Associations of Dietary Patterns and Vitamin D Levels with Iron Status in Pregnant Women: A Cross-Sectional Study in Taiwan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population Demographics
2.2. Dietary Assessment
2.3. Anthropometric and Biochemical Data Collection
2.4. Anthropometric and Biochemical Parameters in Gestational Anemia
2.5. Statistical Analysis
3. Results
3.1. Sociodemographic and Anthropometric Characteristics of the Women
3.2. Biochemical Characteristics of the Women
3.3. Dietary Intake of the Women
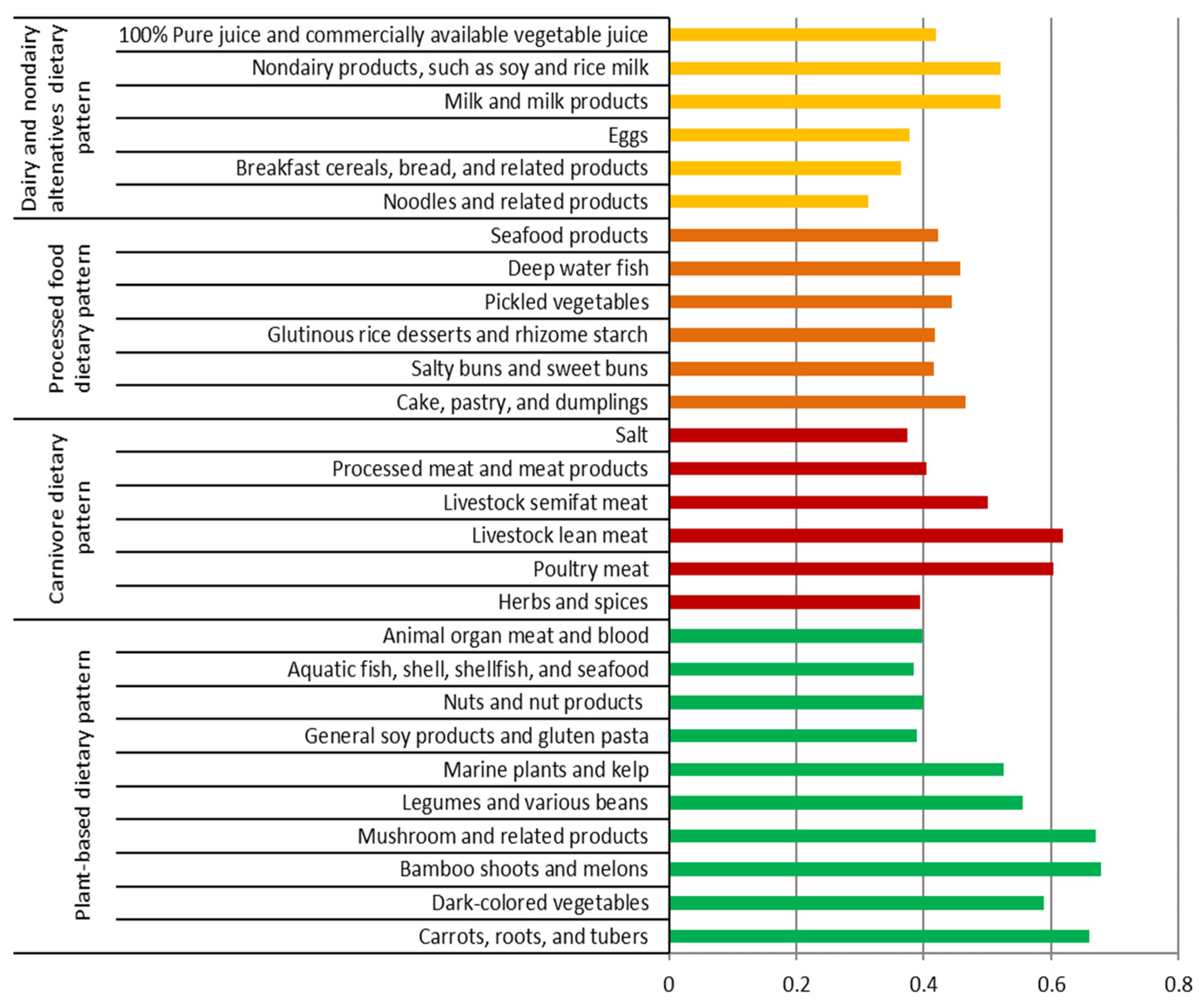
3.4. Dietary Patterns
3.5. Association of DPs with Serum-Anemia-Related Biochemical Variables
3.6. Association of DPs with the Risk of Low-Anemia-Related Biomarkers
4. Discussion
4.1. Association of Serum Vitamin D with Other Serum-Anemia-Related Biomarkers
4.2. Association of DPs with Serum-Anemia-Related Biomarkers
4.3. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, T.; Khaskheli, M.S.; Ansari, S.; Lakhan, H.; Shaikh, F.; Zardari, A.A.; Warsi, J.; Rind, N.A.; Rind, K.H.; Shar, A.H. Gestational anemia and its effects on neonatal outcome, in the population of Hyderabad, Sindh, Pakistan. Saudi J. Biol. Sci. 2022, 29, 83–87. [Google Scholar] [CrossRef]
- Imai, K. Parity-based assessment of anemia and iron deficiency in pregnant women. J. Obstet. Gynecol. 2020, 59, 838–841. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Centers for Disease Control. CDC criteria for anemia in children and childbearing-aged women. MMWR Morb. Mortal. Wkly. Rep. 1989, 38, 400–404. [Google Scholar]
- Anlaakuu, P.; Anto, F. Anaemia in pregnancy and associated factors: A cross sectional study of antenatal attendants at the Sunyani Municipal Hospital, Ghana. BMC Res. Notes 2017, 10, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wedderburn, C.J.; Ringshaw, J.E.; Donald, K.A.; Joshi, S.H.; Subramoney, S.; Fouche, J.-P.; Stadler, J.A.M.; Barnett, W.; Rehman, A.M.; Hoffman, N.; et al. Association of maternal and child anemia with brain structure in early life in South Africa. JAMA Netw. Open 2022, 5, e2244772. [Google Scholar] [CrossRef]
- Uta, M.; Neamtu, R.; Bernad, E.; Mocanu, A.G.; Gluhovschi, A.; Popescu, A.; Dahma, G.; Dumitru, C.; Stelea, L.; Citu, C.; et al. The influence of nutritional supplementation for iron deficiency anemia on pregnancies associated with SARS-CoV-2 infection. Nutrients 2022, 14, 836. [Google Scholar] [CrossRef]
- Elema, T.B.; Yimam, K.B.; Waka, F.C.; Olana, B.N. Folate and vitamin B-12 status of anemic pregnant women and association to hemoglobin during antenatal care, 17–37 weeks in Ambo Hospital, Oromia, Ethiopia, a multi regression analysis of socio-economic and serum folate and vitamin B-12. J. Nutr. Hum. Health 2018, 1, 28–34. [Google Scholar] [CrossRef] [Green Version]
- Behere, R.V.; Deshmukh, A.S.; Otiv, S.; Gupte, M.D.; Yajnik, C.S. Maternal vitamin B12 status during pregnancy and its association with outcomes of pregnancy and health of the offspring: A systematic review and implications for policy in India. Front. Endocrinol. 2021, 12, 619176. [Google Scholar] [CrossRef]
- Finkelstein, J.L.; Fothergill, A.; Krisher, J.T.; Thomas, T.; Kurpad, A.V.; Dwarkanath, P. Maternal vitamin B12 deficiency and perinatal outcomes in Southern India. PLoS ONE 2021, 16, e0248145. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Bell, S.J.; Guan, Y.; Yu, Y.H. Folic acid supplementation and pregnancy: More than just neural tube defect prevention. Rev. Obstet. Gynecol. 2011, 4, 52–59. [Google Scholar]
- Warner, M.J.; Kamran, M.T. Iron Deficiency Anemia; StatPearls Publishing: Tampa, FL, USA, 2017. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448065/ (accessed on 7 November 2022).
- Diana, A.; Purnamasari, D.M.; Rahmannia, S.; Luftimas, D.E.; Haszard, J.J.; Gibson, R.S.; Houghton, L.A. Multimicronutrient biomarkers are related to anemia during infancy in Indonesia: A repeated cross-sectional study. Curr. Dev. Nutr. 2019, 3, nzz022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daru, J.; Colman, K.; Stanworth, S.J.; De La Salle, B.; Wood, E.M.; Pasricha, S.R. Serum ferritin as an indicator of iron status: What do we need to know? Am. J. Clin. Nutr. 2017, 106, 1634S–1639S. [Google Scholar] [CrossRef] [Green Version]
- Crispin, P.; Stephens, B.; McArthur, E.; Sethna, F. First trimester ferritin screening for pre-delivery anaemia as a patient blood management strategy. Transfus. Apher. Sci. 2019, 58, 50–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rahman, S.M.; Siraj, M.S.; Islam, M.R.; Rahman, A.; Ekström, E.C. Association between maternal plasma ferritin level and infants’ size at birth: A prospective cohort study in rural Bangladesh. Glob. Health Action 2021, 1, 1870421. [Google Scholar] [CrossRef] [PubMed]
- Abu-Ouf, N.M.; Jan, M.M. The impact of maternal iron deficiency and iron deficiency anemia on child’s health. Saudi Med. J. 2015, 36, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, G.; Wu, W.; Zhang, M.; Liu, W.; Chen, Q.; Wang, X. The efficacy and safety of vitamin C for iron supplementation in adult patients with iron deficiency anemia: A randomized clinical trial. JAMA Netw. Open 2020, 3, e2023644. [Google Scholar] [CrossRef]
- Heffernan, A.; Evans, C.; Holmes, M.; Moore, J.B. The regulation of dietary iron bioavailability by vitamin C: A systematic review and meta-analysis. Proc. Nutr. Soc. 2017, 76, E182. [Google Scholar] [CrossRef] [Green Version]
- Mogire, R.M.; Muriuki, J.M.; Morovat, A.; Mentzer, A.J.; Webb, E.L.; Kimita, W.; Ndungu, F.M.; Macharia, A.W.; Cutland, C.L.; Sirima, S.B.; et al. Vitamin D deficiency and its association with iron deficiency in African children. Nutrients 2022, 14, 1372. [Google Scholar] [CrossRef]
- Smith, E.M.; Tangpricha, V. Vitamin D and anemia: Insights into an emerging association. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Arabi, S.M.; Ranjbar, G.; Bahrami, L.S.; Vafa, M.; Norouzy, A. The effect of vitamin D supplementation on hemoglobin concentration: A systematic review and meta-analysis. Nutr. J. 2020, 19, 11. [Google Scholar] [CrossRef]
- Qiu, F.; Li, R.; Gu, S.; Zhao, Y.; Yang, L. The effect of iron dextran on vitamin D3 metabolism in SD rats. Nutr. Metab. 2022, 19, 47. [Google Scholar] [CrossRef]
- Si, S.; Peng, Z.; Cheng, H.; Zhuang, Y.; Chi, P.; Alifu, X.; Zhou, H.; Mo, M.; Yu, Y. Association of vitamin D in different trimester with hemoglobin during pregnancy. Nutrients 2022, 14, 2455. [Google Scholar] [CrossRef] [PubMed]
- Mayasari, N.R.; Bai, C.H.; Hu, T.Y.; Chao, J.C.; Chen, Y.C.; Huang, Y.L.; Wang, F.F.; Tinkov, A.A.; Skalny, A.V.; Chang, J.S. Associations of food and nutrient intake with serum hepcidin and the risk of gestational iron-deficiency anemia among pregnant women: A population-based study. Nutrients 2021, 13, 3501. [Google Scholar] [CrossRef] [PubMed]
- Michalski, E.S.; Nguyen, P.H.; Gonzalez-Casanova, I.; Nguyen, S.V.; Martorell, R.; Tangpricha, V.; Ramakrishnan, U. Serum 25-hydroxyvitamin D but not dietary vitamin D intake is associated with hemoglobin in women of reproductive age in rural Northern Vietnam. J. Clin. Transl. Endocrinol. 2017, 8, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.S.; Tung, K.T.S.; Chan, Y.W.K.; Chan, B.N.K.; Leung, W.C.; Yam, J.C.; Ip, P. Adequate dietary intake and vitamin D supplementation: A study of their relative importance in determining serum vitamin D and ferritin concentrations during pregnancy. Nutrients 2022, 14, 3083. [Google Scholar] [CrossRef]
- Zhang, F.; Tapera, T.M.; Gou, J. Application of a new dietary pattern analysis method in nutritional epidemiology. BMC Med. Res. Methodol. 2018, 18, 119. [Google Scholar] [CrossRef]
- Zang, J.; Luo, B.; Chang, S.; Jin, S.; Shan, C.; Ma, L.; Zhu, Z.; Guo, C.; Zou, S.; Jia, X.; et al. Validity and reliability of a food frequency questionnaire for assessing dietary intake among Shanghai residents. Nutr. J. 2019, 18, 30. [Google Scholar] [CrossRef] [Green Version]
- Schwedhelm, C.; Iqbal, K.; Knüppel, S.; Schwingshackl, L.; Boeing, H. Contribution to the understanding of how principal component analysis–derived dietary patterns emerge from habitual data on food consumption. Am. J. Clin. Nutr. 2018, 107, 227–235. [Google Scholar] [CrossRef] [Green Version]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, C.M.; Looker, A.C. Laboratory methodologies for indicators of iron status: Strengths, limitations, and analytical challenges. Am. J. Clin. Nutr. 2017, 106, 1606S–1614S. [Google Scholar] [CrossRef] [Green Version]
- Yamanishi, H.; Iyama, S.; Yamaguchi, Y.; Kanakura, Y.; Iwatani, Y. Total iron-binding capacity calculated from serum transferrin concentration or serum iron concentration and unsaturated iron-binding capacity. Clin. Chem. 2003, 49, 175–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shane, B. Folate status assessment history: Implications for measurement of biomarkers in NHANES. Am. J. Clin. Nutr. 2011, 94, 337S–342S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karmi, O.; Zayed, A.; Baraghethi, S.; Qadi, M.; Ghanem, R. Measurement of vitamin B12 concentration: A review on available methods. IIOAB J. 2011, 2, 23–32. [Google Scholar]
- Abdel-Wareth, L.; Haq, A.; Turner, A.; Khan, S.; Salem, A.; Mustafa, F.; Hussein, N.; Pallinalakam, F.; Grundy, L.; Patras, G.; et al. Total vitamin D assay comparison of the Roche Diagnostics “Vitamin D total” electrochemiluminescence protein binding assay with the Chromsystems HPLC method in a population with both D2 and D3 forms of vitamin D. Nutrients 2013, 5, 971–980. [Google Scholar] [CrossRef]
- Health Promotion Administration, Ministry of Health and Welfare. Taiwan’s Obesity Prevention and Management Strategy; Health Promotion Administration, Ministry of Health and Welfare: Taipei, Taiwan, 2018; p. 55.
- Bellanger, R.A. Iron deficiency anemia in women. US Pharm. 2010, 35, 50–58. [Google Scholar]
- Sukla, S.K.; Mohanty, P.K.; Patel, S.; Das, K.; Hiregoudar, M.; Soren, U.K.; Meher, S. Iron profile of pregnant sickle cell anemia patients in Odisha, India. Hematol. Transfus. Cell Ther. 2021; in press. [Google Scholar] [CrossRef]
- Word Health Organization. WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- World Health Organization. Archived: Iron Deficiency Anemia: Assessment, Prevention and Control; World Health Organization: Geneva, Switzerland, 2001; pp. 47–62. [Google Scholar]
- World Health Organization. Serum and Red Blood Cell Folate Concentrations for Assessing Folate Status in Populations. Vitamin and Mineral Nutrition Information System; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Kurniawan, A.; Hsu, C.-Y.; Rau, H.; Lin, L.-Y.; Chao, J. Dietary patterns in relation to testosterone levels and severity of impaired kidney function among middle-aged and elderly men in Taiwan: A cross-sectional study. Nutr. J. 2019, 18, 42. [Google Scholar] [CrossRef] [Green Version]
- Szumilas, M. Explaining odds ratios. J. Can. Acad. Child Adolesc. Psychiatry 2010, 19, 227–229. [Google Scholar]
- Judistiani, R.T.D.; Gumilang, L.; Nirmala, S.A.; Irianti, S.; Wirhana, D.; Permana, I.; Sofjan, L.; Duhita, H.; Tambunan, L.A.; Gurnadi, J.I.; et al. Association of colecalciferol, ferritin, and anemia among pregnant women: Result from cohort study on vitamin D status and its impact during pregnancy and childhood in Indonesia. Anemia 2018, 2018, 2047981. [Google Scholar] [CrossRef]
- Perzia, B.M.; Ying, G.-S.; Dunaief, J.L.; Dunaief, D.M. Reduction in ferritin concentrations among patients consuming a dark-green leafy vegetable-rich, low inflammatory foods everyday (LIFE) diet. Curr. Dev. Nutr. 2022, 6, nzac095. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Kim, E.-Y.; Lindsay, E.; Han, O. Bioactive dietary polyphenols inhibit heme iron absorption in a dose-dependent manner in human intestinal Caco-2 cells. J. Food Sci. 2011, 76, H143–H150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piskin, E.; Cianciosi, D.; Gulec, S.; Tomas, M.; Capanoglu, E. Iron absorption: Factors, limitations, and improvement methods. ACS Omega 2022, 7, 20441–20456. [Google Scholar] [CrossRef]
- Koebnick, C.; Heins, U.A.; Hoffmann, I.; Dagnelie, P.C.; Leitzmann, C. Folate status during pregnancy in women is improved by long-term high vegetable intake compared with the average western diet. J. Nutr. 2001, 131, 733–739. [Google Scholar] [CrossRef] [Green Version]
- Specker, B.L.; Tsang, R.C.; Ho, M.; Miller, D. Effect of vegetarian diet on serum 1,25-dihydroxyvitamin D concentrations during lactation. Obstet. Gynecol. 1987, 70, 870–874. [Google Scholar] [PubMed]
- Bhatnagar, R.S.; Padilla-Zakour, O.I. Plant-based dietary practices and socioeconomic factors that influence anemia in India. Nutrients 2021, 13, 3538. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, R.; Berger, J.; Hines, I. Iron status of vegetarian adults: A review of literature. Am. J. Lifestyle Med. 2018, 12, 486–498. [Google Scholar] [CrossRef]
- Jackson, J.; Williams, R.; McEvoy, M.; MacDonald-Wicks, L.; Patterson, A. Is higher consumption of animal flesh foods associated with better iron status among adults in developed countries? A systematic review. Nutrients 2016, 8, 89. [Google Scholar] [CrossRef] [Green Version]
- Tuntipopipat, S.; Zeder, C.; Siriprapa, P.; Charoenkiatkul, S. Inhibitory effects of spices and herbs on iron availability. Int. J. Food Sci. Nutr. 2009, 60, 43–55. [Google Scholar] [CrossRef]
- Broderstad, A.R.; Melhus, M.; Brustad, M.; Lund, E. Iron stores in relation to dietary patterns in a multiethnic population: The SAMINOR study. Public Health Nutr. 2011, 14, 1039–1046. [Google Scholar] [CrossRef] [Green Version]
- Denissen, K.F.M.; Heil, S.G.; Eussen, S.J.P.M.; Heeskens, J.P.J.; Thijs, C.; Mommers, M.; Smits, L.J.M.; van Dongen, M.C.J.M.; Dagnelie, P.C. Intakes of vitamin B-12 from dairy food, meat, and fish and shellfish are independently and positively associated with vitamin B-12 biomarker status in pregnant Dutch women. J. Nutr. 2019, 149, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGowan, C.A.; Byrne, J.; Walsh, J.; McAuliffe, F.M. Insufficient vitamin D intakes among pregnant women. Eur. J. Clin. Nutr. 2011, 65, 1076–1078. [Google Scholar] [CrossRef] [PubMed]
- Gille, D.; Schmid, A. Vitamin B12 in meat and dairy products. Nutr. Rev. 2015, 73, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Paula, W.O.; Gonçalves, V.S.S.; Patriota, E.S.O.; Franceschini, S.C.C.; Pizato, N. Impact of ultra-processed food consumption on quality of diet among Brazilian pregnant women assisted in primary health care. Int. J. Environ. Res. Public Health 2023, 20, 1015. [Google Scholar] [CrossRef]
- Cifelli, C.J.; Agarwal, S.; Fulgoni, V.L., III. Association between intake of total dairy and individual dairy foods and markers of folate, vitamin B6 and vitamin B12 status in the U.S. Population. Nutrients 2022, 14, 2441. [Google Scholar] [CrossRef]
- Matte, J.J.; Britten, M.; Girard, C.L. The importance of milk as a source of vitamin B12 for human nutrition. Anim. Front. 2014, 4, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Polzonetti, V.; Pucciarelli, S.; Vincenzetti, S.; Polidori, P. Dietary intake of vitamin D from dairy products reduces the risk of osteoporosis. Nutrients 2020, 12, 1743. [Google Scholar] [CrossRef]
- Dasgupta, A.; Saikia, U.; Sarma, D. Status of 25(OH)D levels in pregnancy: A study from the North Eastern part of India. Indian J. Endocrinol. Metab. 2012, 16, S405–S407. [Google Scholar] [CrossRef]
- Huang, Y.-L.; Pham, T.T.M.; Chen, Y.-C.; Chang, J.-S.; Chao, J.C.-J.; Bai, C.-H. Effects of climate, sun exposure, and dietary intake on vitamin D concentrations in pregnant women: A population-based study. Nutrients 2023, 15, 1182. [Google Scholar] [CrossRef]
- Pagani, A.; Nai, A.; Silvestri, L.; Camaschella, C. Hepcidin and anemia: A tight relationship. Front. Physiol. 2019, 10, 1294. [Google Scholar] [CrossRef]
- Moran-Lev, H.; Weisman, Y.; Cohen, S.; Deutsch, V.; Cipok, M.; Bondar, E.; Lubetzky, R.; Mandel, D. The interrelationship between hepcidin, vitamin D, and anemia in children with acute infectious disease. Pediatr. Res. 2018, 84, 62–65. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, M.G.; Milte, C.M.; Crawford, D.; McNaughton, S.A. A comparison of the dietary patterns derived by principal component analysis and cluster analysis in older Australians. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, L.C.; Lin, C.F.; Chang, F.H.; Chen, H.F.; Lo, C.C.; Ho, H.F. Meal distribution, relative validity and reproducibility of a meal-based food frequency questionnaire in Taiwan. Asia Pac. J. Clin. Nutr. 2007, 16, 766–776. [Google Scholar] [PubMed]
| Variables | Total (n) | Tertiles of Serum Vitamin D 2 | |||
|---|---|---|---|---|---|
| T1 (n = 505) | T2 (n = 486) | T3 (n = 511) | p-Value 3 | ||
| Sociodemographic Data | |||||
| Age (years) | 1502 | 32.0 ± 4.7 a | 32.7 ± 4.7 ab | 32.9 ± 4.9 b | 0.008 |
| Region of residence | 1499 | 0.000 | |||
| Northern | 231 (45.8) | 153 (31.5) | 117 (23.0) | ||
| Central | 130 (25.8) | 124 (25.5) | 117 (23.0) | ||
| Southern | 51 (10.1) | 77 (15.8) | 163 (32.0) | ||
| Eastern and other | 92 (18.3) | 132 (27.2) | 112 (22.0) | ||
| Parity | 1497 | 0.002 | |||
| 1 | 306 (60.7) | 267 (55.2) | 251 (49.3) | ||
| 2 | 164 (32.5) | 170 (35.1) | 193 (37.9) | ||
| ≥3 | 34 (6.8) | 47 (9.7) | 65 (12.8) | ||
| Trimester | 1502 | 0.000 | |||
| First, weeks 0–12 | 172 (34.1) | 125 (25.7) | 78 (15.3) | ||
| Second, weeks 13–26 | 164 (32.5) | 159 (32.7) | 162 (31.7) | ||
| Third, weeks 27–40 | 169 (33.4) | 202 (41.6) | 271 (53.0) | ||
| Education | 1493 | 0.165 | |||
| ≤Junior high school | 68 (13.5) | 72 (15.0) | 97 (19.1) | ||
| College or university | 355 (70.3) | 330 (68.8) | 340 (66.9) | ||
| Graduate school | 82 (16.2) | 78 (16.2) | 71 (14.0) | ||
| Monthly income (NTD) | 1474 | 0.117 | |||
| <30,000 | 63 (12.6) | 69 (14.8) | 80 (15.8) | ||
| 30,000–59,999 | 209 (41.7) | 187 (40.1) | 238 (46.9) | ||
| 60,000–99,999 | 162 (32.3) | 150 (32.2) | 131 (25.8) | ||
| ≥100,000 | 67 (13.4) | 60 (12.9) | 58 (11.5) | ||
| Sun exposure (min/d) | 1498 | 0.676 | |||
| <10 | 158 (31.3) | 147 (30.4) | 155 (30.5) | ||
| 10 to <20 | 155 (30.7) | 150 (31.0) | 144 (28.2) | ||
| 20 to <60 | 172 (34.0) | 158 (32.6) | 179 (35.2) | ||
| ≥60 | 20 (4.0) | 29 (6.0) | 31 (6.1) | ||
| Anthropometric Data | |||||
| Pre-pregnant BMI (kg/m2) | 1479 | 22.4 ± 3.9 | 22.9 ± 4.2 | 22.8 ± 4.0 | 0.082 |
| <18.5 | 52 (10.5) | 45 (9.4) | 44 (8.7) | 0.739 | |
| 18.5 to <24.0 | 312 (62.9) | 285 (59.6) | 309 (61.2) | ||
| 24.0 to <27.0 | 71 (14.3) | 76 (15.9) | 84 (16.6) | ||
| ≥27 | 61 (12.3) | 72 (15.1) | 68 (13.5) | ||
| Serum Variables | Tertiles of Serum Vitamin D 2 | |||
|---|---|---|---|---|
| T1 (n = 505) | T2 (n = 486) | T3 (n = 511) | p-Value 3 | |
| Hemoglobin (mmol/L) | 7.2 ± 1.1 a | 7.3 ± 1.2 ab | 7.4 ± 1.3 b | 0.038 |
| <4.34 | 4 (0.8) | 5 (1.0) | 1 (0.2) | 0.051 |
| 4.34–6.14 | 38 (7.5) | 43 (8.9) | 42 (8.2) | |
| 6.15–6.76 | 80 (15.8) | 50 (10.3) | 59 (11.5) | |
| 6.77–8.69 | 364 (72.1) | 369 (75.9) | 376 (73.6) | |
| >8.69 | 19 (3.8) | 19 (3.9) | 33 (6.5) | |
| Iron (µmol/L) | 12.0 ± 6.8 a | 12.8 ± 6.6 a | 13.9 ± 7.8 b | 0.000 |
| Ferritin (nmol/L) | 0.06± 0.15 a | 0.05 ± 0.06 ab | 0.05 ± 0.05 b | 0.028 |
| TIBC (µmol/L) | 81.5 ± 19.9 a | 83.2 ± 17.2 ab | 85.6 ± 17.1 b | 0.001 |
| Transferrin saturation (%) | 16.2 ± 9.4 | 16.6 ± 10.1 | 16.7 ± 10.2 | 0.779 |
| Folate (nmol/L) | 25.5 ± 15.6 a | 29.2 ± 16.6 b | 32.3 ± 17.0 c | 0.000 |
| <6.8 | 13 (2.6) | 10 (2.0) | 10 (2.0) | 0.000 |
| 6.8–13.4 | 101 (20.0) | 65 (13.4) | 54 (10.6) | |
| 13.5–45.3 | 342 (67.7) | 346 (71.2) | 349 (68.3) | |
| >45.3 | 49 (9.7) | 65 (13.4) | 98 (19.2) | |
| Vitamin B12 (pmol/L) | 215.1 ± 154.9 a | 232.1 ± 108.9 ab | 249.0 ± 169.8 b | 0.001 |
| Vitamin D (nmol/L) | 42.4 ± 8.0 a | 62.3 ± 5.2 b | 89.1 ± 15.1 c | 0.000 |
| Variables | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | |
| Hemoglobin (mmol/L) | −0.04 (−1.76, 1.50) | 0.01 (−1.42, 1.85) | 0.00 (−1.52, 1.13) |
| Iron (µmol/L) | −0.04 (−0.46, 0.08) | −0.03 (−0.45, 1.01) | −0.03 (−0.46, 0.09) |
| Ferritin (nmol/L) | −0.06 (−0.29, −0.01) * | −0.02 (−0.20, 0.07) | −0.02 (−0.19, 0.09) |
| TIBC (µmol/L) | 0.09 (0.02, 0.10) *** | 0.02 (−0.01, 0.03) | 0.02 (−0.01, 0.03) |
| Transferrin saturation (%) | 0.00 (−0.01, 0.01) | 0.00 (−0.01, 0.01) | −0.00 (−0.01, 0.01) |
| Folate (nmol/L) | 0.01 (−0.02, 0.03) | 0.02 (−0.01, 0.03) | 0.02 (−0.01, 0.03) |
| Vitamin B12 (pmol/L) | −0.04 (−0.36, 0.04) | −0.02 (−0.28, 0.11) | −0.02 (−0.27, 0.13) |
| Vitamin D (nmol/L) | 0.08 (0.02, 0.08) ** | 0.06 (0.00, 0.06) * | 0.04 (−0.00, 0.05) * |
| Variables | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | |
| Hemoglobin (mmol/L) | −0.03 (−1.82, 0.43) | −0.02 (−1.56, 0.74) | −0.02 (−1.60, 0.69) |
| Iron (µmol/L) | −0.08 (−0.49, −0.10) ** | −0.07 (−0.47, −0.07) ** | −0.08 (−0.50, −0.11) ** |
| Ferritin (nmol/L) | −0.06 (−0.46, −0.04) * | −0.03 (−0.34, 0.06) | −0.03 (−0.32, 0.08) |
| TIBC (µmol/L) | 0.08 (0.02, 0.10) ** | 0.02 (−0.01, 0.04) | 0.02 (−0.01, 0.04) |
| Transferrin saturation (%) | 0.01 (−0.02, 0.01) | −0.01 (−0.02, 0.01) | −0.02 (−0.02, 0.01) |
| Folate (nmol/L) | −0.02 (−0.05, 0.02) | −0.01 (−0.00, 0.00) | −0.00 (−0.03, 0.03) |
| Vitamin B12 (pmol/L) | 0.00 (−0.29, 0.29) | 0.02 (−0.18, 0.39) | 0.01 (−0.21, 0.36) |
| Vitamin D (nmol/L) | 0.08 (0.02, 0.10) ** | 0.06 (0.00, 0.08) * | 0.04 (−0.00, 0.07) * |
| Variables | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| β (95% CI) | β (95% CI) | β (95% CI) | |
| Hemoglobin (mmol/L) | −0.03 (−1.75, 0.33) | −0.03 (−0.30, 0.06) | −0.03 (−1.70, 0.41) |
| Iron (µmol/L) | −0.04 (−0.29, 0.05) | −0.03 (−0.30, 0.06) | −0.04 (−0.32, 0.04) |
| Ferritin (nmol/L) | −0.04 (−0.41, 0.03) | −0.02 (−0.30, 0.12) | −0.04 (−0.31, 2.00) |
| TIBC (µmol/L) | 0.08 (0.02, 0.10) ** | 0.02 (−0.01, 0.05) | 0.02 (−0.01, 0.05) |
| Transferrin saturation (%) | 0.01 (−0.02, 0.02) | 0.01 (−0.02, 0.02) | 0.01 (−0.02, 0.02) |
| Folate (nmol/L) | 0.03 (−0.02, 0.05) | 0.04 (−0.01, 0.06) | 0.04 (−0.01, 0.06) |
| Vitamin B12 (pmol/L) | −0.01 (−0.38, 0.24) | 0.01 (−0.25, 0.35) | 0.01 (−0.27, 0.34) |
| Vitamin D (nmol/L) | 0.05 (0.02, 0.09) * | 0.04 (−0.00, 0.08) * | 0.03 (−0.01, 0.07) |
| Variables 2 | Plant-Based Dietary Pattern 3 | |||||
|---|---|---|---|---|---|---|
| Model 1 OR (95% Confidence Interval) | Model 2 OR (95% Confidence Interval) | Model 3 OR (95% Confidence Interval) | ||||
| T2 | T3 | T2 | T3 | T2 | T3 | |
| Hemoglobin (mmol/L) | 1.40 (0.44, 4.45) | 1.00 (0.35, 2.87) | 1.53 (0.47, 4.96) | 0.94 (0.32, 2.77) | 1.12 (0.32, 3.87) | 0.65 (0.20, 2.04) |
| Iron (µmol/L) | 0.98 (0.76, 1.25) | 1.17 (0.91, 1.50) | 0.98 (0.75, 1.27) | 1.14 (0.88, 1.49) | 0.96 (0.73, 1.25) | 1.14 (0.87, 1.49) |
| Ferritin (nmol/L) | 0.98 (0.76, 1.25) | 0.73 (0.57, 0.94) * | 0.92 (0.69, 1.22) | 1.17 (0.87, 1.57) | 0.89 (0.67, 1.20) | 1.16 (0.86, 1.56) |
| TIBC (µmol/L) | 1.00 (0.02, 1.28) | 1.33 (0.04, 1.52) | 1.07 (0.14, 1.48) | 0.95 (0.12, 1.34) | 1.06 (0.14, 1.48) | 0.97 (0.12, 1.37) |
| Transferrin saturation (%) | 1.06 (0.82, 1.36) | 0.84 (0.65, 1.07) | 1.04 (0.81, 1.34) | 0.83 (0.65, 1.07) | 1.03 (0.80, 1.33) | 0.83 (0.64, 1.07) |
| Folate (nmol/L) | 0.61 (0.44, 0.85) ** | 0.66 (0.48, 0.92) * | 0.61 (0.42, 0.88) ** | 0.68 (0.47,0.98) * | 0.60 (0.41, 0.87) ** | 0.69 (0.47, 1.00) |
| Vitamin B12 (pmol/L) | 0.86 (0.64, 1.16) | 1.01 (0.76, 1.35) | 0.86 (0.64, 1.18) | 0.95 (0.70, 1.29) | 0.88 (0.67, 1.24) | 1.04 (0.77, 1.42) |
| Vitamin D (nmol/L) | 0.69 (0.52,0.92) * | 0.75 (0.57, 0.99) * | 0.68 (0.51, 0.91) * | 0.81 (0.60, 1.08) | 0.69 (0.52, 0.93) * | 0.84 (0.62, 1.13) |
| Variables 2 | Carnivore Dietary Pattern 3 | |||||
|---|---|---|---|---|---|---|
| Model 1 OR (95% Confidence Interval) | Model 2 OR (95% Confidence Interval) | Model 3 OR (95% Confidence Interval) | ||||
| T2 | T3 | T2 | T3 | T2 | T3 | |
| Hemoglobin (mmol/L) | 0.55 (0.18, 1.66) | 1.00 (0.29, 3.48) | 0.51 (0.17, 1.58) | 1.03 (0.29, 3.68) | 0.47 (0.14, 1.56) | 0.93 (0.24, 3.54) |
| Iron (µmol/L) | 1.36 (1.06,1.76) * | 1.33 (1.03, 1.72) * | 1.24 (0.95, 1.63) | 1.33 (1.02, 1.74) * | 1.30 (0.99, 1.72) | 1.33 (1.02, 1.75) * |
| Ferritin (nmol/L) | 1.29 (1.00, 1.66) | 1.30 (1.00, 1.68) | 1.24 (0.92, 1.66) | 1.15 (0.86, 1.54) | 1.24 (0.92, 1.67) | 1.16 (0.86, 1.56) |
| TIBC (µmol/L) | 0.83 (0.63, 1.08) | 0.78 (0.59, 1.01) | 0.92 (0.65, 1.28) | 1.00 (0.14, 1.11) | 1.00 (0.13, 1.27) | 1.04 (0.14, 1.11) |
| Transferrin saturation (%) | 0.86 (0.66, 1.10) | 0.93 (0.72, 1.20) | 0.84 (0.65, 1.08) | 0.92 (0.71, 1.19) | 0.70 (0.54, 0.91) ** | 0.93 (0.72, 1.21) |
| Folate (nmol/L) | 1.18 (0.84, 1.68) | 1.30 (0.92, 1.84) | 1.15 (0.79, 1.69) | 1.25 (0.85, 1.83) | 1.15 (0.78, 1.70) | 1.26 (0.86, 1.86) |
| Vitamin B12 (pmol/L) | 0.85 (0.63, 1.14) | 0.89 (0.67, 1.19) | 0.56 (0.37, 0.84) ** | 0.54 (0.35, 0.82) ** | 0.68 (0.52, 0.90) ** | 0.25(0.17, 0.37) *** |
| Vitamin D (nmol/L) | 0.69 (0.52, 0.92) * | 0.55 (0.41, 0.74) *** | 0.70 (0.52, 0.94) * | 0.58 (0.43, 0.78) *** | 0.70 (0.52, 0.95) ** | 0.59 (0.44, 0.80) ** |
| Variables 2 | Dairy and Nondairy Alternatives Dietary Pattern 3 | |||||
|---|---|---|---|---|---|---|
| Model 1 OR (95% Confidence Interval) | Model 2 OR (95% Confidence Interval) | Model 3 OR (95% Confidence Interval) | ||||
| T2 | T3 | T2 | T3 | T2 | T3 | |
| Hemoglobin (mmol/L) | 0.63 (0.24, 1.65) | 0.69 (0.35, 1.67) | 0.73 (0.27, 1.95) | 1.12 (0.72, 1.83) | 0.61 (0.21, 1.78) | 0.98 (0.41, 1.89) |
| Iron (µmol/L) | 0.83 (0.65, 1.07) | 0.90 (0.70, 1.16) | 0.81 (0.62, 1.06) | 0.84 (0.64, 1.10) | 0.83 (0.63, 1.08) | 0.85 (0.65, 1.12) |
| Ferritin (nmol/L) | 0.91 (0.71, 1.17) | 1.11 (0.86, 1.42) | 0.91 (0.71, 1.17) | 1.11 (0.86, 1.42) | 0.85 (0.64, 1.15) | 0.87 (0.64, 1.17) |
| TIBC (µmol/L) | 1.03 (0.14, 1.26) | 0.71 (0.54, 0.93) * | 1.10 (0.15, 1.30) | 0.94 (0.12, 1.24) | 1.00 (0.16, 1.31) | 0.95 (0.12, 1.25) |
| Transferrin saturation (%) | 1.04 (0.81, 1.33) | 0.96 (0.75, 1.23) | 1.02 (0.79, 1.31) | 0.95 (0.74, 1.22) | 1.02 (0.79, 1.31) | 0.96 (0.74, 1.24) |
| Folate (nmol/L) | 0.72 (0.52, 1.01) | 0.75 (0.53, 1.04) | 0.80 (0.55, 1.15) | 0.67 (0.47, 0.97) * | 0.80 (0.55, 1.16) | 0.67 (0.46, 0.98) * |
| Vitamin B12 (pmol/L) | 0.80 (0.60, 1.07) | 0.73 (0.54, 0.97) * | 0.79 (0.59, 1.07) | 0.63 (0.46, 0.85) ** | 0.81 (0.60, 1.10) | 0.66 (0.48, 0.90) * |
| Vitamin D (nmol/L) | 0.79 (0.60, 1.05) | 0.72 (0.54, 0.96) * | 0.79 (0.59, 1.06) | 0.72 (0.54, 0.97) * | 0.80 (0.60, 1.08) | 0.75 (0.55, 1.02) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, A.; Bai, C.-H.; Chang, J.-S.; Huang, Y.-L.; Wang, F.-F.; Chen, Y.-C.; Chao, J.C.-J. Associations of Dietary Patterns and Vitamin D Levels with Iron Status in Pregnant Women: A Cross-Sectional Study in Taiwan. Nutrients 2023, 15, 1805. https://doi.org/10.3390/nu15081805
Das A, Bai C-H, Chang J-S, Huang Y-L, Wang F-F, Chen Y-C, Chao JC-J. Associations of Dietary Patterns and Vitamin D Levels with Iron Status in Pregnant Women: A Cross-Sectional Study in Taiwan. Nutrients. 2023; 15(8):1805. https://doi.org/10.3390/nu15081805
Chicago/Turabian StyleDas, Arpita, Chyi-Huey Bai, Jung-Su Chang, Ya-Li Huang, Fan-Fen Wang, Yi-Chun Chen, and Jane C.-J. Chao. 2023. "Associations of Dietary Patterns and Vitamin D Levels with Iron Status in Pregnant Women: A Cross-Sectional Study in Taiwan" Nutrients 15, no. 8: 1805. https://doi.org/10.3390/nu15081805
APA StyleDas, A., Bai, C.-H., Chang, J.-S., Huang, Y.-L., Wang, F.-F., Chen, Y.-C., & Chao, J. C.-J. (2023). Associations of Dietary Patterns and Vitamin D Levels with Iron Status in Pregnant Women: A Cross-Sectional Study in Taiwan. Nutrients, 15(8), 1805. https://doi.org/10.3390/nu15081805










