The Prospective Associations of Egg Consumption with the Risk of Total Cerebrovascular Disease Morbidity among Chinese Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.3. Outcome Variables
2.4. Statistical Methods
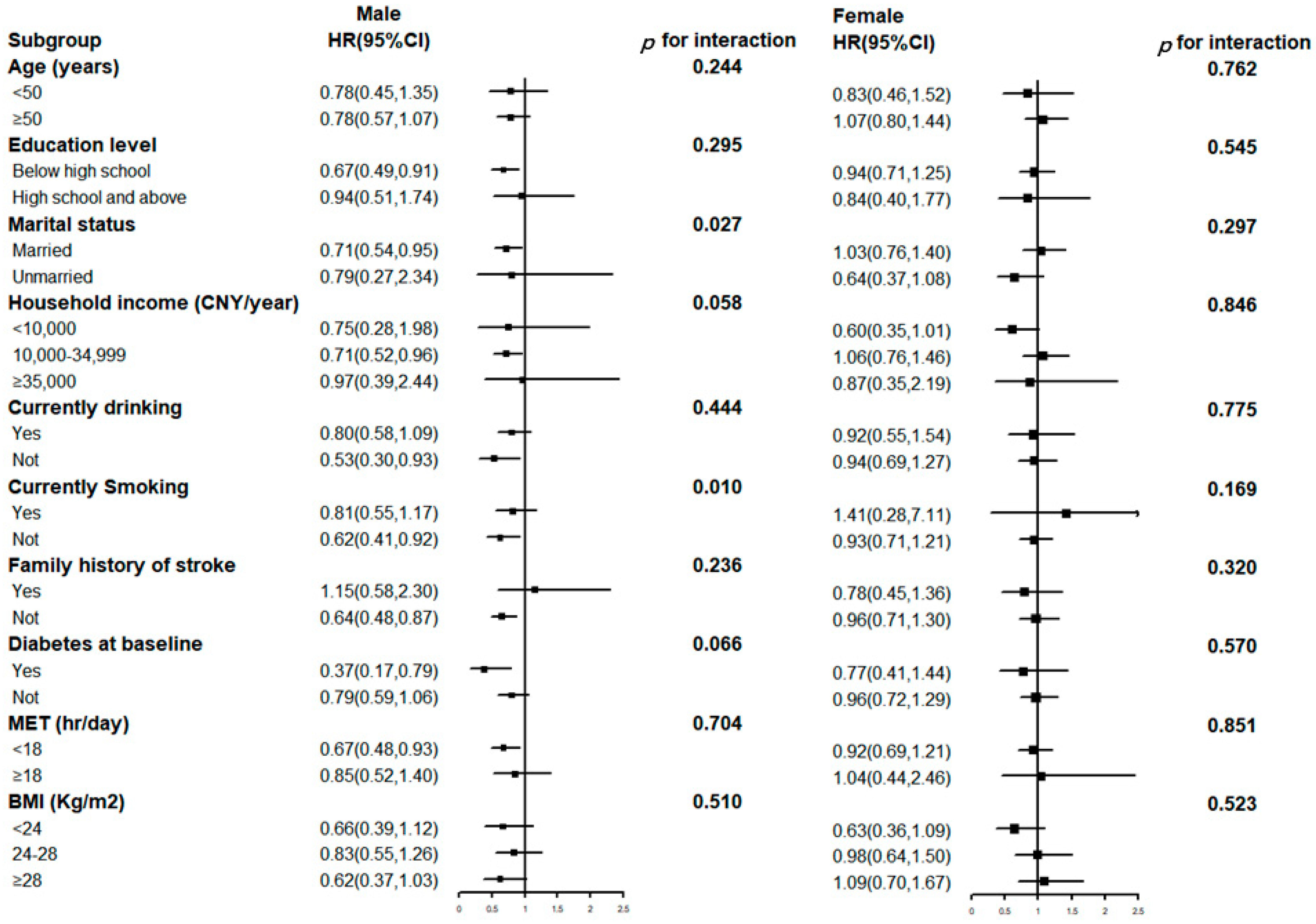
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vos, T.; Lim, S.S.; Abbafati, C.; Abbas, K.M.; Abbasi, M.; Abbasifard, M.; Abbasi-Kangevari, M.; Abbastabar, H.; Abd-Allah, F.; Abdelalim, A.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Feigin, V.L.; Stark, B.; Johnson, C.O.; Roth, G.; Bisignano, C.; Abady, G.G.; Abbasifard, M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abedi, V.; et al. Global, regional, and national burden of stroke and its risk factors, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021, 20, 795–820. [Google Scholar] [CrossRef]
- Report on Cardiovascular Health and Diseases in China 2021: An Updated Summary. Biomed. Environ. Sci. BES 2022, 35, 573–603. [CrossRef]
- Ma, Q.; Li, R.; Wang, L.; Yin, P.; Wang, Y.; Yan, C.; Ren, Y.; Qian, Z.; Vaughn, M.G.; McMillin, S.E.; et al. Temporal trend and attributable risk factors of stroke burden in China, 1990–2019: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2021, 6, e897–e906. [Google Scholar] [CrossRef]
- Huang, L.; Wang, Z.; Wang, H.; Zhao, L.; Jiang, H.; Zhang, B.; Ding, G. Nutrition transition and related health challenges over decades in China. Eur. J. Clin. Nutr. 2021, 75, 247–252. [Google Scholar] [CrossRef]
- Gui, Z.H.; Zhu, Y.N.; Cai, L.; Sun, F.H.; Ma, Y.H.; Jing, J.; Chen, Y.J. Sugar-Sweetened Beverage Consumption and Risks of Obesity and Hypertension in Chinese Children and Adolescents: A National cross-Sectional Analysis. Nutrients 2017, 9, 1302. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Zhang, B.; Zhai, F.; Wang, H.; Zhang, J.; Du, W.; Su, C.; Zhang, J.; Jiang, H.; Popkin, B.M. Fatty and lean red meat consumption in China: Differential association with Chinese abdominal obesity. Nutr. Metab. Cardiovasc. Dis. NMCD 2014, 24, 869–876. [Google Scholar] [CrossRef] [Green Version]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrients 2019, 11, 684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nimalaratne, C.; Wu, J. Hen Egg as an Antioxidant Food Commodity: A Review. Nutrients 2015, 7, 8274–8293. [Google Scholar] [CrossRef] [Green Version]
- Mutungi, G.; Ratliff, J.; Puglisi, M.; Torres-Gonzalez, M.; Vaishnav, U.; Leite, J.O.; Quann, E.; Volek, J.S.; Fernandez, M.L. Dietary cholesterol from eggs increases plasma HDL cholesterol in overweight men consuming a carbohydrate-restricted diet. J. Nutr. 2008, 138, 272–276. [Google Scholar] [CrossRef] [Green Version]
- Goodrow, E.F.; Wilson, T.A.; Houde, S.C.; Vishwanathan, R.; Scollin, P.A.; Handelman, G.; Nicolosi, R.J. Consumption of one egg per day increases serum lutein and zeaxanthin concentrations in older adults without altering serum lipid and lipoprotein cholesterol concentrations. J. Nutr. 2006, 136, 2519–2524. [Google Scholar] [CrossRef] [Green Version]
- Mazidi, M.; Katsiki, N.; Mikhailidis, D.P.; Pencina, M.J.; Banach, M. Egg Consumption and Risk of Total and Cause-Specific Mortality: An Individual-Based Cohort Study and Pooling Prospective Studies on Behalf of the Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. J. Am. Coll. Nutr. 2019, 38, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Lv, J.; Guo, Y.; Bian, Z.; Si, J.; Yang, L.; Chen, Y.; Zhou, Y.; Zhang, H.; Liu, J.; et al. Associations of egg consumption with cardiovascular disease in a cohort study of 0.5 million Chinese adults. Heart (Br. Card. Soc.) 2018, 104, 1756–1763. [Google Scholar] [CrossRef] [Green Version]
- Tong, T.Y.N.; Appleby, P.N.; Key, T.J.; Dahm, C.C.; Overvad, K.; Olsen, A.; Tjønneland, A.; Katzke, V.; Kühn, T.; Boeing, H.; et al. The associations of major foods and fibre with risks of ischaemic and haemorrhagic stroke: A prospective study of 418 329 participants in the EPIC cohort across nine European countries. Eur. Heart J. 2020, 41, 2632–2640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Ramady, O.; Latifi, A.N.; Treu, T.; Ho, Y.L.; Seshadri, S.; Aparicio, H.J.; Cho, K.; Wilson, P.W.; Gaziano, J.M.; Djoussé, L. Egg consumption and risk of acute stroke in the Million Veteran Program. Clin. Nutr. ESPEN 2022, 50, 178–182. [Google Scholar] [CrossRef]
- Abdollahi, A.M.; Virtanen, H.E.K.; Voutilainen, S.; Kurl, S.; Tuomainen, T.P.; Salonen, J.T.; Virtanen, J.K. Egg consumption, cholesterol intake, and risk of incident stroke in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2019, 110, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Larsson, S.C.; Åkesson, A.; Wolk, A. Egg consumption and risk of heart failure, myocardial infarction, and stroke: Results from 2 prospective cohorts. Am. J. Clin. Nutr. 2015, 102, 1007–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohammadifard, N.; Taheri, M.; Haghighatdoost, F.; Grau, N.; Najafian, J.; Sadeghi, M.; Talaei, M.; Sarrafzadegan, N. Egg consumption and risk of cardiovascular events among Iranians: Results from Isfahan Cohort Study (ICS). Eur. J. Clin. Nutr. 2022, 76, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Drouin-Chartier, J.P.; Chen, S.; Li, Y.; Schwab, A.L.; Stampfer, M.J.; Sacks, F.M.; Rosner, B.; Willett, W.C.; Hu, F.B.; Bhupathiraju, S.N. Egg consumption and risk of cardiovascular disease: Three large prospective US cohort studies, systematic review, and updated meta-analysis. BMJ (Clin. Res. Ed.) 2020, 368, m513. [Google Scholar] [CrossRef] [Green Version]
- Alexander, D.D.; Miller, P.E.; Vargas, A.J.; Weed, D.L.; Cohen, S.S. Meta-analysis of Egg Consumption and Risk of Coronary Heart Disease and Stroke. J. Am. Coll. Nutr. 2016, 35, 704–716. [Google Scholar] [CrossRef]
- Chinese Nutrition Society. The Chinese Dietary Guidelines (2022); People’s Medical Publishing House: Beijing, China, 2022; ISBN 978-977-117-31404-31406. [Google Scholar]
- Chen, Z.; Chen, J.; Collins, R.; Guo, Y.; Peto, R.; Wu, F.; Li, L. China Kadoorie Biobank of 0.5 million people: Survey methods, baseline characteristics and long-term follow-up. Int. J. Epidemiol. 2011, 40, 1652–1666. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Lee, L.; Chen, J.; Collins, R.; Wu, F.; Guo, Y.; Linksted, P.; Peto, R. Cohort profile: The Kadoorie Study of Chronic Disease in China (KSCDC). Int. J. Epidemiol. 2005, 34, 1243–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, C.; Shi, Z.; Lv, J.; Du, H.; Qi, L.; Guo, Y.; Bian, Z.; Chang, L.; Tang, X.; Jiang, Q.; et al. Major Dietary Patterns in Relation to General and Central Obesity among Chinese Adults. Nutrients 2015, 7, 5834–5849. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Bennett, D.; Li, L.; Whitlock, G.; Guo, Y.; Collins, R.; Chen, J.; Bian, Z.; Hong, L.S.; Feng, S.; et al. Physical activity and sedentary leisure time and their associations with BMI, waist circumference, and percentage body fat in 0.5 million adults: The China Kadoorie Biobank study. Am. J. Clin. Nutr. 2013, 97, 487–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, A.I.; Suri, F.K.; Ahmed, S.; Nasar, A.; Divani, A.A.; Kirmani, J.F. Regular egg consumption does not increase the risk of stroke and cardiovascular diseases. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2007, 13, CR1–CR8. [Google Scholar]
- Xu, L.; Lam, T.H.; Jiang, C.Q.; Zhang, W.S.; Zhu, F.; Jin, Y.L.; Woo, J.; Cheng, K.K.; Thomas, G.N. Egg consumption and the risk of cardiovascular disease and all-cause mortality: Guangzhou Biobank Cohort Study and meta-analyses. Eur. J. Nutr. 2019, 58, 785–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldberg, S.; Gardener, H.; Tiozzo, E.; Ying Kuen, C.; Elkind, M.S.; Sacco, R.L.; Rundek, T. Egg consumption and carotid atherosclerosis in the Northern Manhattan study. Atherosclerosis 2014, 235, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, A.M.; Pan, A.; Rexrode, K.M.; Stampfer, M.; Hu, F.B.; Mozaffarian, D.; Willett, W.C. Dietary protein sources and the risk of stroke in men and women. Stroke 2012, 43, 637–644. [Google Scholar] [CrossRef] [Green Version]
- Scrafford, C.G.; Tran, N.L.; Barraj, L.M.; Mink, P.J. Egg consumption and CHD and stroke mortality: A prospective study of US adults. Public Health Nutr. 2011, 14, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, Y.; Okamura, T.; Tamaki, S.; Kadowaki, T.; Hayakawa, T.; Kita, Y.; Okayama, A.; Ueshima, H. Egg consumption, serum cholesterol, and cause-specific and all-cause mortality: The National Integrated Project for Prospective Observation of Non-communicable Disease and Its Trends in the Aged, 1980 (NIPPON DATA80). Am. J. Clin. Nutr. 2004, 80, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Djoussé, L.; Gaziano, J.M. Egg consumption in relation to cardiovascular disease and mortality: The Physicians’ Health Study. Am. J. Clin. Nutr. 2008, 87, 964–969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Xiong, Y.; Wang, P.; Liu, R.; Jia, X.; Kong, Y.; Li, F.; Chen, C.; Zhang, X.; Zheng, Y. Risk factors of cardiovascular and cerebrovascular diseases in young and middle-aged adults: A meta-analysis. Medicine 2022, 101, e32082. [Google Scholar] [CrossRef] [PubMed]
- Banks, E.; Joshy, G.; Korda, R.J.; Stavreski, B.; Soga, K.; Egger, S.; Day, C.; Clarke, N.E.; Lewington, S.; Lopez, A.D. Tobacco smoking and risk of 36 cardiovascular disease subtypes: Fatal and non-fatal outcomes in a large prospective Australian study. BMC Med. 2019, 17, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onor, I.O.; Stirling, D.L.; Williams, S.R.; Bediako, D.; Borghol, A.; Harris, M.B.; Darensburg, T.B.; Clay, S.D.; Okpechi, S.C.; Sarpong, D.F. Clinical Effects of Cigarette Smoking: Epidemiologic Impact and Review of Pharmacotherapy Options. Int. J. Environ. Res. Public Health 2017, 14, 1147. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, P.; Wu, F.; Mao, L.; Zhu, F.; Zhang, Y.; Chen, X.; Jiao, J.; Zhang, Y. Egg and cholesterol consumption and mortality from cardiovascular and different causes in the United States: A population-based cohort study. PLoS Med. 2021, 18, e1003508. [Google Scholar] [CrossRef]
- Zhong, V.W.; Van Horn, L.; Cornelis, M.C.; Wilkins, J.T.; Ning, H.; Carnethon, M.R.; Greenland, P.; Mentz, R.J.; Tucker, K.L.; Zhao, L.; et al. Associations of Dietary Cholesterol or Egg Consumption with Incident Cardiovascular Disease and Mortality. JAMA 2019, 321, 1081–1095. [Google Scholar] [CrossRef]
- Berger, S.; Raman, G.; Vishwanathan, R.; Jacques, P.F.; Johnson, E.J. Dietary cholesterol and cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2015, 102, 276–294. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Kovacs-Nolan, J.; Yang, C.; Archbold, T.; Fan, M.Z.; Mine, Y. Hen egg lysozyme attenuates inflammation and modulates local gene expression in a porcine model of dextran sodium sulfate (DSS)-induced colitis. J. Agric. Food Chem. 2009, 57, 2233–2240. [Google Scholar] [CrossRef]
- Coveney, S.; Murphy, S.; Belton, O.; Cassidy, T.; Crowe, M.; Dolan, E.; de Gaetano, M.; Harbison, J.; Horgan, G.; Marnane, M.; et al. Inflammatory cytokines, high-sensitivity C-reactive protein, and risk of one-year vascular events, death, and poor functional outcome after stroke and transient ischemic attack. Int. J. Stroke Off. J. Int. Stroke Soc. 2022, 17, 163–171. [Google Scholar] [CrossRef]
- Lazzaroni, M.G.; Piantoni, S.; Masneri, S.; Garrafa, E.; Martini, G.; Tincani, A.; Andreoli, L.; Franceschini, F. Coagulation dysfunction in COVID-19: The interplay between inflammation, viral infection and the coagulation system. Blood Rev. 2021, 46, 100745. [Google Scholar] [CrossRef]
- Ratliff, J.; Leite, J.O.; de Ogburn, R.; Puglisi, M.J.; VanHeest, J.; Fernandez, M.L. Consuming eggs for breakfast influences plasma glucose and ghrelin, while reducing energy intake during the next 24 hours in adult men. Nutr. Res. 2010, 30, 96–103. [Google Scholar] [CrossRef]
- Matulewicz, N.; Karczewska-Kupczewska, M. Insulin resistance and chronic inflammation. Postep. Hig. I Med. Dosw. (Online) 2016, 70, 1245–1258. [Google Scholar]
- Ragab, A.; Abousamra, N.K.; Higazy, A.; Saleh, O. Relationship between insulin resistance and some coagulation and fibrinolytic parameters in patients with metabolic syndrome. Lab. Hematol. Off. Publ. Int. Soc. Lab. Hematol. 2008, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Żebrowska, E.; Chabowski, A. Insulin Resistance and Oxidative Stress in the Brain: What’s New? Int. J. Mol. Sci. 2019, 20, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Ding, X.; Yue, Q.; Wang, X.; Chen, Z.; Cai, Z.; Li, W.; Cai, Z.; Chen, G.; Lan, Y.; et al. Triglyceride-glucose index trajectory and stroke incidence in patients with hypertension: A prospective cohort study. Cardiovasc. Diabetol. 2022, 21, 141. [Google Scholar] [CrossRef]
- Zierenberg, O.; Grundy, S.M. Intestinal absorption of polyenephosphatidylcholine in man. J. Lipid Res. 1982, 23, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Bunea, R.; El Farrah, K.; Deutsch, L. Evaluation of the effects of Neptune Krill Oil on the clinical course of hyperlipidemia. Altern. Med. Rev. A J. Clin. Ther. 2004, 9, 420–428. [Google Scholar]
- Román, G.C.; Jackson, R.E.; Gadhia, R.; Román, A.N.; Reis, J. Mediterranean diet: The role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Sherratt, S.C.R.; Libby, P.; Budoff, M.J.; Bhatt, D.L.; Mason, R.P. Role of Omega-3 Fatty Acids in Cardiovascular Disease: The Debate Continues. Curr. Atheroscler. Rep. 2023, 25, 1–17. [Google Scholar] [CrossRef]
- Liu, X.; Shao, Y.; Sun, J.; Tu, J.; Wang, Z.; Tao, J.; Chen, J. Egg consumption improves vascular and gut microbiota function without increasing inflammatory, metabolic, and oxidative stress markers. Food Sci. Nutr. 2022, 10, 295–304. [Google Scholar] [CrossRef]
- Liu, G.; Chen, S.; Zhong, J.; Teng, K.; Yin, Y. Crosstalk between Tryptophan Metabolism and Cardiovascular Disease, Mechanisms, and Therapeutic Implications. Oxidative Med. Cell. Longev. 2017, 2017, 1602074. [Google Scholar] [CrossRef] [Green Version]
- Janmohammadi, H.; Hosseintabar-Ghasemabad, B.; Oliyai, M.; Alijani, S.; Gorlov, I.F.; Slozhenkina, M.I.; Mosolov, A.A.; Suarez Ramirez, L.; Seidavi, A.; Laudadio, V.; et al. Effect of Dietary Amaranth (Amaranthus hybridus chlorostachys) Supplemented with Enzyme Blend on Egg Quality, Serum Biochemistry and Antioxidant Status in Laying Hens. Antioxidants 2023, 12, 456. [Google Scholar] [CrossRef] [PubMed]
- Sarvestani, S.; Hosseini, S.M.; Farhangfar, S.H. Effect of aqueous-alcoholic extract of Hibiscus sabdariffa calyx and leaf calyx and leaf on performance, egg quality, immune system and antioxidant balance of laying hens. Iran. J. Appl. Anim. Sci. 2020, 10, 317–325. [Google Scholar]
- Abad, P.; Arroyo-Manzanares, N.; Ariza, J.J.; Baños, A.; García-Campaña, A.M. Effect of Allium Extract Supplementation on Egg Quality, Productivity, and Intestinal Microbiota of Laying Hens. Animals 2020, 11, 41. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | <1 Day/Week | 1–3 Days/Week | 4–6 Days/Week | 7 Days/Week | Overall |
|---|---|---|---|---|---|
| (n = 2193) | (n = 10,658) | (n = 3958) | (n = 18,461) | (n = 35,270) | |
| Age (years) | 49.1 (9.4) | 49.3 (9.6) | 49.5 (9.7) | 52.0 (10.4) | 50.7 (10.1) |
| Men (%) | 45.3 | 46.2 | 43.1 | 42.7 | 44.0 |
| High school and above | 32.0 | 35.4 | 40.4 | 35.2 | 35.7 |
| Married (%) | 91.3 | 92.3 | 94.0 | 92.4 | 92.4 |
| Household income (CNY/year) | |||||
| <10,000 | 10.1 | 7.9 | 5.3 | 8.1 | 7.8 |
| 10,000–34,999 | 75.5 | 76.5 | 77.2 | 74.4 | 75.4 |
| ≥35,000 | 14.4 | 15.6 | 17.5 | 17.5 | 16.7 |
| Current Smoking | 27.9 | 26.1 | 25.8 | 26.4 | 26.3 |
| Current drinking (%) | 63.1 | 63.7 | 63.9 | 63.6 | 63.6 |
| Eating daily (%) | |||||
| rice | 17.0 | 14.4 | 12.3 | 20.5 | 17.5 |
| wheat | 84.9 | 87.9 | 85.9 | 88.7 | 87.9 |
| other staple foods | 3.9 | 2.4 | 2.1 | 4.2 | 3.4 |
| red meat | 58.9 | 56.1 | 45.2 | 68.5 | 61.6 |
| poultry | 2.2 | 2.3 | 3.4 | 1.6 | 2.0 |
| fish | 13.5 | 10.6 | 6.4 | 13.0 | 11.6 |
| dairy products | 27.9 | 24.6 | 20.9 | 43.4 | 34.2 |
| fresh fruit | 48.3 | 47.3 | 47.0 | 60.5 | 54.2 |
| fresh vegetables | 96.9 | 97.2 | 97.4 | 99.0 | 98.1 |
| soybean products | 5.6 | 4.8 | 4.7 | 9.4 | 7.2 |
| preserved vegetables | 32.0 | 25.2 | 17.5 | 35.8 | 30.3 |
| Family history of stroke (%) | 18.7 | 15.3 | 11.5 | 17.2 | 16.1 |
| Diabetes (%) | 3.9 | 4.1 | 3.9 | 8.0 | 6.1 |
| MET (MET-h/day) | 19.4 (11.9) | 18.7 (11.6) | 19.2 (11.8) | 17.5 (11.2) | 18.1 (11.4) |
| BMI (kg/m2) | 25.7 (3.6) | 25.7 (3.5) | 25.6 (3.5) | 25.7 (3.5) | 25.7 (3.5) |
| Egg Consumption | p for Trend | ||||
|---|---|---|---|---|---|
| All | <1 Day/Week | 1–3 Days/Week | 4–6 Days/Week | 7 Days/Week | |
| Overall cohort | |||||
| Cases | 121 | 532 | 186 | 1109 | |
| PYs | 19,070 | 90,422 | 31,439 | 158,115 | |
| Cases/PYs (1/1000) | 6.3 | 5.9 | 5.9 | 7.0 | |
| Model 1 | 1.00 | 0.91 (0.75, 1.11) | 0.91 (0.72, 1.15) | 0.81 (0.67, 0.98) * | 0.004 |
| Model 2 | 1.00 | 0.91 (0.75, 1.11) | 0.91 (0.72, 1.15) | 0.82 (0.68, 1.00) | 0.014 |
| Model 3 | 1.00 | 0.91 (0.75, 1.11) | 0.92 (0.73, 1.16) | 0.83 (0.69, 1.01) | 0.027 |
| Men | |||||
| Cases | 59 | 237 | 92 | 477 | |
| PYs | 8745 | 42,273 | 13,681 | 68,411 | |
| Cases/PYs (1/1000) | 6.7 | 5.6 | 6.7 | 7.0 | |
| Model 1 | 1.00 | 0.82 (0.62, 1.09) | 0.92 (0.66, 1.28) | 0.71 (0.54, 0.94) * | 0.008 |
| Model 2 | 1.00 | 0.83 (0.62, 1.11) | 0.97 (0.69, 1.35) | 0.72 (0.54, 0.94) * | 0.009 |
| Model 3 | 1.00 | 0.83 (0.63, 1.11) | 0.97 (0.70, 1.35) | 0.72 (0.55, 0.95) * | 0.012 |
| Women | |||||
| Cases | 62 | 295 | 94 | 632 | |
| PYs | 10,325 | 48,149 | 17,758 | 89,704 | |
| Cases/PYs (1/1000) | 6.0 | 6.1 | 5.3 | 7.0 | |
| Model 1 | 1.00 | 1.00 (0.76, 1.31) | 0.90 (0.65, 1.24) | 0.90 (0.69, 1.18) | 0.161 |
| Model 2 | 1.00 | 0.98 (0.74, 1.29) | 0.86 (0.62, 1.19) | 0.91 (0.70, 1.19) | 0.316 |
| Model 3 | 1.00 | 0.97 (0.74, 1.28) | 0.87 (0.62, 1.20) | 0.93 (0.71, 1.21) | 0.452 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, C.; Sun, X.; Song, J.; Yu, C.; Guo, Y.; Wang, S.; Gao, R.; Ning, F.; Pang, Z.; Chen, Z.; et al. The Prospective Associations of Egg Consumption with the Risk of Total Cerebrovascular Disease Morbidity among Chinese Adults. Nutrients 2023, 15, 1808. https://doi.org/10.3390/nu15081808
Pan C, Sun X, Song J, Yu C, Guo Y, Wang S, Gao R, Ning F, Pang Z, Chen Z, et al. The Prospective Associations of Egg Consumption with the Risk of Total Cerebrovascular Disease Morbidity among Chinese Adults. Nutrients. 2023; 15(8):1808. https://doi.org/10.3390/nu15081808
Chicago/Turabian StylePan, Chi, Xiaohui Sun, Jiahui Song, Canqing Yu, Yu Guo, Shaojie Wang, Ruqin Gao, Feng Ning, Zengchang Pang, Zhengming Chen, and et al. 2023. "The Prospective Associations of Egg Consumption with the Risk of Total Cerebrovascular Disease Morbidity among Chinese Adults" Nutrients 15, no. 8: 1808. https://doi.org/10.3390/nu15081808
APA StylePan, C., Sun, X., Song, J., Yu, C., Guo, Y., Wang, S., Gao, R., Ning, F., Pang, Z., Chen, Z., & Li, L. (2023). The Prospective Associations of Egg Consumption with the Risk of Total Cerebrovascular Disease Morbidity among Chinese Adults. Nutrients, 15(8), 1808. https://doi.org/10.3390/nu15081808






