Association of Oral or Intravenous Vitamin C Supplementation with Mortality: A Systematic Review and Meta-Analysis
Abstract
:1. Background
2. Methods
2.1. Eligibility Criteria
2.2. Exclusion Criteria
2.3. Information Sources and Search Strategy
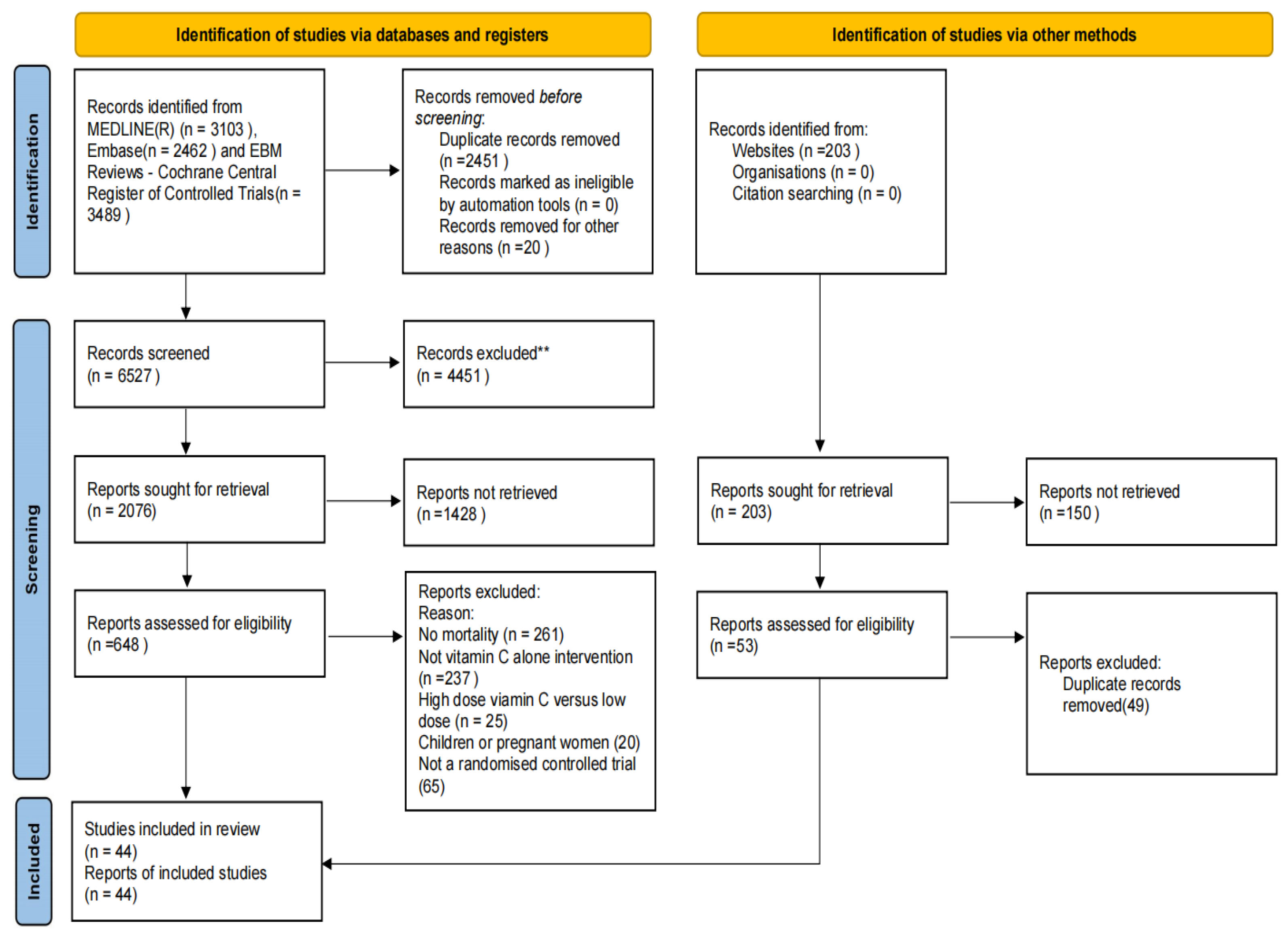
2.4. Selection Process and Items
2.5. Outcomes
2.6. Risk of Bias and Quality of Evidence
2.7. Effect Measures
2.8. Data Synthesis
2.9. Sensitivity Analyses
2.10. Patient and Public Involvement
3. Results
4. Discussion
4.1. Principal Findings
4.2. Strengths and Limitations
4.3. Comparisons with Other Studies
4.4. Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Study Registration
References
- Granger, M.; Eck, P. Dietary Vitamin C in Human Health, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Volume 83. [Google Scholar]
- Wilson, J.X. Mechanism of action of vitamin C in sepsis: Ascorbate modulates redox signaling in endothelium. BioFactors 2009, 35, 5–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, S.Y.; Lee, M.T.; Baek, M.S.; Kim, W.Y. Vitamin C for ≥5 days is associated with decreased hospital mortality in sepsis subgroups: A nationwide cohort study. Crit. Care 2022, 26, 3. [Google Scholar] [CrossRef] [PubMed]
- Rosengrave, P.; Spencer, E.; Williman, J.; Mehrtens, J.; Morgan, S.; Doyle, T.; Van Der Heyden, K.; Morris, A.; Shaw, G.; Carr, A.C. Intravenous vitamin C administration to patients with septic shock: A pilot randomised controlled trial. Crit. Care 2022, 26, 26. [Google Scholar] [CrossRef]
- Mohamed, Z.U.; Prasannan, P.; Moni, M.; Edathadathil, F.; Prasanna, P.; Menon, A.; Nair, S.; Greeshma, C.R.; Sathyapalan, D.T.; Menon, V.; et al. Vitamin c therapy or routine care in septic shock (ViCTOR) trial: Effect of intravenous vitamin C, thiamine, and hydrocortisone administration on inpatient mortality among patients with septic shock. Indian J. Crit. Care Med. 2020, 24, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Vandervelden, S.; Wauters, L.; Breuls, J.; Fieuws, S.; Vanhove, P.; Hubloue, I.; Bartiaux, M.; Creteur, J.; Stifkens, F.; Monsieurs, K.; et al. Early administration of Vitamin C in patients with sepsis or septic shock in emergency departments: A multicenter, double blinded, randomized controlled trial: The C-EASIE trial protocol. PLoS ONE 2021, 16, e0259699. [Google Scholar] [CrossRef] [PubMed]
- Ap, G.R.; Daga, M.K.; Mawari, G.; Koner, B.C.; Singh, V.K.; Kumar, N.; Rohatgi, I.; Mishra, R. Effect of Supplementation of Vitamin C and Thiamine on the Outcome in Sepsis: South East Asian Region. J. Assoc. Physicians India 2022, 70, 11–12. [Google Scholar]
- Block, K.I.; Mead, M.N. Vitamin C in Alternative Cancer Treatment: Historical Background. Integr. Cancer Ther. 2003, 2, 147–154. [Google Scholar] [CrossRef]
- Yeom, C.H.; Lee, G.; Park, J.H.; Yu, J.; Park, S.; Yi, S.Y.; Lee, H.; Hong, Y.S.; Yang, J.; Lee, S. High dose concentration administration of ascorbic acid inhibits tumor growth in BALB/C mice implanted with sarcoma 180 cancer cells via the restriction of angiogenesis. J. Transl. Med. 2009, 7, 70. [Google Scholar] [CrossRef] [Green Version]
- Verrax, J.; Calderon, P.B. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic. Biol. Med. 2009, 47, 32–40. [Google Scholar] [CrossRef]
- Cameron, E.; Pauling, L. Supplemental ascorbate in the supportive treatment of cancer: Reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA 1978, 75, 4538–4542. [Google Scholar] [CrossRef] [Green Version]
- Moertel, C.G.; Fleming, T.R.; Creagan, E.T.; Rubin, J.; O’Connell, M.J.; Ames, M.M. High-Dose Vitamin C versus Placebo in the Treatment of Patients with Advanced Cancer Who Have Had No Prior Chemotherapy. N. Engl. J. Med. 1985, 312, 137–141. [Google Scholar] [CrossRef]
- Lin, J.; Cook, N.R.; Albert, C.; Zaharris, E.; Gaziano, J.M.; Van Denburgh, M.; Buring, J.E.; Manson, J.E. Vitamins C and E and beta carotene supplementation and cancer risk: A randomized controlled trial. J. Natl. Cancer Inst. 2009, 101, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffer, L.J.; Robitaille, L.; Zakarian, R.; Melnychuk, D.; Kavan, P.; Agulnik, J.; Cohen, V.; Small, D.; Miller, W.H. High-dose intravenous vitamin C combined with cytotoxic chemotherapy in patients with advanced cancer: A phase I-II clinical trial. PLoS ONE 2015, 10, e0120228. [Google Scholar] [CrossRef]
- Harris, H.R.; Orsini, N.; Wolk, A. Vitamin C and survival among women with breast cancer: A Meta-analysis. Eur. J. Cancer 2014, 50, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.S.; Wilkes, J.G.; Schroeder, S.R.; Buettner, G.R.; Wagner, B.A.; Du, J.; Gibson-Corley, K.; O’Leary, B.R.; Spitz, D.R.; Buatti, J.M.; et al. Pharmacologic ascorbate reduces radiation-induced normal tissue toxicity and enhances tumor radiosensitization in pancreatic cancer. Cancer Res. 2018, 78, 6838–6851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Krishnan, U.M. The emergence of COVID-19 as a global pandemic: Understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie 2020, 179, 85–100. [Google Scholar] [CrossRef]
- Ozgunay, S.E.; Ceylan, İ.; Ökmen, K.; Sayan, H.E.; Eminoglu, Ş.; Karasu, D.; Yavuz, S. The use of vitamin C in the intensive care unit during the COVID-19 pandemic. Eur. Res. J. 2021, 7, 425–431. [Google Scholar] [CrossRef]
- Rawat, D.; Roy, A.; Maitra, S.; Gulati, A.; Khanna, P.; Baidya, D.K. Vitamin C and COVID-19 treatment: A systematic review and meta-analysis of randomized controlled trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102324. [Google Scholar] [CrossRef]
- Tian, X.; Chen, W.Q.; Liu, X.L.; Chen, H.; Liu, B.L.; Pi, Y.P. Comparative efficacy of combination of 1L polyethylene glycol, castor oil and ascorbic acid versus 2L polyethylene glycol plus castor oil versus 3L polyethylene glycol for colon cleansing before colonoscopy: Study protocol of a randomized, double-blind, single-center study. Medicine 2018, 97, e0481. [Google Scholar] [CrossRef]
- Emadi, N.; Nemati, M.H.; Ghorbani, M.; Allahyari, E. The effect of high-dose vitamin c on biochemical markers of myocardial injury in coronary artery bypass surgery. Braz. J. Cardiovasc. Surg. 2019, 34, 517–524. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyatt, G.H.; Oxman, A.D.; Vist, G. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. Br. Med. J. 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brok, J.; Thorlund, K.; Gluud, C.; Wetterslev, J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J. Clin. Epidemiol. 2008, 61, 763–769. [Google Scholar] [CrossRef]
- Lv, S.J.; Zhang, G.H.; Xia, J.M.; Yu, H.; Zhao, F. Early use of high-dose vitamin C is beneficial in treatment of sepsis. Ir. J. Med. Sci. 2021, 190, 1183–1188. [Google Scholar] [CrossRef]
- Fowler, A.A.; Syed, A.A.; Knowlson, S.; Sculthorpe, R.; Farthing, D.; DeWilde, C.; Farthing, C.A.; Larus, T.L.; Martin, E.; Brophy, D.F.; et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014, 12, 32. [Google Scholar] [CrossRef] [Green Version]
- Bailey, D.M.; Raman, S.; McEneny, J.; Young, I.S.; Parham, K.L.; Hullin, D.A.; Davies, B.; McKeeman, G.; McCord, J.M.; Lewis, M.H. Vitamin C prophylaxis promotes oxidative lipid damage during surgical ischemia-reperfusion. Free Radic. Biol. Med. 2006, 40, 591–600. [Google Scholar] [CrossRef]
- Knodell, R.G.; Tate, M.A.; Akl, B.F.; Wilson, J.W. Vitamin C prophylaxis for posttransfusion hepatitis: Lack of effect in a controlled trial. Am. J. Clin. Nutr. 1981, 34, 20–23. [Google Scholar] [CrossRef]
- Antonic, M.; Lipovec, R.; Gregorcic, F.; Juric, P.; Kosir, G. Perioperative ascorbic acid supplementation does not reduce the incidence of postoperative atrial fibrillation in on-pump coronary artery bypass graft patients. J. Cardiol. 2017, 69, 98–102. [Google Scholar] [CrossRef] [Green Version]
- Kumari, P.; Dembra, S.; Dembra, P.; Bhawna, F.; Gul, A.; Ali, B.; Sohail, H.; Kumar, B.; Memon, M.K.; Rizwan, A. The Role of Vitamin C as Adjuvant Therapy in COVID-19. Cureus 2020, 12, 10–13. [Google Scholar] [CrossRef] [PubMed]
- JamaliMoghadamSiahkali, S.; Zarezade, B.; Koolaji, S.; SeyedAlinaghi, S.A.; Zendehdel, A.; Tabarestani, M.; Sekhavati Moghadam, E.; Abbasian, L.; Dehghan Manshadi, S.A.; Salehi, M.; et al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: A randomized open-label clinical trial. Eur. J. Med. Res. 2021, 26, 20. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, F.; Masse, M.-H.; Menard, J.; Sprague, S.; Pinto, R.; Heyland, D.K.; Cook, D.J.; Battista, M.-C.; Day, A.G.; Guyatt, G.H.; et al. Intravenous Vitamin C in Adults with Sepsis in the Intensive Care Unit. N. Engl. J. Med. 2022, 386, 2387–2398. [Google Scholar] [CrossRef]
- Razmkon, A.; Sadidi, A.; Sherafat-Kazemzadeh, E.; Mehrafshan, A.; Jamali, M.; Malekpour, B.; Saghafinia, M. Administration of vitamin C and vitamin E in severe head injury: A randomized double-blind controlled trial. Clin. Neurosurg. 2011, 58, 133–137. [Google Scholar] [CrossRef] [Green Version]
- Fowler, A.A.; Truwit, J.D.; Hite, R.D.; Morris, P.E.; Dewilde, C.; Priday, A.; Fisher, B.; Thacker, L.R.; Natarajan, R.; Brophy, D.F.; et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients with Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial. J. Am. Med. Assoc. 2019, 322, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Zabet, M.; Mohammadi, M.; Ramezani, M.; Khalili, H. Effect of high-dose Ascorbic acid on vasopressor′s requirement in septic shock. J. Res. Pharm. Pract. 2016, 5, 94. [Google Scholar] [CrossRef]
- Thomas, S.; Patel, D.; Bittel, B.; Wolski, K.; Wang, Q.; Kumar, A.; Il’Giovine, Z.J.; Mehra, R.; McWilliams, C.; Nissen, S.E.; et al. Effect of High-Dose Zinc and Ascorbic Acid Supplementation vs Usual Care on Symptom Length and Reduction among Ambulatory Patients with SARS-CoV-2 Infection: The COVID A to Z Randomized Clinical Trial. JAMA Netw. Open 2021, 4, e210369. [Google Scholar] [CrossRef]
- Wacker, D.A.; Burton, S.L.; Berger, J.P.; Hegg, A.J.; Heisdorffer, J.; Wang, Q.; Medcraft, E.J.; Reilkoff, R.A. Evaluating Vitamin C in Septic Shock: A Randomized Controlled Trial of Vitamin C Monotherapy. Crit. Care Med. 2022, 50, E458–E467. [Google Scholar] [CrossRef]
- El Driny, W.A.; Esmat, I.M.; Shaheen, S.M.; Sabri, N.A. Efficacy of High-Dose Vitamin C Infusion on Outcomes in Sepsis Requiring Mechanical Ventilation: A Double-Blind Randomized Controlled Trial. Anesthesiol. Res. Pract. 2022, 2022, 4057215. [Google Scholar] [CrossRef]
- Zhang, J.; Rao, X.; Li, Y.; Zhu, Y.; Liu, F.; Guo, G.; Luo, G.; Meng, Z.; De Backer, D.; Xiang, H.; et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann. Intensive Care 2021, 11, 3–14. [Google Scholar] [CrossRef]
- Reddy, P.R.; Samavedam, S.; Aluru, N.; Yelle, S.; Rajyalakshmi, B. Metabolic resuscitation using hydrocortisone ascorbic acid thiamine: Do individual components influence reversal of shock independently? Indian J. Crit. Care Med. 2020, 24, 649–652. [Google Scholar] [CrossRef] [PubMed]
- Coppock, D.; Violet, P.C.; Vasquez, G.; Belden, K.; Foster, M.; Mullin, B.; Magee, D.; Mikell, I.; Shah, L.; Powers, V.; et al. Pharmacologic Ascorbic Acid as Early Therapy for Hospitalized Patients with COVID-19: A Randomized Clinical Trial. Life 2022, 12, 453. [Google Scholar] [CrossRef] [PubMed]
- Aisa-Alvarez, A.; Soto, M.E.; Guarner-Lans, V.; Camarena-Alejo, G.; Franco-Granillo, J.; Martínez-Rodríguez, E.A.; Ávila, R.G.; Pech, L.M.; Pérez-Torres, I. Usefulness of antioxidants as adjuvant therapy for septic shock: A randomized clinical trial. Medicina 2020, 56, 619. [Google Scholar] [CrossRef] [PubMed]
- Majidi, N.; Rabbani, F.; Gholami, S.; Gholamalizadeh, M.; BourBour, F.; Rastgoo, S.; Hajipour, A.; Shadnoosh, M.; Akbari, M.E.; Bahar, B.; et al. The Effect of Vitamin C on Pathological Parameters and Survival Duration of Critically Ill Coronavirus Disease 2019 Patients: A Randomized Clinical Trial. Front. Immunol. 2021, 12, 619. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; O’Kane, C.M.; Stevenson, M.; Young, I.S.; Harkin, D.W.; Mullan, B.A.; McAuley, D.F. A randomized clinical trial of ascorbic acid in open abdominal aortic aneurysm repair. Intensive Care Med. Exp. 2015, 3, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niu, J.; Qin, B.; Yang, K.; Fan, Q.; Liu, W.; Wang, C.; Provincial, H. Effects of early vitamin C supplementation on the prognosis of patients with sepsis. Chin. J. Mod. Med. 2019, 29, 65–69. [Google Scholar]
- Dachs, G.U.; Gandhi, J.; Wohlrab, C.; Carr, A.C.; Morrin, H.R.; Pullar, J.M.; Bayer, S.B.; Eglinton, T.W.; Robinson, B.A.; Vissers, M.C.M. Vitamin C Administration by Intravenous Infusion Increases Tumor Ascorbate Content in Patients with Colon Cancer: A Clinical Intervention Study. Front. Oncol. 2021, 10, 600715. [Google Scholar] [CrossRef]
- Ferrón-Celma, I.; Mansilla, A.; Hassan, L.; Garcia-Navarro, A.; Comino, A.M.; Bueno, P.; Ferrón, J.A. Effect of Vitamin C Administration on Neutrophil Apoptosis in Septic Patients After Abdominal Surgery. J. Surg. Res. 2009, 153, 224–230. [Google Scholar] [CrossRef]
- Samir Bazan, N.; Hesham El-Sherazy, N.; Mahmoud Shaheen, S.A.; Sabri, N. Impact of ascorbic acid in reducing the incidence of vancomycin associated nephrotoxicity in critically ill patients: A preliminary randomized controlled trial. F1000Research 2021, 10, 929. [Google Scholar] [CrossRef]
- Roberts, L.J.; Traber, M.G.; Frei, B. Vitamins E and C in the prevention of cardiovascular disease and cancer in men. Free Radic. Biol. Med. 2009, 46, 1558. [Google Scholar] [CrossRef]
- Yanase, F.; Bitker, L.; Hessels, L.; Osawa, E.; Naorungroj, T.; Cutuli, S.L.; Young, P.J.; Ritzema, J.; Hill, G.; Latimer-Bell, C.; et al. A Pilot, Double-Blind, Randomized, Controlled Trial of High-Dose Intravenous Vitamin C for Vasoplegia after Cardiac Surgery. J. Cardiothorac. Vasc. Anesth. 2020, 34, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.B.; Ahmed, I.; Omran, G.; Megahed, M.; Habib, T. Role of ascorbic acid infusion in critically ill patients with transfusion-related acute lung injury. Br. J. Clin. Pharmacol. 2022, 88, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Matsuda, T.; Miyagantani, Y.; Yukioka, T.; Matsuda, H.; Shimazaki, S. Reduction of resuscitation fluid volumes in severely burned patients using ascorbic acid administration: A randomized, prospective study. Arch. Surg. 2000, 135, 326–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjordahl, P.M.; Helmer, S.D.; Gosnell, D.J.; Wemmer, G.E.; O’Hara, W.W.; Milfeld, D.J. Perioperative supplementation with ascorbic acid does not prevent atrial fibrillation in coronary artery bypass graft patients. Am. J. Surg. 2012, 204, 862–867. [Google Scholar] [CrossRef]
- Mahmoodpoor, A.; Shadvar, K.; Sanaie, S.; Hadipoor, M.R.; Pourmoghaddam, M.A.; Saghaleini, S.H. Effect of Vitamin C on mortality of critically ill patients with severe pneumonia in in-tensive care unit: A preliminary study. BMC Infect. Dis. 2021, 21, 616. [Google Scholar] [CrossRef]
- Das, D.; Sen, C.; Goswami, A. Effect of Vitamin C on adrenal suppression by etomidate induction in patients undergoing cardiac surgery: A randomized controlled trial. Ann. Card. Anaesth. 2016, 19, 410–417. [Google Scholar] [CrossRef]
- Wang, D.; Wang, M.; Zhang, H.; Zhu, H.; Zhang, N.; Liu, J. Effect of intravenous injection of vitamin c on postoperative pulmonary complications in patients undergoing cardiac surgery: A double-blind, randomized trial. Drug Des. Devel. Ther. 2020, 14, 3263–3270. [Google Scholar] [CrossRef]
- Tehrani, S.; Yadegarynia, D.; Abrishami, A.; Moradi, H.; Gharaei, B.; Rauofi, M.; Maghsoudi Nejad, F.; Sali, S.; Khabiri, N.; Abolghasemi, S. An investigation into the Effects of Intravenous Vitamin C on Pulmonary CT Findings and Clinical Outcomes of Patients with COVID 19 Pneumonia A Randomized Clinical Trial. Urol. J. 2021, 6863, 460–465. [Google Scholar] [CrossRef]
- Nabil Habib, T.; Ahmed, I. Early Adjuvant Intravenous Vitamin C Treatment in Septic Shock may Resolve the Vasopressor Dependence. Int. J. Microbiol. Adv. Immunol. 2017, 05, 77–81. [Google Scholar] [CrossRef]
- ter Riet, G.; Kessels, A.G.H.; Knipschild, P.G. Randomized clinical trial of ascorbic acid in the treatment of pressure ulcers. J. Clin. Epidemiol. 1995, 48, 1453–1460. [Google Scholar] [CrossRef]
- Donovan, P.C. Prophylaxis to Reduce Postoperative Atrial Fibrillation in Cardiac Surgery. 2012. Available online: https://clinicaltrials.gov/ct2/show/results/NCT00953212 (accessed on 26 April 2018).
- Jahan, K.; Ahmad, K.; Ali, M.A. Effect of ascorbic acid in the treatment of tetanus. Bangla-Desh Med. Res. Counc. Bull. 1984, 10, 24–28. [Google Scholar]
- Creagon, E.T.; Moertel, C.G.; O’Fallon, J.R.; Schutt, A.J.; O’Connell, M.J.; Rubin, J.; Frytak, S. Failure of high-dose vitamin C (ascorbic acid) therapy to benefit patients with advanced cancer. A controlled trial. N. Engl. J. Med. 1979, 301, 687–690. [Google Scholar] [CrossRef]
- Norio, K.; Wikström, M.; Salmela, K.; Kyllönen, L.; Lindgren, L. Ascorbic acid against reperfu-sion injury in human renal transplantation. Transpl. Int. 2003, 16, 578–583. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and Fill: A Simple Funnel-Plot-Based Method. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Fong, K.M.; Au, S.Y.; Ng, G.W.Y. Steroid, ascorbic acid, and thiamine in adults with sepsis and septic shock: A systematic review and component network meta-analysis. Sci. Rep. 2021, 11, 15777. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zeng, J.; Li, D.; Yang, G.; Wang, K.; Deng, H.i; Jiang, H. Efficacy of intravenous vitamin C intervention for septic patients: A systematic review and meta-analysis based on randomized controlled trials. Am. J. Emerg. Med. 2021, 50, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Olczak-pruc, M.; Swieczkowski, D.; Ladny, J.R.; Pruc, M.; Juarez-vela, R.; Rafique, Z.; Peacock, F.W.; Szarpak, L. Vitamin C Supplementation for the Treatment of COVID-19: A Systematic Review and Meta-Analysis. Syst. Rev. Meta-Anal. 2022, 14, 4217. [Google Scholar]
- Darban, M.; Malek, F.; Memarian, M.; Gohari, A.; Kiani, A.; Emadi, A.; Lavvaf, S.; Bagheri, B. Efficacy of high dose Vitamin C, melatonin and zinc in Iranian patients with acute respiratory syndrome due to coronavirus infection: A pilot randomized trial. J. Cell. Mol. Anesth. 2021, 6, 164–167. [Google Scholar] [CrossRef]
- Spargias, K.; Alexopoulos, E.; Kyrzopoulos, S.; Iacovis, P.; Greenwood, D.C.; Manginas, A.; Voudris, V.; Pavlides, G.; Buller, C.E.; Kremastinos, D.; et al. Ascorbic acid prevents contrast-mediated nephropathy in patients with renal dysfunction undergoing coronary angiography or intervention. Circulation 2004, 110, 2837–2842. [Google Scholar] [CrossRef] [Green Version]
- Hu, X.; Yuan, L.; Wang, H.; Li, C.; Cai, J.; Hu, Y.; Ma, C. Efficacy and safety of vitamin C for atrial fibrillation after cardiac surgery: A meta-analysis with trial sequential analysis of randomized controlled trials. Int. J. Surg. 2017, 37, 58–64. [Google Scholar] [CrossRef]
- Cortés-Jofré, M.; Rueda, J.R.; Asenjo-Lobos, C.; Madrid, E.; Bonfill Cosp, X. Drugs for preventing lung cancer in healthy people. Cochrane Database Syst. Rev. 2020, 3, CD002141. [Google Scholar] [CrossRef] [PubMed]
- Greenlee, H.; Kwan, M.L.; Kushi, L.H.; Song, J.; Castillo, A.; Weltzien, E.; Quesenberry, C.P.; Caan, B.J. Antioxidant supplement use after breast cancer diagnosis and mortality in the Life after Cancer Epidemiology (LACE) cohort. Cancer 2012, 118, 2048–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Eligible Studies: | No of Trials (No of Participants) |
|---|---|
| Total No of trials (No of participants) | 44 (28,540) |
| Median follow-up (days) | 150 (days) |
| Follow-up less than 1 month | 35 (3730) |
| Median No of participants | 603 (40–121) |
| Total No of deaths | 1842 |
| Median% male | 71% |
| Median age (years) | 64.3 |
| Country | |
| European | 5 (257) |
| American | 13 (24,496) |
| Asian-Pacific | 7 (604) |
| International country | 19 (1183) |
| Dose | |
| Dose ≥ 4 g daily | 22 (16,779) |
| Dose < 4 g daily | 22 (9861) |
| Subgroup Title | No of Trials | No of Participants | I2 (%) | Risk Ratio (95% CI) | p for Interaction |
|---|---|---|---|---|---|
| Overall | 44 | 26,540 | 31 | 0.84 (0.75–0.95) | 0.03 |
| No of participants: | |||||
| 100 | 16 | 25,109 | 0 | 0.89 (0.77–1.04) | 0.01 |
| 100 | 28 | 1431 | 51 | 0.76 (0.62–0.93) | 0.62 |
| Year of publication | |||||
| In or after 2015 | 30 | 3081 | 18 | 0.75 (0.65–0.88) | 0.20 |
| Before 2015 | 14 | 23,459 | 1 | 0.84 (0.75–0.95) | 0.44 |
| Follow-up | |||||
| At least 30 days | 9 | 24,686 | 0 | 0.84 (0.75–0.95) | 0.80 |
| Less than 30 days | 35 | 3730 | 16 | 0.74 (0.63–0.86) | 0.21 |
| Age (years): | |||||
| 19 | 24,434 | 22 | 1.01 (0.92–1.10) | 0.18 | |
| 25 | 2106 | 0 | 0.71 (0.60–0.83) | 0.82 | |
| Daily dose(g) | |||||
| 22 | 16,779 | 32 | 0.84 (0.69–1.02) | 0.07 | |
| 22 | 5378 | 31 | 0.84 (0.71–0.99) | 0.08 | |
| Latitude: | |||||
| 0 | 17 | 24,472 | 31 | 1.02 (0.99–1.05) | 0.30 |
| 0 | 27 | 2068 | 0 | 0.74 (0.64–0.86) | 0.57 |
| country | |||||
| Asian-Pacific | 7 | 604 | 0 | 1.02 (0.99–1.05) | 0.46 |
| International countries | 19 | 1183 | 0 | 0.73 (0.60–0.88) | 0.51 |
| American | 13 | 24,496 | 25 | 1.02 (0.99–1.05) | 0.19 |
| European | 5 | 257 | 0 | 1.10 (0.55–2.17) | 0.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Yi, T.; Tan, S.; Xu, H.; Hu, Y.; Ma, J.; Xu, J. Association of Oral or Intravenous Vitamin C Supplementation with Mortality: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 1848. https://doi.org/10.3390/nu15081848
Xu C, Yi T, Tan S, Xu H, Hu Y, Ma J, Xu J. Association of Oral or Intravenous Vitamin C Supplementation with Mortality: A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(8):1848. https://doi.org/10.3390/nu15081848
Chicago/Turabian StyleXu, Chongxi, Tong Yi, Siwen Tan, Hui Xu, Yu Hu, Junpeng Ma, and Jianguo Xu. 2023. "Association of Oral or Intravenous Vitamin C Supplementation with Mortality: A Systematic Review and Meta-Analysis" Nutrients 15, no. 8: 1848. https://doi.org/10.3390/nu15081848





