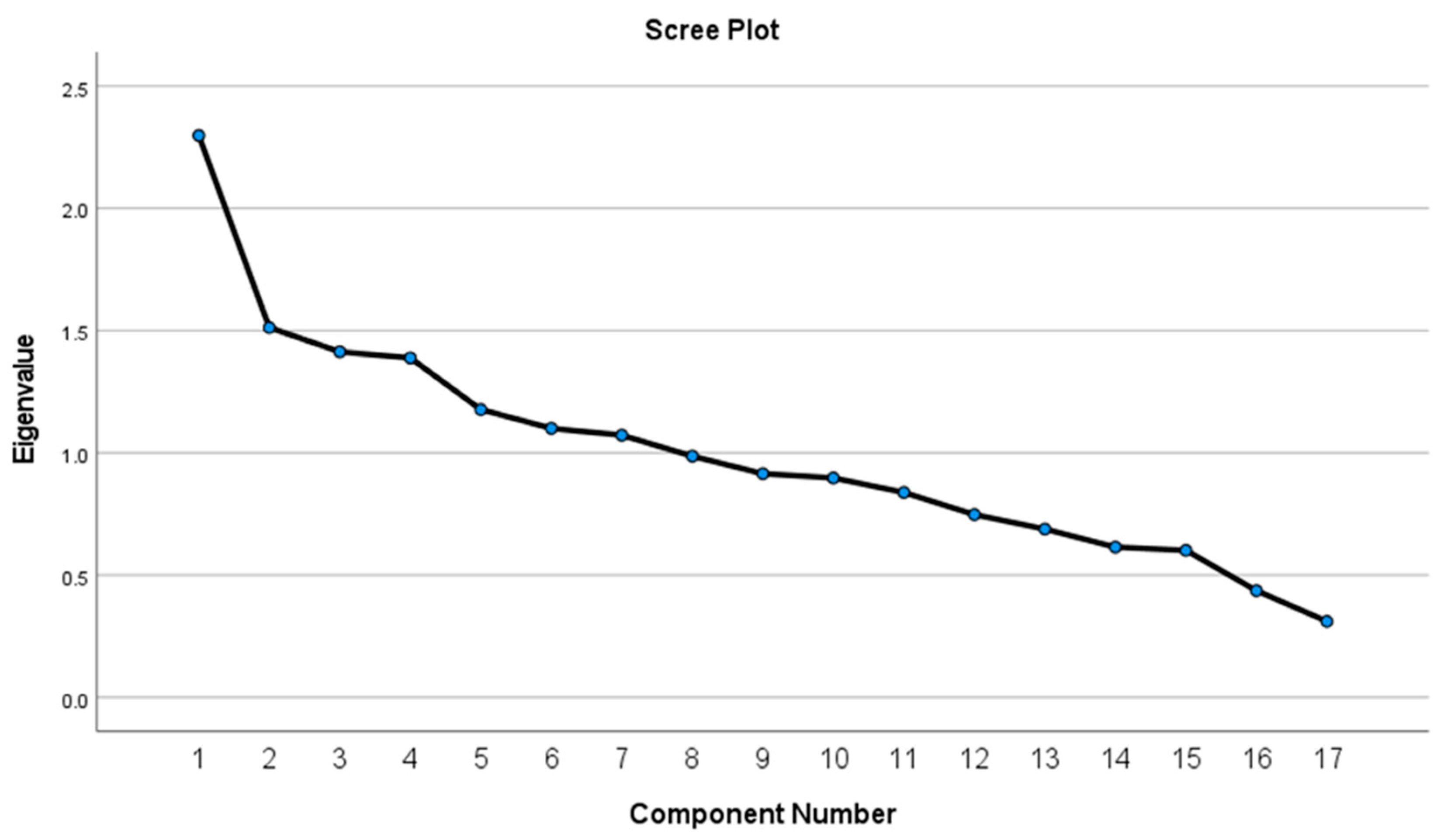
3.4. A Posteriori Analysis: Dietary Patterns
3.5. Association between PCA-Derived Dietary Patterns and Cardiometabolic Risk Profiles
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poó, F.M.; Ledesma, R.D.; López, S.S. The taxi industry: Working conditions and health of drivers, a literature review. Transp. Rev. 2018, 38, 394–411. [Google Scholar] [CrossRef]
- Paulley, N.; Balcombe, R.; Mackett, R.; Titheridge, H.; Preston, J.; Wardman, M.; Shires, J.; White, P. The demand for public transport: The effects of fares, quality of service, income and car ownership. Transp. Policy 2006, 13, 295–306. [Google Scholar] [CrossRef] [Green Version]
- Poumanyvong, P.; Kaneko, S.; Dhakal, S. Impacts of urbanization on national transport and road energy use: Evidence from low, middle and high income countries. Energy Policy 2012, 46, 268–277. [Google Scholar] [CrossRef]
- Fenta, T. Demands for Urban Public Transportation in Addis Ababa. J. Intell. Transp. Urban Plan. 2014, 2, 81–88. [Google Scholar] [CrossRef]
- Mabetwa, E.M.; Mokwena, K.E.; Mphekgwana, P.M.; Modjadji, P. Metabolic Syndrome and Its Components among Taxi Drivers in the City of Tshwane, South Africa. Appl. Sci. 2022, 12, 1767. [Google Scholar] [CrossRef]
- Appiah, C.A.; Afriyie, E.O.; Hayford, F.E.A.; Frimpong, E. Prevalence and lifestyle-associated risk factors of metabolic syndrome among commercial motor vehicle drivers in a metropolitan city in Ghana. Pan Afr. Med. J. 2020, 36, 136. [Google Scholar] [CrossRef] [PubMed]
- Kurosaka, K.; Daida, H.; Muto, T.; Watanabe, Y.; Kawai, S.; Yamaguchi, H. Characteristics of Coronary Heart Disease in Japanese Taxi Drivers as Determined by Coronary Angiographic Analyses. Ind. Health 2000, 38, 15–23. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Song, Y.M.; Ko, H.; Sung, J.; Lee, K.; Shin, J.; Shin, S. Educational Disparities in Risk for Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2018, 16, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Woolley, A.K.; Chudasama, Y.; Seidu, S.I.; Gillies, C.; Schreder, S.; Davies, M.J.; Khunti, K. Influence of sociodemographic characteristics on the preferred format of health education delivery in individuals with type 2 diabetes mellitus and or cardiovascular disease: A questionnaire study. Diabet. Med. J. Br. Diabet. Assoc. 2020, 37, 982–990. [Google Scholar] [CrossRef] [Green Version]
- Niessen, L.W.; Mohan, D.; Akuoku, J.K.; Mirelman, A.J.; Ahmed, S.; Koehlmoos, T.P.; Trujillo, A.; Khan, J.; Peters, D.H. Tackling socioeconomic inequalities and non-communicable diseases in low-income and middle-income countries under the Sustainable Development agenda. Lancet 2018, 391, 2036–2046. [Google Scholar] [CrossRef] [Green Version]
- Miranda, J.J.; Barrientos-Gutiérrez, T.; Corvalan, C.; Hyder, A.A.; Lazo-Porras, M.; Oni, T.; Wells, J.C.K. Understanding the rise of cardiometabolic diseases in low- and middle-income countries. Nat. Med. 2019, 25, 1667–1679. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, G.; Sousa, S.; Lança de Morais, I.; Gelormini, M.; Motta, C.; Gonzales, G.B.; Ovezov, A.; Damasceno, A.; Moreira, P.; Breda, J.; et al. Nutritional Characterization of Street Food in Urban Turkmenistan, Central Asia. Front. Public Health 2022, 10, 877906. [Google Scholar] [CrossRef]
- Sousa, S.; Gelormini, M.; Damasceno, A.; Lopes, S.A.; Maló, S.; Chongole, C.; Muholove, P.; Casal, S.; Pinho, O.; Moreira, P.; et al. Street food in Maputo, Mozambique: Availability and nutritional value of homemade foods. Nutr. Health 2019, 25, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Steyn, N.P.; Labadarios, D.; Nel, J.H. Factors which influence the consumption of street foods and fast foods in South Africa—A national survey. Nutr. J. 2011, 10, 104. [Google Scholar] [CrossRef] [PubMed]
- Steyn, N.P.; McHiza, Z.; Hill, J.; Davids, Y.D.; Venter, I.; Hinrichsen, E.; Opperman, M.; Rumbelow, J.; Jacobs, P. Nutritional contribution of street foods to the diet of people in developing countries: A systematic review. Public Health Nutr. 2014, 17, 1363–1374. [Google Scholar] [CrossRef] [Green Version]
- Aljuraiban, G.S.; Gibson, R.; Oude Griep, L.M.; Okuda, N.; Steffen, L.M.; Van Horn, L.; Chan, Q. Perspective: The Application of A Priori Diet Quality Scores to Cardiovascular Disease Risk-A Critical Evaluation of Current Scoring Systems. Adv. Nutr. 2020, 11, 10–24. [Google Scholar] [CrossRef] [Green Version]
- Satija, A.; Bhupathiraju, S.N.; Rimm, E.B.; Spiegelman, D.; Chiuve, S.E.; Borgi, L.; Willett, W.C.; Manson, J.E.; Sun, Q.; Hu, F.B. Plant-based dietary patterns and incidence of type 2 diabetes in US men and women: Results from three prospective cohort studies. PLoS Med. 2016, 13, e1002039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satija, A.; Bhupathiraju, S.N.; Spiegelman, D.; Chiuve, S.E.; Manson, J.E.; Willett, W.; Rexrode, K.M.; Rimm, E.B.; Hu, F.B. Healthful and unhealthful plant-based diets and the risk of coronary heart disease in U.S. adults. J. Am. Coll. Cardiol. 2017, 70, 411–422. [Google Scholar] [CrossRef]
- Satija, A.; Hu, F.B. Plant-based diets and cardiovascular health. Trends Cardiovasc. Med. 2018, 28, 437–441. [Google Scholar] [CrossRef]
- Del Re, A.; Aspry, K. Update on Plant-Based Diets and Cardiometabolic Risk. Curr. Atheroscler. Rep. 2022, 24, 173–183. [Google Scholar] [CrossRef]
- Ramezankhani, A.; Hosseini-Esfahani, F.; Mirmiran, P.; Azizi, F.; Hadaegh, F. The association of priori and posteriori dietary patterns with the risk of incident hypertension: Tehran Lipid and Glucose Study. J. Transl. Med. 2021, 19, 44. [Google Scholar] [CrossRef] [PubMed]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Alpha-priori and alpha-posterior dietary pattern analyses have similar estimating and discriminating ability in predicting 5-Y incidence of cardiovascular disease: Methodological issues in nutrition assessment. J. Food Sci. 2009, 74, H218–H224. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Flock, M.R.; Richter, C.K.; Mukherjea, R.; Slavin, J.L.; Kris-Etherton, P.M. Healthy Dietary Patterns for Preventing Cardiometabolic Disease: The Role of Plant-Based Foods and Animal Products. Curr. Dev. Nutr. 2017, 1, cdn-117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Li, Z.; Gao, Q.; Zhao, H.; Chen, S.; Huang, L.; Wang, W.; Wang, T. A review of statistical methods for dietary pattern analysis. Nutr. J. 2021, 20, 37. [Google Scholar] [CrossRef]
- Hill, J.; Mchiza, Z.; Fourie, J.; Puoane, T.; Steyn, N. Consumption patterns of street food consumers in Cape Town. JFECS 2016, 2016, 25–35. [Google Scholar]
- Shisana, O.; Labadarios, D.; Rehle, T.; Simbayi, L.; Zuma, K.; Dhansay, A.; Reddy, P.; Parker, W.; Hoosain, E.; Naidoo, P.; et al. The South African National Health and Nutrition Examination Survey, 2012: SANHANES-1: The Health and Nutritional Status of the Nation; HSRC Press: Cape Town, Sonth Africa, 2015. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- FoodFinder: Dietary Analysis Software, Version 1.0 [Computer Software]; South African Medical Research Council: Cape Town, South Africa, 2020. Available online: https://foodfinder.samrc.ac.za/(accessed on 20 July 2022).
- South African Department of Health. Guidelines for Healthy Eating. Information for Nutrition Educators. Available online: https://www.nutritionweek.co.za/NNW2014/docs/NNW-2013-Nutrition%20Educators%20Guideline.pdf (accessed on 19 July 2022).
- Shaw, K.A.; Zello, G.A.; Rodgers, C.D.; Warkentin, T.D.; Baerwald, A.R.; Chilibeck, P.D. Benefits of a plant-based diet and considerations for the athlete. Eur. J. Appl. Physiol. 2022, 122, 1163–1178. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R.; Fresán, U.; Marsh, K.; Miles, F.L.; Saunders, A.V.; Haddad, E.H.; Heskey, C.E.; Johnston, P.; Larson-Meyer, E.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients 2021, 13, 4144. [Google Scholar] [CrossRef]
- Burggraf, C.; Teuber, R.; Brosig, S.; Meier, T. Review of a priori dietary quality indices in relation to their construction criteria. Nutr. Rev. 2018, 76, 747–764. [Google Scholar] [CrossRef] [Green Version]
- Unger, T.; Borghi, C.; Charchar, F.; Khan, N.A.; Poulter, N.R.; Prabhakaran, D.; Ramirez, A.; Schlaich, M.; Stergiou, G.S.; Tomaszewski, M.; et al. 2020 International Society of Hypertension Global Hypertension Practice Guidelines. Hypertension 2020, 75, 1334–1357. [Google Scholar] [CrossRef]
- Whelton, P.K.; Carey, R.M.; Mancia, G.; Kreutz, R.; Bundy, J.D.; Williams, B. Harmonization of the American College of Cardiology/American Heart Association and European Society of Cardiology/European Society of Hypertension Blood Pressure/Hypertension Guidelines: Comparisons, Reflections, and Recommendations. Circulation 2022, 146, 868–877. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization; International Diabetes Federation. Definition and Diagnosis of Diabetes and Intermediate Hyperglycaemia: Report of a WHO/IDF Consultation; World Health Organization; International Diabetes Federation: Geneva, Switzerland, 2006; pp. 1–50.
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar] [CrossRef] [PubMed]
- Klug, E.; Raal, F.J.; Marais, A.D.; Smuts, C.M.; Schamroth, C.; Jankelow, D.; Blom, D.J.; Webb, D.A. South African dyslipidaemia guideline consensus statement: 2018 update A joint statement from the South African Heart Association (SA Heart) and the Lipid and Atherosclerosis Society of Southern Africa (LASSA). S. Afr. Med. J. 2018, 108, 973–1000. [Google Scholar] [CrossRef]
- WHO Consultation on Obesity; World Health Organization. Obesity: Preventing and Managing the Global Epidemic. Report of a WHO Consultation; World Health Organization: Geneva, Switzerland, 2000.
- Pearson, T.A.; Mensah, G.A.; Alexander, R.W.; Anderson, J.L.; Cannon, R.O., 3rd; Criqui, M.; Fadl, Y.Y.; Fortmann, S.P.; Hong, Y.; Myers, G.L.; et al. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation 2003, 107, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Della Corte, K.W.; Perrar, I.; Penczynski, K.J.; Schwingshackl, L.; Herder, C.; Buyken, A.E. Effect of Dietary Sugar Intake on Biomarkers of Subclinical Inflammation: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2018, 10, 606. [Google Scholar] [CrossRef] [Green Version]
- Herder, C.; Færch, K.; Carstensen-Kirberg, M.; Lowe, G.D.; Haapakoski, R.; Witte, D.R.; Brunner, E.J.; Roden, M.; Tabák, A.G.; Kivimäki, M.; et al. Biomarkers of subclinical inflammation and increases in glycaemia, insulin resistance and beta-cell function in non-diabetic individuals: The Whitehall II study. Eur. J. Endocrinol. 2016, 175, 367–377. [Google Scholar] [CrossRef] [Green Version]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Food and Nutrition Board; Committee to Review the Dietary Reference Intakes for Sodium and Potassium. Oria, M., Harrison, M.l., Stallings, V.A., Eds.; Dietary Reference Intakes for Sodium and Potassium; National Academies Press (US): Washington, DC, USA, 2019; Appendix J, Dietary Reference Intakes Summary Tables. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545442/ (accessed on 24 February 2023).
- Kim, H.; Rebholz, C.M.; Garcia-Larsen, V.; Steffen, L.M.; Coresh, J.; Caulfield, L.E. Operational differences in plant-based diet indices affect the ability to detect associations with incident hypertension in middle-aged US adults. J. Nutr. 2020, 150, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.S.; Oh, K.; Kim, H.C. Dietary assessment methods in epidemiologic studies. Epidemiol. Health 2014, 36, e2014009. [Google Scholar] [CrossRef] [Green Version]
- Teufel, N.I. Development of culturally competent food-frequency questionnaires. Am. J. Clin. Nutr. 1997, 65 (Suppl. 4), 1173s–1178s. [Google Scholar] [CrossRef] [Green Version]
- Berrougui, H.; Momo, C.N.; Khalil, A. Health benefits of high-density lipoproteins in preventing cardiovascular diseases. J. Clin. Lipidol. 2012, 6, 524–533. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.; Mandes, T.; Crimarco, A. A plant-based diet for overweight and obesity prevention and treatment. J. Geriatr. Cardiol. 2017, 14, 369–374. [Google Scholar] [PubMed]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-Metabolic Benefits of Plant-Based Diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef] [Green Version]
- Mchiza, Z.; Hill, J.; Steyn, N. Foods currently sold by street food vendors in the Western Cape, South Africa, do not foster good health. In Fast Foods: Consumption Patterns, Role of Globalization and Health Effects; Nova Science Publishers: Hauppauge, NY, USA, 2014; pp. 91–118. [Google Scholar]
- Jambi, H.; Enani, S.; Malibary, M.; Bahijri, S.; Eldakhakhny, B.; Al-Ahmadi, J.; Al Raddadi, R.; Ajabnoor, G.; Boraie, A.; Tuomilehto, J. The Association Between Dietary Habits and Other Lifestyle Indicators and Dysglycemia in Saudi Adults Free of Previous Diagnosis of Diabetes. Nutr. Metab. Insights 2020, 13, 1178638820965258. [Google Scholar] [CrossRef]
- Fretts, A.M.; Follis, J.L.; Nettleton, J.A.; Lemaitre, R.N.; Ngwa, J.S.; Wojczynski, M.K.; Kalafati, I.P.; Varga, T.V.; Frazier-Wood, A.C.; Houston, D.K.; et al. Consumption of meat is associated with higher fasting glucose and insulin concentrations regardless of glucose and insulin genetic risk scores: A meta-analysis of 50,345 Caucasians. Am. J. Clin. Nutr. 2015, 102, 1266–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damigou, E.; Kosti, R.I.; Panagiotakos, D.B. White Meat Consumption and Cardiometabolic Risk Factors: A Review of Recent Prospective Cohort Studies. Nutrients 2022, 14, 5213. [Google Scholar] [CrossRef]
- Hill, J.; McHiza, Z.; Puoane, T.; Steyn, N.P. The development of an evidence-based street food vending model within a socioecological framework: A guide for African countries. PLoS ONE 2019, 14, e0223535. [Google Scholar] [CrossRef]
- Xi, B.; Huang, Y.; Reilly, K.H.; Li, S.; Zheng, R.; Barrio-Lopez, M.T.; Martinez-Gonzalez, M.A.; Zhou, D. Sugar-sweetened beverages and risk of hypertension and CVD: A dose-response meta-analysis. Br. J. Nutr. 2015, 113, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.T.; Kao, Y.H.; Sothern, M.S.; Seal, D.W.; Lee, C.H.; Lin, H.Y.; Chen, T.; Tseng, T.S. The association between sugar-sweetened beverages intake, body mass index, and inflammation in US adults. Int. J. Public Health 2020, 65, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Sajadi Hezaveh, Z.; Sikaroudi, M.K.; Vafa, M.; Clayton, Z.S.; Soltani, S. Effect of egg consumption on inflammatory markers: A systematic review and meta-analysis of randomized controlled clinical trials. J. Sci. Food Agric. 2019, 99, 6663–6670. [Google Scholar] [CrossRef]
- Richter, C.K.; Skulas-Ray, A.C.; Champagne, C.M.; Kris-Etherton, P.M. Plant protein and animal proteins: Do they differentially affect cardiovascular disease risk? Adv. Nutr. 2015, 6, 712–728. [Google Scholar] [CrossRef] [Green Version]
- Aycart, D.F.; Acevedo, S.; Eguiguren-Jimenez, L.; Andrade, J.M. Influence of Plant and Animal Proteins on Inflammation Markers among Adults with Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 1660. [Google Scholar] [CrossRef] [PubMed]
| Food Groups | Number of Servings | Quantiles of Consumption | Scoring System |
|---|---|---|---|
| Whole grains | 0 | Low consumption = 1 | HEALTHY PLANT FOODS PDI: positive scores hPDI: positive scores uPDI: reverse scores |
| 0.01–4.00 | Med consumption = 2 | ||
| 4.01–12.63 | High consumption = 3 | ||
| Fruits | 0 | Low consumption = 1 | |
| 0.01–1.00 | Med consumption = 2 | ||
| 1.01–8.42 | High consumption = 3 | ||
| Vegetables | 0 | Low consumption = 1 | |
| 0.01–1.00 | Med consumption = 2 | ||
| 1.01–5.26 | High consumption = 3 | ||
| Nuts | 0 | Low consumption = 1 | |
| 0.01–3.16 | High consumption = 2 | ||
| Legumes | 0 | Low consumption = 1 | |
| 0.01–0.55 | Med consumption = 2 | ||
| 0.56–2.11 | High consumption = 3 | ||
| Tea and coffee | 0 | Low consumption = 1 | |
| 0.01–1.00 | Med consumption = 2 | ||
| 1.01–4.21 | High consumption = 3 | ||
| Refined grains | 0.00–5.00 | Low consumption = 1 | LESS HEALTHY PLANT FOODS PDI: positive scores hPDI: reverse scores uPDI: positive scores |
| 5.01–8.00 | Med consumption = 2 | ||
| 8.01–37.89 | High consumption = 3 | ||
| Potatoes | 0 | Low consumption = 1 | |
| 0.01–1.00 | Med consumption = 2 | ||
| 1.01–5.26 | High consumption = 3 | ||
| Fruit juices | 0 | Low consumption = 1 | |
| 0.01–2.11 | High consumption = 2 | ||
| SSBs | 0 | Low consumption = 1 | |
| 0.01–1.00 | Med consumption = 2 | ||
| 1.01–6.32 | High consumption = 3 | ||
| Sweets and desserts | 0 | Low consumption = 1 | |
| 0.01–8.42 | High consumption = 2 | ||
| Animal fat | 0 | Low consumption = 2 | ANIMAL FOODS PDI: reverse scores hPDI: reverse scores uPDI: reverse scores |
| 0.01–6.32 | High consumption = 1 | ||
| Egg | 0 | Low consumption = 2 | |
| 0.01–6.32 | High consumption = 1 | ||
| Dairy | 0 | Low consumption = 3 | |
| 0.01–1.00 | Med consumption = 2 | ||
| 1.01–5.26 | High consumption = 1 | ||
| Fish/seafood | 0 | Low consumption = 2 | |
| 0.01–4.21 | High consumption = 1 | ||
| Meat | 0.00–2.00 | Low consumption = 3 | |
| 2.01–4.00 | Med consumption = 2 | ||
| 4.01–14.74 | High consumption = 1 | ||
| Miscellaneous animal foods | 0 | High consumption = 1 | |
| 0.01–4.21 | Low consumption = 2 |
| Parameters | Overall |
|---|---|
| Sociodemographic risk factors | |
| Age in years, median (25th percentile; 75th percentile) | 38 (32–49) |
| Education, n (%) | |
| No schooling | 7 (4) |
| Attended primary school | 55 (29) |
| Attended high school | 91 (48) |
| Matriculated (Grade 12) | 32 (17) |
| Diploma | 4 (2) |
| Marital status, n (%) | |
| Single/separated/divorced | 90 (48) |
| Married/living as married | 99 (52) |
| Behavioural risk factors | |
| Current smoker, n (%) | 83 (44) |
| Current alcohol drinker, n (%) | 102 (54) |
| Cardiometabolic risk factors | |
| Hypertension, n (%) | 74 (40) |
| Dysglycaemia, n (%) | 43 (23) |
| Low HDL-C, n (%) | 75 (40) |
| Raised LDL-C, n (%) | 70 (37) |
| Raised TG, n (%) | 51 (27) |
| Obesity, n (%) | 69 (37) |
| Subclinical inflammation, n (%) | 57 (30) |
| Parameters | DRIs | Tertile 1 | Tertile 2 | Tertile 3 | p-Value |
|---|---|---|---|---|---|
| PDI | RDA/AI * | ||||
| Macronutrients | |||||
| Total protein, g | 56 | 106.6 (74.5; 138.7) | 96.4 (71.9; 132.7) | 127.0 (76.1; 173.9) | 0.300 |
| Plant protein, g | 27.8 (18.4; 41.0) | 30.5 (18.8; 48.0) | 39.5 (21.0; 60.0) | 0.126 | |
| Animal protein, g | 56.4 (31.6; 85.7) | 44.0 (26.3; 70.3) | 44.0 (14.9; 65.9) | 0.174 | |
| Carbohydrates, total, g | 130 | 380.9 (250.1; 497.4) | 362.2 (257.9; 575.0) | 578.0 (366.7; 787.9) | 0.003 |
| Total sugars, g | 23.0 (9.1; 48.6) | 45.2 (20.3; 73.2) | 69.3 (35.6; 107.9) | <0.001 | |
| Saturated fatty acids, g | 20.06 (14.31; 30.20) | 19.86 (12.06; 29.71) | 21.52 (9.14; 47.38) | 0.693 | |
| MUFA, g | 22.99 (12.98; 43.68) | 24.51 (14.91; 38.81) | 38.10 (15.72; 54.82) | 0.283 | |
| PUFA, g | 21.44 (7.60; 37.55) | 21.87 (10.60; 43.26) | 21.35 (9.14; 47.38) | 0.266 | |
| Total dietary fibre, g | 30 */38 * | 30.2 (20.7; 39.6) | 32.4 (23.7; 55.7) | 57.2 (45.4; 74.0) | <0.001 |
| Minerals | |||||
| Calcium, mg | 1000 | 604 (291; 839) | 678 (355; 1120) | 957 (701; 1206) | <0.001 |
| Iron, mg | 8.0 | 12.9 (9.8; 19.2) | 16.1 (11.1; 29.7) | 26.4 (17.1; 38.5) | <0.001 |
| Zinc, mg | 11.0 | 12.34 (8.87; 17.78) | 12.18 (8.48; 18.73) | 16.24 (10.60; 25.56) | 0.038 |
| Vitamins | |||||
| Vitamin B2, mg | 1.3 | 1.37 (0.91; 1.95) | 1.33 (1.01; 2.28) | 1.88 (1.33; 2.88) | 0.013 |
| Vitamin B12, mcg | 2.4 µg | 3.6 (1.8; 7.1) | 3.6 (1.6; 6.7) | 3.2 (1.9; 6.8) | 0.978 |
| Vitamin D, mcg | 5–15 * µg | 6.48 (1.21; 13.79) | 8.17 (1.73; 14.52) | 10.75 (6.88; 15.49) | 0.027 |
| hPDI | |||||
| Macronutrients | |||||
| Total protein, g | 56 | 139.5 (105.2; 191.0) | 93.8 (76.3; 127.4) | 71.8 (35.5; 96.3) | <0.001 |
| Plant protein, g | 36.2 (24.5; 45.9) | 29.9 (16.3; 45.5) | 20.6 (8.5; 33.1) | <0.001 | |
| Animal protein, g | 76.9 (48.8; 101.0) | 43.3 (28.1; 61.6) | 21.0 (12.0; 45.3) | <0.001 | |
| Carbohydrates, total, g | 130 | 525.1 (380.2; 753.8) | 356.8 (253.1; 495.9) | 279.6 (185.3; 378.2) | <0.001 |
| Total sugars, g | 48.6 (24.0; 92.4) | 33.7 (12.2; 58.0) | 24.9 (13.4; 59.4) | 0.017 | |
| Saturated fatty acids, g | 28.44 (21.26; 44.11) | 17.68 (12.36; 27.47) | 10.95 (6.57; 18.03) | <0.001 | |
| MUFA, g | 43.68 (27.02; 62.94) | 21.40 (13.30; 34.33) | 13.05 (6.26; 19.94) | <0.001 | |
| PUFA, g | 39.53 (24.88; 58.07) | 18.22 (9.45; 39.75) | 9.04 (4.57; 15.94) | <0.001 | |
| Total dietary fibre, g | 30 */38 * | 40.2 (26.7; 51.5) | 32.5 (20.9; 45.4) | 29.9 (22.3; 50.2) | 0.104 |
| Minerals | |||||
| Calcium, mg | 1000 | 813 (535; 1130) | 619 (354; 965) | 585 (232; 949) | 0.020 |
| Iron, mg | 8.0 | 18.6 (12.9; 31.0) | 14.6 (10.7; 23.9) | 13.0 (9.8; 19.1) | 0.001 |
| Zinc, mg | 11.0 | 16.93 (12.34; 23.10) | 12.01 (8.69; 18.14) | 9.84 (5.98; 12.13) | <0.001 |
| Vitamins | |||||
| Vitamin B2, mg | 1.3 | 1.76 (1.29; 2.72) | 1.34 (0.86; 2.04) | 1.17 (0.71; 1.58) | <0.001 |
| Vitamin B12, mcg | 2.4 µg | 5.4 (2.9; 15.4) | 3.6 (1.6; 5.8) | 2.2 (1.2; 3.4) | <0.001 |
| Vitamin D, mcg | 5–15 * µg | 10.44 (4.86; 18.12) | 6.59 (1.21; 13.70) | 7.12 (1.22; 12.76) | 0.010 |
| uPDI | |||||
| Macronutrients | |||||
| Total protein, g | 56 | 98.2 (75.3; 130.1) | 104.4 (56.5; 142.7) | 122.4 (85.0; 202.9) | 0.046 |
| Plant protein, g | 29.0 (19.2; 42.4) | 30.6 (16.0; 45.3) | 32.2 (23.2; 42.8) | 0.776 | |
| Animal protein, g | 46.8 (26.3; 65.7) | 49.0 (27.5; 76.9) | 56.4 (30.3; 126.0) | 0.116 | |
| Carbohydrates, total, g | 130 | 365.5 (273.9; 481.3) | 370.2 (238.3; 528.9) | 597.4 (379.6; 756.8) | <0.001 |
| Total sugars, g | 31.0 (16.7; 64.6) | 25.5 (9.4; 52.3) | 68.8 (46.6; 169.6) | <0.001 | |
| Saturated fatty acids, g | 18.03 (13.50; 25.54) | 20.59 (9.58; 30.44) | 24.63 (15.51; 44.84) | 0.054 | |
| MUFA, g | 20.80 (13.96; 38.75) | 23.79 (11.71; 38.81) | 47.43 (18.31; 70.07) | 0.003 | |
| PUFA, g | 15.83 (9.04; 28.44) | 21.79 (8.15; 41.00) | 44.08 (18.13; 76.12) | <0.001 | |
| Total dietary fibre, g | 30 */38 * | 37.5 (28.3; 50.9) | 29.1 (18.4; 44.7) | 42.0 (25.6; 54.2) | 0.022 |
| Minerals | |||||
| Calcium, mg | 1000 | 782 (515; 1061) | 545 (266; 965) | 803 (509; 1040) | 0.035 |
| Iron, mg | 8.0 | 15.1 (12.3; 24.3) | 15.3 (9.6; 23.1) | 18.6 (11.4; 35.5) | 0.118 |
| Zinc, mg | 11.0 | 12.01 (10.11; 17.37) | 13.00 (7.55; 19.00) | 16.42 (9.27; 27.24) | 0.088 |
| Vitamins | |||||
| Vitamin B2, mg | 1.3 | 1.49 (1.17; 1.92) | 1.34 (0.76; 2.01) | 1.45 (0.99; 3.09) | 0.429 |
| Vitamin B12, mcg | 2.4 µg | 3.5 (2.3; 5.1) | 3.5 (1.2; 6.9) | 3.5 (1.7; 16.8) | 0.577 |
| Vitamin D, mcg | 5–15 * µg | 9.50 (6.55; 15.50) | 5.50 (0.89; 13.70) | 7.74 (2.36; 14.49) | 0.010 |
| Parameters | Normal Range | Tertile 1 | Tertile 2 | Tertile 3 | p-Value |
|---|---|---|---|---|---|
| PDI | |||||
| SBP, mmHg | ≤140 | 134 (122; 143) | 132 (126; 139) | 125.50 (116.00; 145.00) | 0.470 |
| DBP, mmHg | ≤90 | 84 (74; 92) | 80 (76; 91) | 81.50 (73.00; 97.00) | 0.905 |
| FBG, mmol/L | ≤7.0 | 5.77 (5.15; 6.67) | 5.83 (5.06; 7.36) | 5.47 (5.11; 6.68) | 0.782 |
| HDL-C, mmol/L | ≥1.0 | 1.05 (0.93; 1.12) | 1.07 (0.89; 1.34) | 1.04 (0.87; 1.25) | 0.671 |
| LDL-C, mmol/L | ≤3.0 | 2.76 (2.23; 3.29) | 2.59 (2.17; 3.29) | 2.78 (2.44; 3.24) | 0.481 |
| TG, mmol/L | ≤1.5 | 1.06 (0.72; 1.46) | 1.03 (0.62; 1.51) | 1.10 (0.81; 1.68) | 0.492 |
| BMI, kg/m2 | ≤30 | 28.05 (23.44; 32.13) | 28.25 (24.90; 32.74) | 27.91 (24.11; 32.88) | 0.961 |
| hs-CRP, mg/L | ≤3.0 | 2.60 (1.10–5.10) | 2.20 (1.10; 4.90) | 2.20 (1.20; 4.90) | 0.969 |
| hPDI | |||||
| SBP, mmHg | ≤140 | 133.00 (123.00; 145.00) | 132.00 (122.00; 140.00) | 131.00 (121.00; 142.00) | 0.636 |
| DBP, mmHg | ≤90 | 84.00 (79.00; 97.00) | 79.50 (74.00; 90.00) | 77.00 (71.00; 90.00) | 0.056 |
| FBG, mmol/L | ≤7.0 | 5.50 (5.08; 6.52) | 5.77 (5.06; 6.61) | 6.22 (5.25; 7.36) | 0.235 |
| HDL-C, mmol/L | ≥1.0 | 1.05 (0.95; 1.26) | 0.99 (0.89; 1.20) | 1.09 (0.99; 1.24) | 0.352 |
| LDL-C, mmol/L | ≤3.0 | 2.69 (2.23; 3.28) | 2.80 (2.28; 3.36) | 2.55 (2.04; 3.20) | 0.700 |
| TG, mmol/L | ≤1.5 | 1.04 (0.74; 1.47) | 1.16 (0.64; 1.65) | 0.99 (0.69; 1.35) | 0.521 |
| BMI, kg/m2 | ≤30 | 28.90 (25.42; 32.46) | 27.96 (24.18; 31.05) | 25.71 (21.71; 32.88) | 0.172 |
| hs-CRP, mg/L | ≤3.0 | 2.70 (1.20; 5.90) | 2.60 (1.10; 4.50) | 2.20 (1.10; 4.80) | 0.608 |
| uPDI | |||||
| SBP, mmHg | ≤140 | 134.00 (121.00; 143.00) | 130.00 (121.00; 139.00) | 138.00 (129.00; 145.00) | 0.188 |
| DBP, mmHg | ≤90 | 80.00 (71.50; 91.00) | 82.00 (75.00; 90.00) | 90.00 (80.00; 100.00) | 0.026 |
| FBG, mmol/L | ≤7.0 | 5.98 (5.13; 7.03) | 5.72 (5.14; 6.85) | 5.63 (4.99; 6.63) | 0.966 |
| HDL-C, mmol/L | ≥1.0 | 1.05 (0.89; 1.27) | 1.02 (0.89; 1.20) | 1.09 (0.95; 1.39) | 0.396 |
| LDL-C, mmol/L | ≤3.0 | 2.65 (2.28; 3.24) | 2.87 (1.97; 3.29) | 2.61 (2.23; 3.21) | 0.783 |
| TG, mmol/L | ≤1.5 | 0.92 (0.70; 1.33) | 1.04 (0.69; 1.61) | 1.36 (0.92; 1.75) | 0.095 |
| BMI, kg/m2 | ≤30 | 26.54 (23.32; 32.63) | 28.13 (23.60; 31.46) | 30.66 (26.18; 32.64) | 0.165 |
| hs-CRP, mg/L | ≤3.0 | 2.20 (1.10; 4.90) | 2.30 (0.90; 4.80) | 3.90 (1.50; 6.80) | 0.136 |
| hPDI | Tertile 2 | Tertile 3 | p-Value |
| Hypertension | 1.31 (0.65–2.68) | 1.48 (0.65–3.46) | 0.593 |
| Model 1 | 1.14 (0.54–2.40) | 1.42 (0.61–3.38) | 0.718 |
| Model 2 | 1.08 (0.50–2.31) | 1.40 (0.59–3.40) | 0.740 |
| Model 3 | 1.01 (0.46–2.21) | 1.37 (0.57–3.38) | 0.755 |
| Dysglycaemia | 0.97 (0.40–2.34) | 0.46 (0.18–1.12) | 0.178 |
| Model 1 | 0.88 (0.35–2.22) | 0.41 (0.16–1.04) | 0.144 |
| Model 2 | 0.83 (0.33–2.11) | 0.40 (0.15–1.03) | 0.147 |
| Model 3 | 0.85 (0.33–2.19) | 0.41 (0.16–1.06) | 0.159 |
| Low HDL-C | 0.64 (0.32–1.28) | 1.62 (0.70–3.90) | 0.090 |
| Model 1 | 0.66 (0.32–1.37) | 1.66 (0.71–4.06) | 0.110 |
| Model 2 | 0.62 (0.30–1.30) | 1.57 (0.65–3.91) | 0.115 |
| Model 3 | 0.65 (0.30–1.37) | 1.63 (0.68–4.11) | 0.120 |
| Raised LDL-C | 0.95 (0.47–1.92) | 1.43 (0.62–3.37) | 0.611 |
| Model 1 | 0.95 (0.46–1.98) | 1.42 (0.62–3.39) | 0.623 |
| Model 2 | 0.98 (0.47–2.05) | 1.43 (0.62–3.42) | 0.646 |
| Model 3 | 0.94 (0.45–1.97) | 1.39 (0.60–3.35) | 0.650 |
| Raised TG | 0.50 (0.23–1.08) | 1.06 (0.41–2.90) | 0.136 |
| Model 1 | 0.40 (0.17–0.90) | 0.97 (0.37–2.70) | 0.053 |
| Model 2 | 0.38 (0.16–0.86) | 0.99 (0.37–2.81) | 0.038 |
| Model 3 | 0.36 (0.15–0.83) | 0.96 (0.35–2.71) | 0.034 |
| Obesity | 2.00 (0.97–4.20) | 1.82 (0.80–4.28) | 0.129 |
| Model 1 | 1.74 (0.82–3.73) | 1.72 (0.74–4.11) | 0.269 |
| Model 2 | 1.67 (0.78–3.62) | 1.70 (0.73–4.11) | 0.312 |
| Model 3 | 1.62 (0.76–3.53) | 1.66 (0.71–4.02) | 0.358 |
| Subclinical inflammation | 1.95 (0.91–4.31) | 1.12 (0.49–2.61) | 0.206 |
| Model 1 | 2.00 (0.90–4.59) | 1.09 (0.47–2.61) | 0.210 |
| Model 2 | 1.96 (0.87–4.53) | 1.12 (0.48–2.70) | 0.242 |
| Model 3 | 2.00 (0.88–4.70) | 1.15 (0.48–2.79) | 0.235 |
| Food Groups | Component 1 | Component 2 | Component 3 | Component 4 | Component 5 | Component 6 | Component 7 |
| Whole grains | −0.416 | −0.110 | −0.066 | −0.111 | 0.702 | 0.218 | −0.012 |
| Fruits | −0.131 | 0.647 | −0.165 | −0.002 | 0.179 | −0.168 | −0.030 |
| Vegetables | −0.252 | 0.017 | −0.022 | 0.455 | −0.003 | 0.020 | 0.391 |
| Nuts | 0.053 | −0.230 | 0.324 | −0.228 | −0.033 | −0.050 | −0.337 |
| Legumes | 0.110 | −0.086 | 0.663 | −0.126 | 0.004 | −0.009 | −0.085 |
| Tea and coffee | −0.286 | −0.005 | 0.108 | 0.185 | 0.160 | 0.700 | −0.250 |
| Refined grains | 0.845 | −0.038 | −0.003 | 0.193 | 0.047 | −0.092 | −0.073 |
| Potatoes | 0.327 | −0.172 | 0.246 | 0.606 | 0.135 | 0.108 | −0.057 |
| Fruit juices | −0.112 | 0.755 | 0.091 | −0.040 | −0.085 | 0.185 | −0.085 |
| SSBs | 0.213 | −0.202 | 0.304 | −0.170 | 0.116 | 0.033 | 0.649 |
| Sweets and desserts | 0.055 | 0.413 | 0.534 | 0.110 | 0.028 | 0.320 | 0.174 |
| Animal fat | 0.288 | −0.056 | −0.509 | −0.262 | 0.260 | 0.408 | −0.022 |
| Egg | −0.073 | −0.018 | −0.138 | 0.012 | −0.122 | −0.053 | 0.642 |
| Dairy | 0.211 | 0.197 | 0.002 | 0.082 | 0.752 | −0.227 | −0.056 |
| Fish/seafood | 0.049 | 0.071 | −0.220 | 0.708 | −0.072 | −0.114 | −0.063 |
| Meat | 0.726 | −0.327 | 0.149 | −0.124 | −0.101 | 0.127 | 0.055 |
| Miscellaneous animal foods | −0.133 | −0.061 | 0.052 | 0.138 | 0.206 | −0.550 | −0.122 |
| Parameters | OR | 95% CI | p-Value |
|---|---|---|---|
| Refined grains and meat pattern | |||
| Hypertension | 0.89 | 0.65–1.21 | 0.448 |
| Model 1 | 0.86 | 0.62–1.18 | 0.340 |
| Model 2 | 0.83 | 0.59–1.15 | 0.261 |
| Model 3 | 0.782 | 0.56–1.10 | 0.156 |
| Dysglycaemia | 0.70 | 0.48–1.03 | 0.067 |
| Model 1 | 0.69 | 0.47–1.02 | 0.062 |
| Model 2 | 0.67 | 0.45–1.00 | 0.049 |
| Model 3 | 0.67 | 0.44–1.00 | 0.051 |
| Low HDL-C | 0.95 | 0.70–1.28 | 0.713 |
| Model 1 | 0.96 | 0.70–1.31 | 0.807 |
| Model 2 | 0.94 | 0.68–1.28 | 0.685 |
| Model 3 | 0.97 | 0.70–1.33 | 0.833 |
| Raised LDL-C | 0.97 | 0.71–1.31 | 0.829 |
| Model 1 | 0.97 | 0.71–1.32 | 0.849 |
| Model 2 | 0.97 | 0.71–1.32 | 0.845 |
| Model 3 | 0.93 | 0.68–1.28 | 0.660 |
| Raised TG | 0.89 | 0.63–1.25 | 0.509 |
| Model 1 | 0.87 | 0.62–1.24 | 0.450 |
| Model 2 | 0.86 | 0.61–1.23 | 0.415 |
| Model 3 | 0.83 | 0.58–1.19 | 0.314 |
| Obesity | 1.13 | 0.83–1.54 | 0.421 |
| Model 1 | 1.12 | 0.82–1.54 | 0.476 |
| Model 2 | 1.11 | 0.80–1.53 | 0.536 |
| Model 3 | 1.07 | 0.77–1.49 | 0.674 |
| Subclinical inflammation | 1.00 | 0.73–1.39 | 0.979 |
| Model 1 | 1.03 | 0.74–1.44 | 0.858 |
| Model 2 | 1.03 | 0.73–1.45 | 0.866 |
| Model 3 | 0.99 | 0.53–1.86 | 0.986 |
| Fish/seafood, potatoes, and vegetables pattern | |||
| Hypertension | 1.17 | 0.86–1.58 | 0.320 |
| Model 1 | 1.19 | 0.87–1.63 | 0.284 |
| Model 2 | 1.17 | 0.84–1.61 | 0.349 |
| Model 3 | 1.30 | 0.92–1.83 | 0.142 |
| Dysglycaemia | 0.91 | 0.63–1.31 | 0.606 |
| Model 1 | 0.93 | 0.64–1.36 | 0.700 |
| Model 2 | 0.91 | 0.62–1.33 | 0.630 |
| Model 3 | 0.89 | 0.60–1.32 | 0.560 |
| Low HDL-C | 1.40 | 1.03–1.90 | 0.034 |
| Model 1 | 1.41 | 1.03–1.92 | 0.030 |
| Model 2 | 1.43 | 1.04–1.96 | 0.026 |
| Model 3 | 1.37 | 0.99–1.90 | 0.055 |
| Raised LDL-C | 1.31 | 0.96–1.77 | 0.086 |
| Model 1 | 1.31 | 0.97–1.78 | 0.081 |
| Model 2 | 1.34 | 0.98–1.83 | 0.063 |
| Model 3 | 1.44 | 1.04–1.99 | 0.027 |
| Raised TG | 0.88 | 0.62–1.24 | 0.457 |
| Model 1 | 0.89 | 0.62–1.27 | 0.513 |
| Model 2 | 0.87 | 0.60–1.25 | 0.455 |
| Model 3 | 0.93 | 0.64–1.34 | 0.681 |
| Obesity | 1.08 | 0.80–1.47 | 0.613 |
| Model 1 | 1.11 | 0.81–1.52 | 0.529 |
| Model 2 | 1.09 | 0.79–1.51 | 0.585 |
| Model 3 | 1.16 | 0.83–1.63 | 0.377 |
| Subclinical inflammation | 0.79 | 0.56–1.13 | 0.196 |
| Model 1 | 0.81 | 0.56–1.17 | 0.268 |
| Model 2 | 0.79 | 0.55–1.45 | 0.221 |
| Model 3 | 0.76 | 0.52–1.13 | 0.176 |
| SSBs and egg pattern | |||
| Hypertension | 1.37 | 1.00–1.86 | 0.047 |
| Model 1 | 1.34 | 0.98–1.83 | 0.070 |
| Model 2 | 1.34 | 0.97–1.85 | 0.074 |
| Model 3 | 1.26 | 0.91–1.76 | 0.170 |
| Dysglycaemia | 1.19 | 0.86–1.66 | 0.302 |
| Model 1 | 1.17 | 0.83–1.64 | 0.367 |
| Model 2 | 1.17 | 0.84–1.64 | 0.361 |
| Model 3 | 1.15 | 0.81–1.63 | 0.438 |
| Low HDL-C | 0.81 | 0.59–1.10 | 0.181 |
| Model 1 | 0.82 | 0.60–1.11 | 0.199 |
| Model 2 | 0.81 | 0.59–1.12 | 0.199 |
| Model 3 | 0.87 | 0.63–1.20 | 0.393 |
| Raised LDL-C | 1.16 | 0.86–1.56 | 0.330 |
| Model 1 | 1.16 | 0.86–1.57 | 0.334 |
| Model 2 | 1.17 | 0.87–1.59 | 0.301 |
| Model 3 | 1.19 | 0.87–1.63 | 0.283 |
| Raised TG | 1.09 | 0.79–1.50 | 0.615 |
| Model 1 | 1.06 | 0.76–1.47 | 0.743 |
| Model 2 | 1.05 | 0.76–1.46 | 0.766 |
| Model 3 | 0.96 | 0.68–1.36 | 0.816 |
| Obesity | 1.07 | 0.79–1.44 | 0.671 |
| Model 1 | 1.03 | 0.76–1.41 | 0.849 |
| Model 2 | 1.03 | 0.75–1.40 | 0.868 |
| Model 3 | 0.96 | 0.70–1.33 | 0.817 |
| Subclinical inflammation | 1.44 | 1.05–1.98 | 0.023 |
| Model 1 | 1.46 | 1.05–2.04 | 0.023 |
| Model 2 | 1.45 | 1.04–2.01 | 0.029 |
| Model 3 | 1.53 | 1.08–2.16 | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, T.; Zemlin, A.E.; Sekgala, M.D.; Mchiza, Z.J.-R.; Erasmus, R.T.; Kengne, A.P. The Association between Plant-Based Diets and Dietary Patterns with Cardiometabolic Risk in a Sample of Commercial Taxi Drivers in South Africa. Nutrients 2023, 15, 1789. https://doi.org/10.3390/nu15071789
Lopes T, Zemlin AE, Sekgala MD, Mchiza ZJ-R, Erasmus RT, Kengne AP. The Association between Plant-Based Diets and Dietary Patterns with Cardiometabolic Risk in a Sample of Commercial Taxi Drivers in South Africa. Nutrients. 2023; 15(7):1789. https://doi.org/10.3390/nu15071789
Chicago/Turabian StyleLopes, Tatum, Annalise Edith Zemlin, Machoene Derrick Sekgala, Zandile June-Rose Mchiza, Rajiv Timothy Erasmus, and Andre Pascal Kengne. 2023. "The Association between Plant-Based Diets and Dietary Patterns with Cardiometabolic Risk in a Sample of Commercial Taxi Drivers in South Africa" Nutrients 15, no. 7: 1789. https://doi.org/10.3390/nu15071789
APA StyleLopes, T., Zemlin, A. E., Sekgala, M. D., Mchiza, Z. J.-R., Erasmus, R. T., & Kengne, A. P. (2023). The Association between Plant-Based Diets and Dietary Patterns with Cardiometabolic Risk in a Sample of Commercial Taxi Drivers in South Africa. Nutrients, 15(7), 1789. https://doi.org/10.3390/nu15071789








