Human Milk Composition and Nutritional Status of Omnivore Human Milk Donors Compared with Vegetarian/Vegan Lactating Mothers
Abstract
:1. Introduction
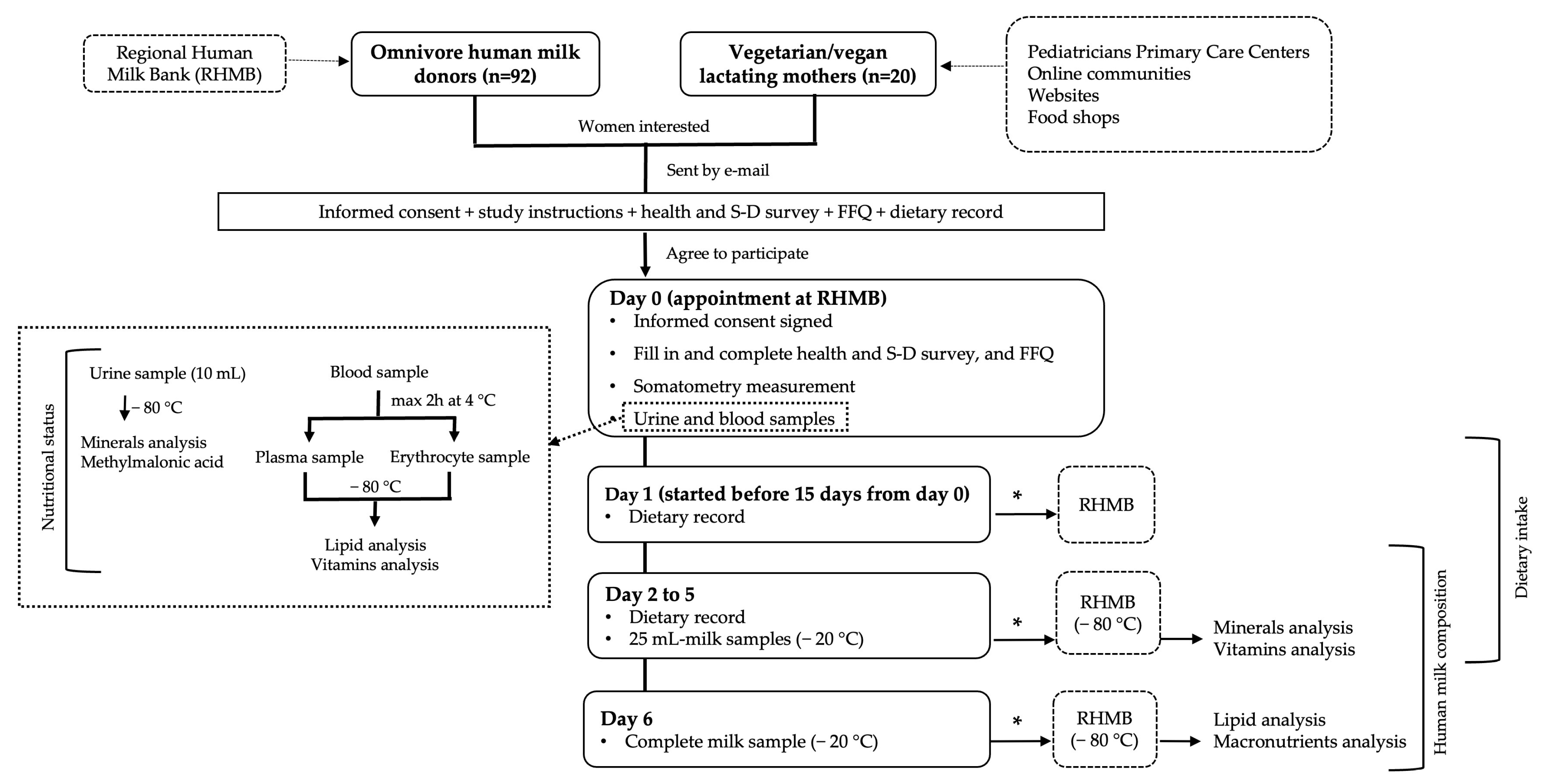
2. Materials and Methods
2.1. Study Design and Subjects
2.1.1. Sample Size Calculation
2.1.2. Study Protocol
2.2. Blood Samples Processing
2.3. 5-Day Dietary Record
2.4. Milk Sample Collection and Processing
2.5. Sample Storage and Shipment
2.6. Lipid Analysis
2.6.1. Fat Extraction
2.6.2. Chromatographic Analyses
Separation and Quantification of Lipid Classes by HPLC Evaporative Light Scattering Detector (ELSD)
Determination of Fatty Acid Methyl Esters (FAMEs) by GC-MS
Determination of TAG Molecular Species by GC-FID
2.7. Vitamins and Minerals Analysis
2.7.1. Minerals
2.7.2. Water-Soluble Vitamins and Vitamin-B12-Associated Biomarkers
2.7.3. Fat-Soluble Vitamins
2.8. Blood Biochemistry, Hemoglobin, and Urine Creatinine
2.9. Human Milk Macronutrients Analysis
2.10. Statistics
3. Results
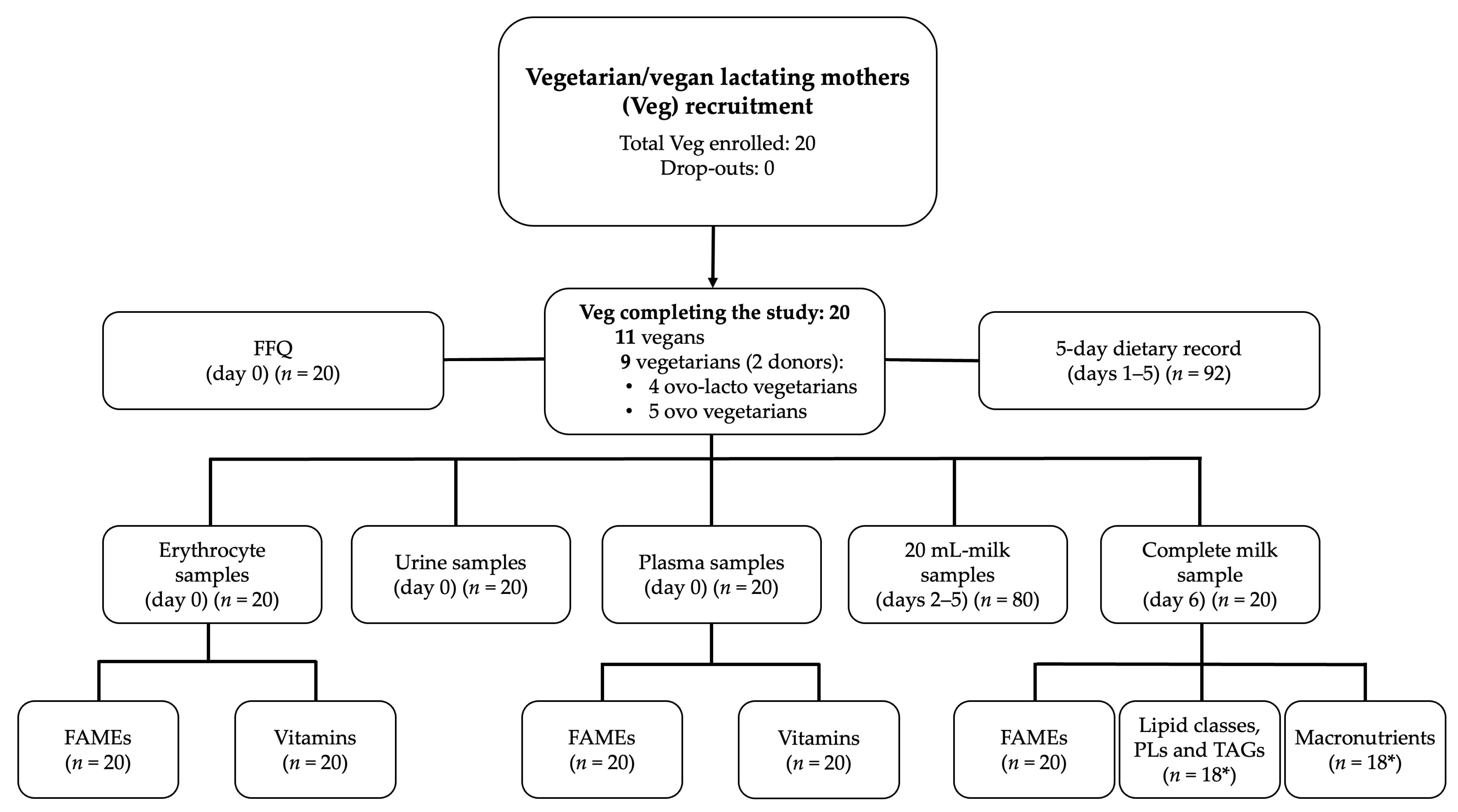
3.1. Population Studied
3.2. Diet Survey
3.3. Nutritional Status
3.4. Human Milk Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Guideline: Protecting, Promoting and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services; World Helath Organization (WHO): Genova, Switzerland, 2017; ISBN 978-92-4-155008-6. Available online: https://www.who.int/publications/i/item/9789241550086 (accessed on 5 April 2023).
- World Health Organization. Guidelines on Optimal Feeding of Low Birth-Weight Infants in Low-and Middle-Income Countries; World Health Organization (WHO): Geneva, Switzerland, 2011; ISBN 978-92-4-154836-6. Available online: https://apps.who.int/iris/handle/10665/85670 (accessed on 5 April 2023).
- AAP Comittee on Nutrition; AAP Section on breastfeeding; AAP Comittee on Fetus and Newbornborn. Donor human milk for the high- risk infant: Preparation, safety, and usage options in the United States. Pediatrics 2017, 139, e20163440. [Google Scholar] [CrossRef]
- Moro, G.E.; Billeaud, C.; Rachel, B.; Calvo, J.; Cavallarin, L.; Christen, L.; Escuder-Vieco, D.; Gaya, A.; Lembo, D.; Wesolowska, A.; et al. Processing of Donor Human Milk: Update and Recommendations from the European Milk Bank Association (EMBA). Front. Pediatr. 2019, 7, 49. [Google Scholar] [CrossRef]
- Perrin, M.T.; Belfort, M.B.; Hagadorn, J.I.; McGrath, J.M.; Taylor, S.N.; Tosi, L.M.; Brownell, E.A. The nutritional composition and energy content of donor human milk: A systematic review. Adv. Nutr. 2020, 11, 960–970. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Smith, E.R.; Lee, S.E.; Vargas, A.J.; Bremer, A.A.; Raiten, D.J. The need to study human milk as a biological system. Am. J. Clin. Nutr. 2021, 113, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Overview of nutrients in human milk. Adv. Nutr. 2018, 9, 278S–294S. [Google Scholar] [CrossRef]
- Hampel, D.; Shahab-Ferdows, S.; Islam, M.M.; Peerson, J.M.; Allen, L.H. Vitamin Concentrations in Human Milk Vary with Time within Feed, Circadian Rhythm, and Single-Dose Supplementation. J. Nutr. 2017, 147, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Bravi, F.; Wiens, F.; Decarli, A.; Dal Pont, A.; Agostoni, C.; Ferraroni, M. Impact of maternal nutrition on breast-milk composition: A systematic review. Am. J. Clin. Nutr. 2016, 104, 646–662. [Google Scholar] [CrossRef] [PubMed]
- Keikha, M.; Bahreynian, M.; Saleki, M.; Kelishadi, R. Macro- and Micronutrients of Human Milk Composition: Are They Related to Maternal Diet? A Comprehensive Systematic Review. Breastfeed. Med. 2017, 12, 517–527. [Google Scholar] [CrossRef]
- Elorinne, A.L.; Alfthan, G.; Erlund, I.; Kivimäki, H.; Paju, A.; Salminen, I.; Turpeinen, U.; Voutilainen, S.; Laakso, J. Food and nutrient intake and nutritional status of Finnish vegans and non-vegetarians. PLoS ONE 2016, 11, e0148235. [Google Scholar] [CrossRef]
- Karcz, K.; Królak-Olejnik, B. Vegan or vegetarian diet and breast milk composition–a systematic review. Crit. Rev. Food Sci. Nutr. 2021, 61, 1081–1098. [Google Scholar] [CrossRef]
- Weaver, G.; Bertino, E.; Gebauer, C.; Grovslien, A.; Mileusnic-Milenovic, R.; Arslanoglu, S.; Barnett, D.; Boquien, C.Y.; Buffin, R.; Gaya, A.; et al. Recommendations for the establishment and operation of Human Milk Banks in Europe: A consensus statement from the European Milk Bank Association (EMBA). Front. Pediatr. 2019, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.; Mangels, A.; American Dietetic Association. Position of the American Dietetic Association: Vegetarian diets. J. Am. Diet. Assoc. 2009, 109, 1266–1286. [Google Scholar] [CrossRef] [PubMed]
- Perrin, M.T.; Pawlak, R.; Judd, N.; Cooper, J.; Donati, G.L. Major and Trace Mineral Composition of Milk from Lactating Women Following Vegan, Vegetarian, and Omnivore Diets. Br. J. Nutr. 2022, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, M.; Witriw, A. Modelos Visuales de Alimentos y Tablas de Relacion, 1st ed.; Edición del Autor: Buenos Aires, Argentina, 1997; ISBN 978-950-43-8807-4. [Google Scholar]
- Dapcich, V.; Salvador Castell, G.; Ribas Barba, L.; Pérez Rodrigo, C.; Aranceta-Bartrina, J.; Serra Majem, L. Embarazo y lactancia. Necesidades especiales. In Guía de la Alimentación Saludable; Sociedad Española de Nutrición Comunitaria, Ed.; Spanish Society of Community Nutrition: Madrid, Spain, 2004; p. 82. Available online: https://www.nutricioncomunitaria.org/es/otras-publicaciones (accessed on 2 February 2023).
- Ministerio de Sanidad, Gobierno de España. Estilos de Vida Saludable. Available online: https://estilosdevidasaludable.sanidad.gob.es/alimentacionSaludable/queSabemos/enLaPractica/tablaPlanificacion/planificaciones/home.htm (accessed on 3 February 2023).
- López Martínez, R.; Alonso Nieva, N.; Serrat Orús, N.; Gella Tomás, F.; Boned Juliani, B.; Canalias Reverter, F.; Esteve Poblador, S.; González de la Presa, B.; Izquierdo Álvarez, S.; Macías Blanco, C.; et al. Procedimiento para el Estudio de la Interferencia por Hemólisis, Bilirrubina y Turbidez y Para la Verificación de los Índices de Hemólisis, Ictericia y Lipemia. Documento Técnico (2013). Documentos de la SEQC 2014(7)-junio 2014. Available online: https://publicaciones.seqc.es/recopilacion-de-documentos/33-documentos-de-la-seqc-2014-7-junio-2014.html (accessed on 6 April 2023).
- Klem, S.; Klingler, M.; Demmelmair, H.; Koletzko, B. Efficient and specific analysis of red blood cell glycerophospholipid fatty acid composition. PLoS ONE 2012, 7, e33874. [Google Scholar] [CrossRef]
- Ortega, R.; López-Sobaler, A.; Andrés, P.; Requejo, A.; Aparicio, A.; Molinero, L. DIAL Software for Assessing Diets and Food Calculations; Department of Nutrition (UCM) & Alceingeniería, SA: Madrid, Spain, 2014; Available online: http://www.alceingenieria.net/nutricion.htm (accessed on 6 April 2023).
- Red BEDCA del Ministerio de Ciencia e Innovación. Base de Datos Española de Composición de Alimentos. Available online: http://www.bedca.net/bdpub/index.php (accessed on 3 February 2023).
- U.S. Department of Agriculture ARS. FoodData Central. 2019. Available online: https://fdc.nal.usda.gov/ (accessed on 1 February 2023).
- Ortega Anta, R.; Requejo Marcos, A. Nutriguía. Manual de Nutrición Clínica; Editorial Médica Panamericana: Madrid, Spain, 2015; ISBN 9788498358674. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press: Washington, DC, USA, 1997; ISBN 9780309063500. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; The National Academies Press: Washington, DC, USA, 1998; ISBN 9780309064118. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000; ISBN 9780309069496. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; The National Academies Press: Washington, DC, USA, 2001; ISBN 9780309072908. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids; The National Academies Press: Washington, DC, USA, 2005; ISBN 030908525X. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D.; Ross, C.A., Taylor, C.L., Yaktine, A.L., Del Valle, H.B., Institute of Medicine, Eds.; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-16395-8. [Google Scholar]
- EFSA (European Food Safety Authority). Dietary Reference Values for nutrients. Summary report. EFSA Support. Publ. 2017, 98, e15121. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Fats and Fatty Acids in Human Nutrition. Report of an Expert Consultation; FAO Food and Nutrition Paper 91; FAO: Geneva, Switzerland, 2008; ISBN 9789251067338. [Google Scholar]
- Basiotis, P.P.; Carlson, A.; Gerrior, S.A.; Juan, W.Y.; Lino, M. The Healthy Eating Index: 1999–2000; Dinkins, J.M., Ed.; U.S. Department of Agriculture, Center for Nutrition Policy and Promotion: Washington, DC, USA, 2002.
- Kennedy, E.T.; Ohls, J.; Carlson, S.; Fleming, K. The Healthy Eating Index: Design and applications. J. Am. Diet. Assoc. 1995, 95, 1103–1108. [Google Scholar] [CrossRef]
- Löfgren, L.; Ståhlman, M.; Forsberg, G.-B.; Saarinen, S.; Nilsson, R.; Hansson, G.I. The BUME method: A novel automated chloroform-free 96-well total lipid extraction method for blood plasma. J. Lipid Res. 2012, 53, 1690–1700. [Google Scholar] [CrossRef]
- García-Serrano, A.; Tomé-Carneiro, J.; Carmen Crespo, M.; Visitación Calvo, M.; Pereda-Pérez, I.; Baliyan, S.; Burgos-Ramos, E.; Montero, O.; Dávalos, A.; Venero, C.; et al. Concentrates of buttermilk and krill oil improve cognition in aged rats. Prostaglandins Leukot. Essent. Fatty Acids 2020, 155, 102077. [Google Scholar] [CrossRef]
- Castro-Gómez, P.; Montero, O.; Fontecha, J. In-Depth Lipidomic Analysis of Molecular Species of Triacylglycerides, Diacylglycerides, Glycerophospholipids, and Sphingolipids of Buttermilk by GC-MS/FID, HPLC-ELSD, and UPLC-QToF-MS. Int. J. Mol. Sci. 2017, 18, 605. [Google Scholar] [CrossRef]
- Castro-Gómez, P.; Fontecha, J.; Rodríguez-Alcalá, L.M. A high-performance direct transmethylation method for total fatty acids assessment in biological and foodstuff samples. Talanta 2014, 128, 518–523. [Google Scholar] [CrossRef]
- Calvo, M.V.; Martín-Hernández, M.C.; García-Serrano, A.; Castro-Gómez, M.P.; Alonso-Miravalles, L.; García-Martín, R.; Megino-Tello, J.; Alonso, L.; Fontecha, J. Comprehensive characterization of neutral and polar lipids of buttermilk from different sources and its milk fat globule membrane isolates. J. Food Compos. Anal. 2020, 86, 103386. [Google Scholar] [CrossRef]
- Fontecha, J.; Mayo, I.; Toledano, G.; Juárez, M. Triacylglycerol composition of protected designation of origin cheeses during ripening. Authenticity of milk fat. J. Dairy Sci. 2006, 89, 882–887. [Google Scholar] [CrossRef]
- Huynh, D.; Zhou, S.J.; Gibson, R.; Palmer, L.; Muhlhausler, B. Validation of an optimized method for the determination of iodine in human breast milk by inductively coupled plasma mass spectrometry (ICPMS) after tetramethylammonium hydroxide extraction. J. Trace. Elem. Med. Biol. 2015, 29, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Boutin, M.; Presse, N.; Martineau, T.; Perreault, A.; Gaudreau, P.; Auray-Blais, C. Mass spectrometry analysis of urinary methylmalonic acid to screen for metabolic vitamin B12 deficiency in older adults. Bioanalysis 2020, 12, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Romeu-Nadal, M.; Morera-Pons, S.; Castellote, A.I.; López-Sabater, M.C. Rapid high-performance liquid chromatographic method for Vitamin C determination in human milk versus an enzymatic method. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2006, 830, 41–46. [Google Scholar] [CrossRef]
- Robitaille, L.; Hoffer, L.J. A simple method for plasma total vitamin C analysis suitable for routine clinical laboratory use. Nutr. J. 2016, 15, 40. [Google Scholar] [CrossRef] [PubMed]
- Hampel, D.; York, E.R.; Allen, L.H. Ultra-performance liquid chromatography tandem mass-spectrometry (UPLC-MS/MS) for the rapid, simultaneous analysis of thiamin, riboflavin, flavin adenine dinucleotide, nicotinamide and pyridoxal in human milk. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 903, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xiao, H.; Wu, K.; Yu, Z.; Ren, Y.; Zhao, Y.; Li, K.; Li, J.; Li, D. Retinol and α-tocopherol in human milk and their relationship with dietary intake during lactation. Food Funct. 2016, 7, 1985–1991. [Google Scholar] [CrossRef]
- Aronov, P.A.; Hall, L.M.; Dettmer, K.; Stephensen, C.B.; Hammock, B.D. Metabolic profiling of major vitamin D metabolites using Diels-Alder derivatization and ultra-performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2008, 391, 1917–1930. [Google Scholar] [CrossRef]
- Olsen, I.E.; Groveman, S.A.; Lawson, M.L.; Clark, R.H.; Zemel, B.S. New intrauterine growth curves based on United States data. Pediatrics 2010, 125, 214–224. [Google Scholar] [CrossRef]
- World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization (WHO): Geneva, Switzerland, 2006; ISBN 92-4-154693-X. Available online: https://www.who.int/publications/i/item/924154693X (accessed on 5 April 2023).
- Allen, L.H.; Carriquiry, A.L.; Murphy, S.P. Perspective: Proposed Harmonized Nutrient Reference Values for Populations. Adv. Nutr. 2020, 11, 469–483. [Google Scholar] [CrossRef]
- Graham, J.; Peerson, J.; Haskell, M.; Shrestha, R.; Brown, K.; Allen, L. Erythrocyte riboflavin for the detection of riboflavin deficiency in pregnant Nepali women. Clin. Chem. 2005, 51, 2162–2165. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.M.; Shenkin, A.; Schweinlin, A.; Amrein, K.; Augsburger, M.; Biesalski, H.K.; Bischoff, S.C.; Casaer, M.P.; Gundogan, K.; Lepp, H.L.; et al. ESPEN micronutrient guideline. Clin. Nutr. 2022, 41, 1357–1424. [Google Scholar] [CrossRef] [PubMed]
- Hustad, S.; McKinley, M.C.; McNulty, H.; Schneede, J.; Strain, J.J.; Scott, J.M.; Ueland, P.M. Riboflavin, flavin mononucleotide, and flavin adenine dinucleotide in human plasma and erythrocytes at baseline and after low-dose riboflavin supplementation. Clin. Chem. 2002, 48, 1571–1577. [Google Scholar] [CrossRef] [PubMed]
- Andraos, S.; Jones, B.; Wall, C.; Thorstensen, E.; Kussmann, M.; Smith, D.C.; Lange, K.; Clifford, S.; Saffery, R.; Burgner, D.; et al. Plasma B vitamers: Population epidemiology and parent-child concordance in children and adults. Nutrients 2021, 13, 821. [Google Scholar] [CrossRef]
- Ehsanian, R.; Anderson, S.; Schneider, B.; Kennedy, D.; Mansourian, V. Prevalence of low plasma vitamin B1 in the stroke population admitted to acute inpatient rehabilitation. Nutrients 2020, 12, 1034. [Google Scholar] [CrossRef] [PubMed]
- Håglin, L.; Domellöf, M.; Bäckman, L.; Forsgren, L. Low plasma thiamine and phosphate in male patients with Parkinson’s disease is associated with mild cognitive impairment. Clin. Nutr. ESPEN 2020, 37, 93–99. [Google Scholar] [CrossRef]
- Khaksari, M.; Mazzoleni, L.R.; Ruan, C.; Kennedy, R.T.; Minerick, A.R. Determination of water-soluble and fat-soluble vitamins in tears and blood serum of infants and parents by liquid chromatography/mass spectrometry. Exp. Eye Res. 2017, 155, 54–63. [Google Scholar] [CrossRef]
- McCann, A.; Midttun, Ø.; Whitfield, K.C.; Kroeun, H.; Borath, M.; Sophonneary, P.; Ueland, P.M.; Green, T.J. Comparable performance characteristics of plasma thiamine and erythrocyte thiamine diphosphate in response to thiamine fortification in rural cambodian women. Nutrients 2017, 9, 676. [Google Scholar] [CrossRef]
- Anwar, A.; Marini, M.; Abruzzo, P.M.; Bolotta, A.; Ghezzo, A.; Visconti, P.; Thornalley, P.J.; Rabbani, N. Quantitation of plasma thiamine, related metabolites and plasma protein oxidative damage markers in children with autism spectrum disorder and healthy controls. Free Radic. Res. 2016, 50, S85–S90. [Google Scholar] [CrossRef]
- Coats, D.; Frank, E.L.; Reid, J.M.; Ou, K.; Chea, M.; Khin, M.; Preou, C.; Enders, F.T.; Fischer, P.R.; Topazian, M. Thiamine pharmacokinetics in Cambodian mothers and their breastfed infants. Am. J. Clin. Nutr. 2013, 98, 839–844. [Google Scholar] [CrossRef]
- Gangolf, M.; Czerniecki, J.; Radermecker, M.; Detry, O.; Nisolle, M.; Jouan, C.; Martin, D.; Chantraine, F.; Lakaye, B.; Wins, P.; et al. Thiamine status in humans and content of phosphorylated thiamine derivatives in biopsies and cultured cells. PLoS ONE 2010, 5, e13616. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J.; Babaei-Jadidi, R.; Al Ali, H.; Rabbani, N.; Antonysunil, A.; Larkin, J.; Ahmed, A.; Rayman, G.; Bodmer, C.W. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia 2007, 50, 2164–2170. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wei, J.; Gao, W.; Pu, L.; Tang, Z.; Li, L. Plasma riboflavin is a useful marker for studying riboflavin requirement in Chinese male adults. Nutr. Res. 2016, 36, 534–540. [Google Scholar] [CrossRef]
- Petteys, B.J.; Frank, E.L. Rapid determination of vitamin B2 (riboflavin) in plasma by HPLC. Clin. Chim. Acta 2011, 412, 38–43. [Google Scholar] [CrossRef]
- Powers, H.J.; Hill, M.H.; Mushtaq, S.; Dainty, J.R.; Majsak-Newman, G.; Williams, E.A. Correcting a marginal riboflavin deficiency improves hematologic status in young women in the United Kingdom (RIBOFEM). Am. J. Clin. Nutr. 2011, 93, 1274–1284. [Google Scholar] [CrossRef]
- Ibrahim, G.R.; Shah, I.; Gariballa, S.; Yasin, J.; Barker, J.; Ashraf, S.S. Significantly Elevated Levels of Plasma Nicotinamide, Pyridoxal, and Pyridoxamine Phosphate Levels in Obese Emirati Population: A Cross-Sectional Study Ghada. Molecules 2020, 25, 3932. [Google Scholar] [CrossRef] [PubMed]
- Kouassi Nzoughet, J.; Chao de la Barca, J.M.; Guehlouz, K.; Leruez, S.; Coulbault, L.; Allouche, S.; Bocca, C.; Muller, J.; Amati-Bonneau, P.; Gohier, P.; et al. Nicotinamide deficiency in primary open-angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2019, 60, 2509–2514. [Google Scholar] [CrossRef]
- Meisser Redeuil, K.; Longet, K.; Bénet, S.; Munari, C.; Campos-Giménez, E. Simultaneous quantification of 21 water soluble vitamin circulating forms in human plasma by liquid chromatography-mass spectrometry. J. Chromatogr. A 2015, 1422, 89–98. [Google Scholar] [CrossRef]
- Hansen, C.M.; Shultz, T.D.; Kwak, H.K.; Memon, H.S.; Leklem, J.E. Assessment of vitamin B-6 status in young women consuming a controlled diet containing four levels of vitamin B-6 provides an estimated average requirement and recommended dietary allowance. J. Nutr. 2001, 131, 1777–1786. [Google Scholar] [CrossRef]
- Driskell, J.A.; Chrisley, B.M. Plasma B-6 vitamer and plasma and urinary 4-pyridoxic acid concentrations in young women as determined using high performance liquid chromatography. Biomed. Chromatogr. 1991, 5, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Sobczyńska-Malefora, A.; Harrington, D.J. Laboratory assessment of folate (Vitamin B9) status. J. Clin. Pathol. 2018, 71, 949–956. [Google Scholar] [CrossRef]
- Allen, L.H.; Miller, J.W.; De Groot, L.; Rosenberg, I.H.; Smith, A.D.; Refsum, H.; Raiten, D.J. Biomarkers of Nutrition for Development (BOND): Vitamin B-12 Review. J. Nutr. 2018, 148, 1995S–2027S. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, R.; Parrott, S.J.; Raj, S.; Cullum-Dugan, D.; Lucus, D. How prevalent is vitamin B12 deficiency among vegetarians? Nutr. Rev. 2013, 71, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.B.; Stover, P.J.; McNulty, H.; Fenech, M.F.; Gregory, J.F., III; Mills, J.L.; Pfeiffer, C.M.; Fazili, Z.; Zhang, M.; Ueland, P.M.; et al. Biomarkers of nutrition for development-Folate review. J. Nutr. 2015, 145, 1636S–1680S. [Google Scholar] [CrossRef] [PubMed]
- EFSA. NDA Panel. Scientific Opinion on Dietary Reference Values for vitamin C. EFSA J. 2013, 11, 3418. [Google Scholar] [CrossRef]
- De Pee, S.; Dary, O. Biochemical indicators of vitamin A deficiency: Serum retinol and serum retinol binding protein. J. Nutr. 2002, 132, 2895–2901. [Google Scholar] [CrossRef]
- World Health Organization. Serum Retinol Concentrations for Determining the Prevalence of Vitamin A Deficiency in Populations; World Health Organization (WHO): Geneva, Switzerland, 2011. Available online: https://apps.who.int/iris/bitstream/handle/10665/85859/WHO_NMH_NHD_MNM_11.3_eng.pdf?sequence=4&isAllowed=y (accessed on 5 April 2023).
- U.S. Centers for Disease Control and Prevention. Second National Report on Biochemical Indicators of Diet and Nutrition in the U.S. Population; CDC: Atlanta, GA, USA, 2012; ISBN 1499234783. Available online: https://www.cdc.gov/nutritionreport/pdf/nutrition_book_complete508_final.pdf (accessed on 5 April 2023).
- Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture; Department of Agriculture, Agricultural Research Service: Washington, DC, USA, 2015. Available online: https://health.gov/sites/default/files/2019-09/Scientific-Report-of-the-2015-Dietary-Guidelines-Advisory-Committee.pdf (accessed on 5 April 2023).
- Papathakis, P.C.; Rollins, N.C.; Chantry, C.J.; Bennish, M.L.; Brown, K.H. Micronutrient status during lactation in HIV-infected and HIV-uninfected South African women during the first 6 mo after delivery. Am. J. Clin. Nutr. 2007, 85, 182–192. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Vitamin E deficiency in developing countries. Food Nutr. Bull. 2011, 32, 124–143. [Google Scholar] [CrossRef]
- Henjum, S.; Manger, M.; Hampel, D.; Brantsæter, A.L.; Shahab-Ferdows, S.; Bastani, N.E.; Strand, T.A.; Refsum, H.; Allen, L.H. Vitamin B12 concentrations in milk from Norwegian women during the six first months of lactation. Eur. J. Clin. Nutr. 2020, 74, 749–756. [Google Scholar] [CrossRef]
- Norman, E.J.; Morrison, J.A. Screening elderly populations for cobalamin (vitamin B12) deficiency using the urinary methylmalonic acid assay by gas chromatography mass spectrometry. Am. J. Med. 1993, 94, 589–594. [Google Scholar] [CrossRef]
- World Health Organization. Vitamin and Mineral Nutrition Information System. Urinary Iodine Concentrations for Determining Iodine Status Deficiency in Populations; World Health Organization (WHO): Geneva, Switzerland, 2013. Available online: https://apps.who.int/iris/bitstream/handle/10665/85972/WHO_NMH_NHD_EPG_13.1_eng.pdf (accessed on 8 March 2023).
- Ahn, J.; Lee, J.H.; Lee, J.; Baek, J.Y.; Song, E.; Oh, H.S.; Kim, M.; Park, S.; Jeon, M.J.; Kim, T.Y.; et al. Association between Urinary Sodium Levels and Iodine Status in Korea. Korean J. Intern. Med. 2020, 35, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Foley, K.F.; Boccuzzi, L. Urine calcium: Laboratory measurement and clinical utility. Lab. Med. 2010, 41, 683–686. [Google Scholar] [CrossRef]
- Fernández-Ruiz, L.; Rodelo Haad, C.; Rodríguez-Portillo, M.; Santamaría-Olmo, R. Variation of phosphaturia according to phosphorus intake. Actual. Med. 2020, 105, 18–26. [Google Scholar] [CrossRef]
- Giuffrida, F.; Fleith, M.; Goyer, A.; Samuel, T.M.; Elmelegy-Masserey, I.; Fontannaz, P.; Cruz-Hernandez, C.; Thakkar, S.K.; Monnard, C.; De Castro, C.A.; et al. Human milk fatty acid composition and its association with maternal blood and adipose tissue fatty acid content in a cohort of women from Europe. Eur. J. Nutr. 2022, 61, 2167–2182. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Yang, X.; Cheng, Y.; Zhang, H.; Xu, X.; Zhou, J.; Chen, H.; Su, M.; Yang, Y.; et al. Human Milk Lipid Profiles around the World: A Systematic Review and Meta-Analysis. Adv. Nutr. 2022, 13, 2519–2536. [Google Scholar] [CrossRef]
- Gibson, R.S.; Rahmannia, S.; Diana, A.; Leong, C.; Haszard, J.J.; Hampel, D.; Reid, M.; Erhardt, J.; Suryanto, A.H.; Sofiah, W.N.; et al. Association of maternal diet, micronutrient status, and milk volume with milk micronutrient concentrations in Indonesian mothers at 2 and 5 months postpartum. Am. J. Clin. Nutr. 2020, 112, 1039–1050. [Google Scholar] [CrossRef]
- EFSA NDA Panel; Turck, D.; Bresson, J.L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.I.; Mangelsdorf, I.; McArdle, H.J.; et al. Dietary reference values for thiamin. EFSA J. 2016, 14, 4653. [Google Scholar] [CrossRef]
- EFSA NDA Panel; Turck, D.; Bresson, J.-L.; Burlingame, B.; Dean, T.; Fairweather-Tait, S.; Heinonen, M.; Hirsch-Ernst, K.; Mangelsdorf, I.; McArdle, H.; et al. Dietary Reference Values for riboflavin. EFSA J. 2017, 15, 4919. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for niacin. EFSA J. 2014, 12, 3759. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for pantothenic acid. EFSA J. 2014, 12, 3581. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA J. 2013, 11, 3408. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for cobalamin (vitamin B12). EFSA J. 2015, 13, 4150. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for vitamin A. EFSA J. 2015, 13, 4028. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on dietary reference values for vitamin D. EFSA J. 2016, 14, 4547. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for vitamin E as α-tocopherol. EFSA J. 2015, 13, 4149. [Google Scholar] [CrossRef]
- Semba, R.D.; Delange, F. Iodine in human milk: Perspectives for infant health. Nutr. Rev. 2001, 59, 269–278. [Google Scholar] [CrossRef]
- Andersson, M.; Braegger, C.P. The role of iodine for thyroid function in lactating women and infants. Endocr. Rev. 2022, 43, 469–506. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for calcium. EFSA J. 2015, 13, 4101. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for phosphorus. EFSA J. 2015, 13, 4185. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on Dietary Reference Values for selenium. EFSA J. 2014, 12, 3846. [Google Scholar] [CrossRef]
- LASER Analytica. Comprehensive literature search and review of breast milk composition as preparatory work for the setting of dietary reference values for vitamins and minerals. EFSA Support. Publ. 2014, 11, EN-629. [Google Scholar] [CrossRef]
- Davis, B.C.; Kris-Etherton, P.M. Achieving optimal essential fatty acid status in vegetarians: Current knowledge and practical implications. Am. J. Clin. Nutr. 2003, 78, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, B. Plasmalogens: Targets for oxidants and major lipophilic antioxidants. Biochem. Soc. Trans. 2004, 32, 147–150. [Google Scholar] [CrossRef]
- Sanders, T.; Reddy, S. The influence of a vegetarian diet on the fatty acid composition of human milk and the essential fatty acid status of the infant. J. Pediatr. 1992, 120, 71–77. [Google Scholar] [CrossRef]
- Koletzko, B.; Boey, C.C.M.; Campoy, C.; Carlson, S.E.; Chang, N.; Guillermo-Tuazon, M.A.; Joshi, S.; Prell, C.; Quak, S.H.; Sjarif, D.R.; et al. Current information and asian perspectives on long-chain polyunsaturated fatty acids in pregnancy, lactation, and infancy: Systematic review and practice recommendations from an early nutrition academy workshop. Ann. Nutr. Metab. 2014, 65, 49–80. [Google Scholar] [CrossRef] [PubMed]
- Cetin, I.; Koletzko, B. Long-chain ω-3 fatty acid supply in pregnancy and lactation. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Koletzko, B.; Cetin, I.; Brenna, J.T.; Perinatal Lipid Intake Working Group; Child Health Foundation; Diabetic Pregnancy Study Group; European Association of Perinatal Medicine; European Society for Clinical Nutrition and Metabolism; European Society for Paediatric Gastroenterology; Hepatology and Nutrition; et al. Dietary fat intakes for pregnant and lactating women. Br. J. Nutr. 2007, 98, 873–877. [Google Scholar] [CrossRef]
- Lapillonne, A.; Eleni Dit Trolli, S.; Kermorvant-Duchemin, E. Postnatal docosahexaenoic acid deficiency is an inevitable consequence of current recommendations and practice in preterm infants. Neonatology 2010, 98, 397–403. [Google Scholar] [CrossRef]
- Valentine, C.J. Maternal Dietary DHA Supplementation to Improve Inflammatory Outcomes in the Preterm Infant. Adv. Nutr. 2012, 3, 370–376. [Google Scholar] [CrossRef]
- Makrides, M.; Gibson, R.A.; McPhee, A.J.; Collins, C.T.; Davis, P.G.; Doyle, L.W.; Simmer, K.; Colditz, P.B.; Morris, S.; Smithers, L.G.; et al. Neurodevelopmental outcomes of preterm infants fed high-dose docosahexaenoic acid: A randomized controlled trial. JAMA 2009, 301, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellöf, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the european society of paediatric gastroenterology, hepatology and nutrition committee on nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Baack, M.L.; Norris, A.W.; Yao, J.; Colaizy, T. Long Chain Polyunsaturated Fatty Acid Levels in U.S. Donor Human Milk: Meeting the Needs of Premature Infants? J. Perinatol. 2012, 32, 598–603. [Google Scholar] [CrossRef]
- Valentine, C.J.; Morrow, G.; Fernandez, S.; Gulati, P.; Bartholomew, D.; Long, D.; Welty, S.E.; Morrow, A.L.; Rogers, L.K. Docosahexaenoic acid and amino acid contents in pasteurized donor milk are low for preterm infants. J. Pediatr. 2010, 157, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Brenna, J.T.; Varamini, B.; Jensen, R.G.; Diersen-Schade, D.A.; Boettcher, J.A.; Arterburn, L.M. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am. J. Clin. Nutr. 2007, 85, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, S.; Gautier, S.; Salem, N.J. Global estimates of dietary intake of docosahexaenoic acid and arachidonic acid in developing and developed countries. Ann. Nutr. Metab. 2016, 68, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Liu, X.; Zhou, B.; Jiang, A.C.; Chai, L. An updated review of worldwide levels of docosahexaenoic and arachidonic acid in human breast milk by region. Public Health Nutr. 2016, 19, 2675–2687. [Google Scholar] [CrossRef]
- Perrin, M.T.; Pawlak, R.; Dean, L.L.; Christis, A.; Friend, L. A cross-sectional study of fatty acids and brain-derived neurotrophic factor (BDNF) in human milk from lactating women following vegan, vegetarian, and omnivore diets. Eur. J. Clin. Nutr. 2019, 58, 2401–2410. [Google Scholar] [CrossRef]
- Barreiro, R.; Díaz-Bao, M.; Cepeda, A.; Regal, P.; Fente, C.A. Fatty acid composition of breast milk in Galicia (NW Spain): A cross-country comparison. Prostaglandins Leukot. Essent. Fat. Acids 2018, 135, 102–114. [Google Scholar] [CrossRef]
- Valentine, C.J.; Morrow, G.; Pennell, M.; Morrow, A.L.; Hodge, A.; Haban-Bartz, A.; Collins, K.; Rogers, L.K. Randomized controlled trial of docosahexaenoic acid supplementation in midwestern U.S. human milk donors. Breastfeed. Med. 2013, 8, 86–91. [Google Scholar] [CrossRef]
- Makrides, M.; Neumann, M.; Gibson, R. Effect of maternal docosahexaenoic (DHA) supplementation on breast milk composition. Eur. J. Clin. Nutr. 1996, 50, 352–357. [Google Scholar]
- Fidler, N.; Sauerwald, T.; Pohl, A.; Demmelmair, H.; Koletzko, B. Docosahexaenoic acid transfer into human milk after dietary supplementation: A randomized clinical trial. J. Lipid Res. 2000, 41, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.L.; Maude, M.; Anderson, R.E.; Heird, W.C. Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids. Am. J. Clin. Nutr. 2000, 71, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Sherry, C.L.; Oliver, J.S.; Marriage, B.J. Docosahexaenoic acid supplementation in lactating women increases breast milk and plasma docosahexaenoic acid concentrations and alters infant omega 6:3 fatty acid ratio. Prostaglandins Leukot. Essent. Fat. Acids 2015, 95, 63–69. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Li, F.; Xu, F.; Hu, P.; Xie, Z.; Lu, X.; Ding, Y.; Wang, Z. Impact of DHA from Algal Oil on the Breast Milk DHA Levels of Lactating Women: A Randomized Controlled Trial in China. Nutrients 2022, 14, 3410. [Google Scholar] [CrossRef]
- Valencia-Naranjo, A.; Manjarres-Correa, L.M.; Bermúdez-Cardona, J. Pilot study of the effect of EPA + DHA supplementation on the fatty acid profile of erythrocytes and breast milk of lactating women from Sonsón, Colombia. Curr. Res. Food Sci. 2022, 5, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Smithers, L.G.; Markrides, M.; Gibson, R.A. Human milk fatty acids from lactating mothers of preterm infants: A study revealing wide intra- and inter-individual variation. Prostaglandins Leukot. Essent. Fat. Acids 2010, 83, 9–13. [Google Scholar] [CrossRef]
- Burns-Whitmore, B.; Froyen, E.; Heskey, C.; Parker, T.; San Pablo, G. Alpha-linolenic and linoleic fatty acids in the vegan diet: Do they require dietary reference intake/adequate intake special consideration? Nutrients 2019, 11, 2365. [Google Scholar] [CrossRef]
- Henriksen, C.; Haugholt, K.; Lindgren, M.; Aurvåg, A.K.; Rønnestad, A.; Grønn, M.; Solberg, R.; Moen, A.; Nakstad, B.; Berge, R.K.; et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 2008, 121, 1137–1145. [Google Scholar] [CrossRef]
- Sæle, Ø.; Rød, K.; Quinlivan, V.; Li, S.; Farber, S. A novel system to quantify intestinal lipid digestion and transport. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 948–957. [Google Scholar] [CrossRef]
- Ahmed, T.B.; Eggesbø, M.; Criswell, R.; Uhl, O.; Demmelmair, H.; Koletzko, B. Total fatty acid and polar lipid species composition of human milk. Nutrients 2022, 14, 158. [Google Scholar] [CrossRef]
- Demmelmair, H.; Koletzko, B. Lipids in human milk. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 32, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Benoit, B.; Fauquant, C.; Daira, P.; Peretti, N.; Guichardant, M.; Michalski, M.-C. Phospholipid species and minor sterols in French human milks. Food Chem. 2010, 120, 684–691. [Google Scholar] [CrossRef]
- Sala-Vila, A.; Castellote, A.I.; Rodriguez-Palmero, M.; Campoy, C.; López-Sabater, M.C. Lipid composition in human breast milk from Granada (Spain): Changes during lactation. Nutrition 2005, 21, 467–473. [Google Scholar] [CrossRef]
- Ingvordsen Lindahl, I.E.; Artegoitia, V.M.; Downey, E.; O’Mahony, J.A.; O’Shea, C.A.; Ryan, C.A.; Kelly, A.L.; Bertram, H.C.; Sundekilde, U.K. Quantification of human milk phospholipids: The effect of gestational and lactational age on phospholipid composition. Nutrients 2019, 11, 222. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shimizu, Y.; Kaneko, S.; Hanaka, S.; Abe, T.; Shimasaki, H.; Hisaki, H.; Nakajima, H. Comparison of the fatty acid composition of total lipids and phospholipids in breast milk from Japanese women. Pediatr. Int. 2000, 42, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Bitman, J.; Wood, D.L.; Mehta, N.R.; Hamosh, P.; Hamosh, M. Comparison of the phospholipid composition of breast milk from mothers of term and preterm infants during lactation. Am. J. Clin. Nutr. 1984, 40, 1103–1119. [Google Scholar] [CrossRef] [PubMed]
- Dror, D.K.; Allen, L.H. Effect of vitamin B12 deficiency on neurodevelopment in infants: Current knowledge and possible mechanisms. Nutr. Rev. 2008, 66, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Messina, V.; Melina, V.; Mangels, A.R. A new food guide for North American vegetarians. J. Am. Diet. Assoc. 2003, 103, 771–775. [Google Scholar] [CrossRef]
- Rizzo, G.; Laganà, A.S.; Rapisarda, A.M.C.; La Ferrera, G.M.G.; Buscema, M.; Rossetti, P.; Nigro, A.; Muscia, V.; Valenti, G.; Sapia, F.; et al. Vitamin B12 among vegetarians: Status, assessment and supplementation. Nutrients 2016, 8, 767. [Google Scholar] [CrossRef]
- Wagner, K.-H.; Kamal-Eldin, A.; Elmadfa, I. Gamma-tocopherol--an underestimated vitamin? Ann. Nutr. Metab. 2004, 48, 169–188. [Google Scholar] [CrossRef]
- Allen, L.H. B Vitamins in Breast Milk: Relative Importance of Maternal Status and Intake, and Effects on Infant Status and Function. Adv. Nutr. Int. Rev. J. 2012, 3, 362–369. [Google Scholar] [CrossRef]
- Bijur, A.M.; Desai, A.G. Composition of breast milk with reference to vitamin B12 and folic acid in Indian mothers. Indian J. Pediatr. 1985, 52, 147–150. [Google Scholar] [CrossRef]
- Büttner, B.E.; Witthöft, C.M.; Domellöf, M.; Hernell, O.; Öhlund, I. Effect of type of heat treatment of breastmilk on folate content and pattern. Breastfeed. Med. 2014, 9, 86–91. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on Nutritional Status During Pregnancy and Lactation. Nutrition during Lactation; Subcommittee on Nutrition During Lactation: Washington, DC, USA; Committee on Nutritional Status During Pregnancy and Lactation: Washington, DC, USA; Food and Nutrition Board: Washington, DC, USA; Institute of Medicine: Washington, DC, USA; National Academy of Sciences: Washington, DC, USA, 1991; ISBN 0309043913.
- Debski, B.; Finley, D.A.; Picciano, M.F.; Lönnerdal, B.; Milner, J. Selenium content and glutathione peroxidase activity of milk from vegetarian and nonvegetarian women. J. Nutr. 1989, 119, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Ureta-Velasco, N.; Keller, K.; Escuder-Vieco, D.; Serrano, J.C.E.; García-Lara, N.R.; Pallás-Alonso, C.R. Assessment of Iodine Concentration in Human Milk from Donors: Implications for Preterm Infants. Nutrients 2022, 14, 4304. [Google Scholar] [CrossRef] [PubMed]
- Streuling, I.; Beyerlein, A.; Rosenfeld, E.; Schukat, B.; Von Kries, R. Weight gain and dietary intake during pregnancy in industrialized countries—A systematic review of observational studies. J. Perinat. Med. 2011, 39, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, G.; Herranz Barbero, A.; Borrás-Novel, C.; Alsina Casanova, M.; Aldecoa-Bilbao, V.; Andreu-Fernández, V.; Pascual Tutusaus, M.; FerreroMartínez, S.; Gómez Roig, M.D.; García-Algar, O. The effects of vegetarian and vegan diet during pregnancy on the health of mothers and offspring. Nutrients 2019, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Nessel, I.; Khashu, M.; Dyall, S.C. The effects of storage conditions on long-chain polyunsaturated fatty acids, lipid mediators, and antioxidants in donor human milk—A review. Prostaglandins Leukot. Essent. Fat. Acids 2019, 149, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cheng, J.; Yang, J.; Chen, C.; Jin, Q.; Song, J.; Wang, X. Phospholipid composition and fat globule structure change during low temperature storage of human milk. LWT 2021, 150, 112050. [Google Scholar] [CrossRef]
- Leaf, A.; Lansdowne, Z. Vitamins--conventional uses and new insights. World Rev. Nutr. Diet. 2014, 110, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Peila, C.; Moro, G.E.; Bertino, E.; Cavallarin, L.; Giribaldi, M.; Giuliani, F.; Cresi, F.; Coscia, A. The effect of holder pasteurization on nutrients and biologically-active components in donor human milk: A review. Nutrients 2016, 8, 477. [Google Scholar] [CrossRef] [PubMed]
- Escuder-Vieco, D.; Rodríguez, J.M.; Espinosa-Martos, I.; Corzo, N.; Montilla, A.; García-Serrano, A.; Calvo, M.V.; Fontecha, J.; Serrano, J.; Fernández, L.; et al. High-Temperature Short-Time and Holder Pasteurization of Donor Milk: Impact on Milk Composition. Life 2021, 11, 114. [Google Scholar] [CrossRef] [PubMed]
- Young, B.E.; Murphy, K.; Borman, L.L.; Heinrich, R.; Krebs, N.F. Milk Bank Pooling Practices Impact Concentrations and Variability of Bioactive Components of Donor Human Milk. Front. Nutr. 2020, 7, 579115. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Donors (n = 92) | Veg (n = 20) | p Value |
|---|---|---|---|
| Age (years) | 36.1 (4.2); 28–47 | 33.9 (5,2); 24–42 | 0.118 |
| Weight (kg) | 60.8 (55.3, 71.0); 46–122 | 61.1 (52.8, 64.9); 48–93 | 0.365 |
| Height (cm) | 164.2 (6.3); 152–158 | 163.1 (4.4); 155–172 | 0.493 |
| Pre-pregnancy BMI (kg/m2) | 22.3 (21.0, 25.0); 18.2–44.2 | 22.2 (20.1, 23.9); 18.0–34.3 | 0.436 |
| Pre-pregnancy BMI (kg/m2) category | |||
| Underweight (<18.5) | 1 (1.1%) | 2 (10.0%) | 0.135 |
| Normal (18.5–24.9) | 69 (75.0%) | 15 (75.0%) | |
| Overweight (25–29.9) | 12 (13.0%) | 2 (10.0%) | |
| Obese (≥30) | 10 (10.9%) | 1 (5.0%) | |
| Current BMI (kg/m2) | 23.1 (21.2, 25.0); 16.7–42.8 | 22.8 (20.5, 23.7); 18.3–38.9 | 0.287 |
| Current BMI (kg/m2) category | |||
| Underweight (<18.5) | 2 (2.2%) | 1 (5.0%) | 0.624 |
| Normal (18.5–24.9) | 67 (72.8%) | 16 (80.0%) | |
| Overweight (25–29.9) | 12 (13.0%) | 1 (5.0%) | |
| Obese (≥30) | 11 (12.0%) | 2 (10.0%) | |
| Gestational weight gain (kg) | 12.0 (9.2, 15.0); 5–30 | 11.0 (8.5, 12.2); 6–13.6 | 0.024 |
| Postpartum weight retention (kg) | 0.75 (−0.8–2.2); −12–11.8 | 0.7 (−1.8–3.2); −4.2–11.2 | 0.846 |
| Number of children | |||
| 1 | 48 (52.2%) | 14 (70.0%) | 0.557 |
| 2 | 36 (39.1%) | 6 (30.0%) | |
| ≥3 | 8 (8.7%) | 0 (0.0%) | |
| Country of origin: Spain, n (%) | 84 (91.3%) | 19 (95.0%) | 0.581 |
| Education level | |||
| Secondary studies | 2 (2.2%) | 3 (15.0%) | 0.063 |
| Technical studies | 9 (9.8%) | 1 (5.0%) | |
| University studies | 81 (88.0%) | 16 (80.0%) | |
| Currently working | 43 (46.7%) | 14 (70.0%) | 0.059 |
| Physical activity | |||
| Sedentary | 21 (22.8%) | 5 (25.0%) | 0.355 |
| Low active | 49 (53.3%) | 7 (35.0%) | |
| Active/very active | 22 (23.9%) | 8 (40.0%) | |
| Tobacco consumption | |||
| Previously | 18 (19.6%) | 4 (20.0%) | 0.96 |
| Currently | |||
| Passive smoking | 18 (19.6%) | 2 (10.0%) | 0.16 |
| Active smoking | 1 (1.1%) | 1 (5.0%) | |
| Alcohol consumption | |||
| Prior to pregnancy | 47 (51.1%) | 11 (55.0%) | 0.228 |
| During pregnancy | 1 (1.1%) | 1 (5.0%) | |
| Currently | 4 (4.3%) | 3 (15.0%) | |
| Season during the study | |||
| Spring | 24 (26.1%) | 1 (5.0%) | 0.103 |
| Summer | 13 (14.1%) | 6 (30.0%) | |
| Autumn | 33 (35.9%) | 8 (40.0%) | |
| Winter | 22 (23.9%) | 5 (25.0%) |
| Characteristic | Donors (n = 92) | Veg (n = 20) | p Value |
|---|---|---|---|
| Girl | 50 (54.3%) | 7 (35%) | 0.116 |
| Boy | 42 (45.7%) | 13 (65.0%) | |
| Twin pregnancy | 0 (0.0%) | 1 (5.0%) | 0.178 |
| Gestational age (weeks) | 39+6 (39, 40+4); 37+1–42+3 | 39+4 (38+6, 40+3); 36+4–41+4 | 0.082 |
| Birth weight (grams) | 3303.4 (412.7); 2120–4640 | 3125.8 (412.6); 2240–3960 | 0.120 |
| Birth weight percentile 1 | |||
| ≤25 | 23 (25.0%) | 12 (60.0%) | 0.007 |
| 25–75 | 62 (67.4%) | 8 (40.0%) | |
| ≥75 | 7 (7.6%) | 0 (0.0%) | |
| Age of breastfed child (months) | |||
| 0–6 | 34 (37.0%) | 9 (45.0%) | 0.776 |
| 6–12 | 34 (37.0%) | 6 (30.0%) | |
| 12–50 | 24 (26.1%) | 5 (25.0%) | |
| Weight percentile of breastfed child 2 | |||
| ≤15 | 13 (14.1%) | 7 (35.0%) | 0.049 |
| 15–85 | 62 (67.4%) | 12 (60.0%) | |
| ≥85 | 17 (18.5%) | 1 (5.0%) |
| Characteristic | Donors (n = 92) | Veg (n = 20) | p Value |
|---|---|---|---|
| Donor previously | 19 (20.6%) | 1 (5%) | 0.118 |
| Lactation stage (months) | 7.0 (5.0, 13.5); 2–50 | 8.0 [4.5–14.0]; 1–36 | 0.942 |
| Type of lactation | |||
| Exclusive | 40 (43.5%) | 9 (45%) | 0.901 |
| Partial | 52 (56.5%) | 11 (55%) | |
| Sum of child direct breastfeeding times plus daily pumped sessions | |||
| <5 | 8 (8.7%) | 2 (10.0%) | 0.971 |
| 5–10 | 59 (64.1%) | 12 (60.0%) | |
| >10 | 24 (26.1%) | 5 (25.0%) | |
| Missing data | 1 (1.1%) | 1 (5.0%) | |
| Tandem breastfeeding | 5 (5.4%) | 1 (5.0%) | 0.937 |
| Breastfeeding twins | 0 (0%) | 0 (0%) | - |
| Type of milk expression * | |||
| Manual | 7 (7.6%) | 5 (25%) | 0.038 |
| Mechanical breast pump | 10 (10.9%) | 2 (10.0%) | 0.909 |
| Simple electric breast pump | 68 (73.9%) | 14 (70%) | 0.720 |
| Double electric breast pump | 12 (13.0%) | 2 (10%) | 0.709 |
| Pharmacological Supplement | n (%) | p Value | Daily Dose, Mean (SE); Range | p Value | ||
|---|---|---|---|---|---|---|
| Donors (n = 92) | Veg (n = 20) | Donors (n = 92) | Veg (n = 20) | |||
| Vitamin A, mcg | ||||||
| Pregnancy | 12 (13.0) | 3 (15.0) | 0.732 | 527.6 (69.7); 23.0–700.0 | 383.3 (174.0); 100.0–700.0 | 0.389 |
| Lactation | 45 (48.9) | 3 (15.0) | 0.002 | |||
| Previously | 14 (15.2) | 2 (10.0) | 730.9 (49.2); 333.0–1000.0 | 250.0 (150.0); 100.0–400.0 | 0.022 | |
| Currently | 31 (33.7) | 1 (5.0) | 678.1 (45.2); 160.0–1000.0 | 800.0 (-); 800.0–800.0 | 0.729 | |
| Vitamin D, mcg | ||||||
| Pregnancy | 50 (54.3) | 10 (50.0) | 0.687 | 10.61 (0.8); 3.8–30.0 | 17.08 (6.2); 5.0–62.5 | 0.925 |
| Lactation | 52 (56.5) | 9 (45.0) | 0.220 | |||
| Previously | 15 (16.3) | 2 (10.0) | 5.0 (0.4); 2.5–10.0 | 6.2 (3.7); 2.5–10.0 | 0.926 | |
| Currently | 37 (40.2) | 7 (35.0) | 6.0 (0.8); 1.0–25.0 | 27.4 (9.0); 5.0–62.5 | 0.001 | |
| Vitamin E, mg | ||||||
| Pregnancy | 22 (23.9) | 7 (35.0) | 0.318 | 10.7 (0.7); 1.8–15.0 | 11. (2.0); 4.0–20.0 | 0.762 |
| Lactation | 49 (53.3) | 6 (30.0) | 0.030 | |||
| Previously | 15 (16.3) | 2 (10.0) | 11.5 (0.5); 6.0–15.0 | 13.0 (7.0); 6.0–20.0 | 0.926 | |
| Currently | 34 (37.0) | 4 (20.0) | 10.9 (0.6); 2.4–16.0 | 11.0 (0.6); 10.0–12.0 | 0.542 | |
| Vitamin C, mg | ||||||
| Pregnancy | 48 (52.2) | 10 (50.0) | 0.823 | 61.1 (4.2); 12.0–180.0 | 93.6 (30.6); 26.0–358.0 | 0.336 |
| Lactation | 51 (55.4) | 7 (35.0) | 0.050 | |||
| Previously | 16 (17.4) | 3 (15.0) | 80.0 (4.2); 40.0–110.0 | 60.0 (20.0); 40.0–100.0 | 0.260 | |
| Currently | 35 (38.0) | 4 (20.0) | 73.5 (4.2); 16.0–125.0 | 65.0 (8.7); 50.0–80.0 | 0.492 | |
| Vitamin B1, thiamine, mg | ||||||
| Pregnancy | 48 (52.2) | 9 (45.0) | 0.530 | 1.1 (0.0); 0.6–1.5 | 3.3 (1.7); 0.9–17.0 | 0.038 |
| Lactation | 50 (54.3) | 8 (40.0) | 0.149 | |||
| Previously | 16 (17.4) | 3 (15.0) | 1.0 (0.1); 0.6–1.2 | 2.8 (1.1); 0.6–4.3 | 0.221 | |
| Currently | 34 (37.0) | 5 (25.0) | 0.9 (0.1); 0.2–1.1 | 10.8 (9.8); 0.9–50.0 | 0.431 | |
| Vitamin B2, riboflavin, mg | ||||||
| Pregnancy | 48 (52.2) | 9 (45.0) | 0.530 | 1.4 (0.0); 0.7–2.5 | 2.4 (0.8); 1.0–8.5 | 0.060 |
| Lactation | 50 (54.3) | 8 (40.0) | 0.149 | |||
| Previously | 16 (17.4) | 3 (15.0) | 1.3 (0.1); 0.7–1.6 | 2.9 (1.2); 0.7–5.0 | 0.272 | |
| Currently | 34 (37.0) | 5 (25.0) | 1.3 (0.1); 0.7–1.6 | 5.0 (3.8); 1.0–20.0 | 0.750 | |
| Vitamin B3, niacin, mg | ||||||
| Pregnancy | 48 (52.2) | 8 (40.0) | 0.301 | 15.6 (0.4); 4.0–20.0 | 17.4 (2.6); 10.0–33.0 | 0.375 |
| Lactation | 50 (54.3) | 8 (40.0) | 0.149 | |||
| Previously | 16 (17.4) | 3 (15.0) | 15.0 (0.7); 8.0–16.0 | 14.0 (3.1); 8.0–18.0 | 0.718 | |
| Currently | 34 (37.0) | 5 (25.0) | 13.3 (0.7); 3.2–16.0 | 11.4 (2.1); 5.0–16.0 | 0.403 | |
| Vitamin B5, pantothenic, mg | ||||||
| Pregnancy | 48 (52.2) | 8 (40.0) | 0.301 | 5.7 (0.2); 4.0–20.0 | 8.9 (3.2); 5.0–31.0 | 0.923 |
| Lactation | 50 (54.3) | 8 (40.0) | 0.149 | |||
| Previously | 16 (17.4) | 3 (15.0) | 5.6 (0.3); 3.0–6.0 | 6.3 (2.0); 3.0–10.0 | 0.718 | |
| Currently | 34 (37.0) | 5 (25.0) | 5.1 (0.2); 2.8–6.0 | 24.4 (18.9); 5.0–100.0 | 0.488 | |
| Vitamin B6, pyridoxine, mg | ||||||
| Pregnancy | 48 (52.2) | 9 (45.0) | 0.530 | 1.4 (0.0); 0.7–2.2 | 3.6 (1.5); 0.8–2.5 | 0.533 |
| Lactation | 50 (54.3) | 8 (40.0) | 0.149 | |||
| Previously | 16 (17.4) | 3 (15.0) | 1.4 (0.1); 0.7–2.2 | 3.0 (1.4); 0.7–5.4 | 0.249 | |
| Currently | 34 (37.0) | 5 (25.0) | 1.3 (0.1); 0.3–2.0 | 3.6 (2.2); 1.3–12.5 | 0.617 | |
| Vitamin B7, biotin, mcg | ||||||
| Pregnancy | 48 (52.2) | 8 (40.0) | 0.301 | 55.3 (2.9); 25.0–150.0 | 117.1 (20.1); 50.0–187.0 | <0.001 |
| Lactation | 50 (54.3) | 7 (35.0) | 0.063 | |||
| Previously | 16 (17.4) | 2 (10.0) | 47.5 (2.3); 25.0–60.0 | 37.5 (12.5); 25.0–50−0 | 0.208 | |
| Currently | 34 (37.0) | 5 (25.0) | 42.3 (2.1); 10.0–50.0 | 116.0 (27.5); 50.0–180.0 | 0.002 | |
| Vitamin B9, folic acid, mcg | ||||||
| Pregnancy | 89 (96.7) | 20 (100.0) | 0.999 | 603.6 (93.5); 162.0–6200.0 | 636.5 (229.9); 286.0–5000.0 | 0.990 |
| Lactation | 75 (81.5) | 13 (65.0) | 0.022 | |||
| Previously | 19 (20.7) | 6 (30.0) | 272.4 (24.3); 100–400 | 416.7 (91.0); 100.0–800.0 | 0.094 | |
| Currently | 56 (60.9) | 7 (35.0) | 280.1 (16.2); 2.0–400 | 400.0 (21.8); 300.0–500.0 | 0.011 | |
| Vitamin B12, cobalamin, mcg | ||||||
| Pregnancy | 88 (95.7) | 20 (100.0) | 0.999 | 2.3 (0.1); 0.95–4.7 | 213.5 (55.8); 1.2–934.0 | 0.002 |
| Lactation | 74 (80.4) | 18 (90.0) | 0.683 | |||
| Previously | 19 (20.7) | 1 (5.0) | 2.4 (0.1); 1.3–3.5 | 2.0 (-); 2.0–2.0 | 0.213 | |
| Currently | 55 (59.8) | 17 (85.0) | 2.1 (0.1); 1.0–2.5 | 312.1 (48.0); 2.0–857.0 | <0.001 | |
| Iodine, mcg | ||||||
| Pregnancy | 89 (96.7) | 19 (95.0) | 0.452 | 199.0 (3.9); 46.5–400.0 | 177.7 (10.1); 75.0–229.4 | 0.076 |
| Lactation | 78 (84.8) | 12 (60.0) | 0.001 | |||
| Previously | 18 (19.6) | 5 (25.0) | 191.7 (6.1); 100.0–200.0 | 185.8 (22.2); 100.0–229.0 | 0.693 | |
| Currently | 60 (65.2) | 7 (35.0) | 183.2 (5.9); 46.0–300.0 | 157.1 (22.3); 75.0–200.0 | 0.101 | |
| Calcium, mg | ||||||
| Pregnancy | 3 (3.3) | 4 (20.0) | 0.019 | 62.0 (44.1); 12.0–150.0 | 391.8 (149.7); 100.0–650.0 | 0.075 |
| Lactation | 37 (40.2) | 5 (25.0) | 0.144 | |||
| Previously | 12 (13.0) | 2 (10.0) | 180.8 (17.1); 24.0–245.0 | 225.0 (125.0); 100.0–350.0 | 0.749 | |
| Currently | 25 (27.2) | 3 (15.0) | 164.0 (17.9); 40.0–500.0 | 566.7 (83.3); 400.0–650.0 | 0.004 | |
| Iron, mg | ||||||
| Pregnancy | 67 (72.8) | 15 (75.0) | 0.899 | 45.6 (3.6); 6.5–108.0 | 46.9 (6.0); 9.0–80.0 | 0.540 |
| Lactation | 66 (71.7) | 10 (50.0) | 0.021 | |||
| Previously | 26 (28.3) | 5 (25.0) | 39.9 (6.3); 7.0–105.0 | 69.0 (7.0); 47.0–80.0 | 0.061 | |
| Currently | 40 (43.5) | 5 (25.0) | 29.2 (5.3); 3.0–114.0 | 40.4 (16.2); 14.0–80.0 | 0.302 | |
| Zinc, mg | ||||||
| Pregnancy | 44 (47.8) | 8 (40.0) | 0.498 | 9.7 (0.2); 4.3–15.0 | 11.9 (2.1); 7.5–25.0 | 0.603 |
| Lactation | 48 (52.2) | 7 (35.0) | 0.097 | |||
| Previously | 16 (17.4) | 3 (15.0) | 9.2 (0.5); 5.0–10.0 | 13.3 (6.0); 5.0–25.0 | 0.718 | |
| Currently | 32 (34.8) | 4 (20.0) | 8.3 (0.5); 2.0–10.0 | 7.8 (1.0); 5.0–10.0 | 0.302 | |
| Selenium, mcg | ||||||
| Pregnancy | 42 (45.7) | 7 (35.0) | 0.363 | 52.3 (1.3); 27.0–60.0 | 40.0 (8.0); 12.5–60.0 | 0.173 |
| Lactation | 46 (50.0) | 6 (30.0) | 0.054 | |||
| Previously | 15 (16.3) | 2 (10.0) | 26.8 (4.0); 10.0–55.0 | 32.5 (22.5); 10.0–55.0 | 0.937 | |
| Currently | 31 (33.7) | 4 (20.0) | 27.1 (3.4); 4.0–55.0 | 34.0 (12.1); 13.0–55.0 | 0.579 | |
| Omega 3, g | ||||||
| Pregnancy | 48 (52.2) | 6 (30.0%) | 0.065 | 0.23 (0.02); 0.16–0.95 | 0.21 (0.04); 0.10–0.38 | 0.550 |
| Lactation | 48 (52.2) | 7 (35.0%) | 0.097 | |||
| DHA, g | ||||||
| Lactation | ||||||
| Previously | 15 (16.3) | 2 (10.0%) | 0.18 (0.01); 0.08–0.20 | 0.10 (-); 0.10–0.10 | 0.102 | |
| Currently | 33 (35.9) | 5 (25.0%) | 0.18 (0.01); 0.10–0.38 | 0.18 (0.03); 0.10–0.25 | 0.546 | |
| EPA, g | ||||||
| Lactation | ||||||
| Previously | 15 (16.3) | 2 (10.0) | 0.03 (0.00); 0.00–0.04 | 0.02 (-); 0.02–0.02 | 0.332 | |
| Currently | 33 (35.9) | 5 (25.0) | 0.05 (0.02); 0.00–0.50 | 0.15 (0.12); 0.00–0.64 | 0.727 | |
| Donors (n = 92) | Veg (n = 20) | p Value | Recommendations a | ||
|---|---|---|---|---|---|
| EFSA (PRI/AI *) | IOM (RDA/AI *) | ||||
| Energy (Kcal) | 2318.66 (43.49) | 2146.73 (85.06) | 0.079 | b | |
| Protein (g) | 96.36 (1.95) | 67.46 (3.12) | <0.001 | c | 71 |
| Total fat (g) | 102.66 (2.62) | 85.81 (4.55) | 0.004 | ||
| Saturated fat (g) | 33.12 (1.01) | 19.88 (1.51) | <0.001 | ALAP | ALAP |
| Polyunsaturated fat (g) | 16.29 (0.58) | 21.00 (1.42) | 0.001 | ||
| Monounsaturated fat (g) | 43.94 (1.21) | 38.06 (2.48) | 0.040 | ||
| PUFAs/SFAs | 0.54 (0.02) | 1.28 (0.11) | <0.001 | ||
| (PUFAs + MUFAs)/SFAs | 1.94 (0.05) | 3.54 (0.23) | <0.001 | ||
| Kcal from carbohydrate (%) | 43.76 (0.60) | 51.83 (0.92) | <0.001 | 45–60 ** | 45–65 ** |
| Kcal from protein (%) | 16.84 (0.26) | 12.65 (0.45) | <0.001 | 10–35 ** | |
| Kcal from fat (%) | 39.24 (0.60) | 35.29 (0.95) | 0.003 | 20–35 ** | 20–35 ** |
| Kcal from saturated fat (%) | 12.66 (0.23) | 8.18 (0.54) | <0.001 | ||
| Kcal from polyunsaturated fat (%) | 6.30 (0.19) | 8.72 (0.35) | <0.001 | ||
| Kcal from monounsaturated fat (%) | 16.81 (0.33) | 15.72 (0.67) | 0.239 | ||
| Kcal from n-3 fatty acids (%) | 0.84 (0.03) | 0.89 (0.10) | 0.858 | 0.5 | 0.6–1.2 |
| n-6 fatty acids (g) | 13.64 (0.51) | 18.27 (1.28) | <0.001 | 13 * | |
| n-3 fatty acids (g) | 2.11 (0.09) | 2.37 (0.31) | 0.846 | 1.3 * | |
| n-6/n-3 fatty acids | 7.77 (0.27) | 9.90 (0.69) | 0.002 | ||
| Myristic acid C14:0 (g) | 2.86 (0.14) | 1.28 (0.26) | <0.001 | ||
| Palmitic acid C16:0 (g) | 16.52 (0.50) | 8.40 (0.67) | <0.001 | ||
| Palmitoleic acid C16:1 n7 (g) | 1.55 (0.06) | 0.42 (0.06) | <0.001 | ||
| Stearic acid C18:0 (g) | 7.07 (0.23) | 3.53 (0.33) | <0.001 | ||
| Oleic acid C18:1n9c (g) | 40.58 (1.16) | 36.40 (2.35) | 0.106 | ||
| Linoleic acid C18:2n6c (g) | 13.45 (0.51) | 18.25 (1.28) | <0.001 | ||
| Linolenic acid C18:3n3 (g) | 1.58 (0.07) | 2.24 (0.30) | 0.016 | ||
| Eicosapentaenoic acid C20:5n3 (g) | 0.15 (0.02) | 0.04 (0.02) | <0.001 | ||
| Docosapentaenoic acid C22:5n3 (g) | 0.08 (0.03) | 0.00 (0.00) | <0.001 | ||
| Docosahexaenoic acid C22:6n3 (g) | 0.38 (0.03) | 0.11 (0.03) | <0.001 | +0.10–0.20 *d | |
| EPA + DHA (g) | 0.53 (0.04) | 0.14 (0.04) | <0.001 | 0.25 * | |
| Trans fatty acids (g) | 0.44 (0.02) | 0.13 (0.03) | <0.001 | ALAP | ALAP |
| Cholesterol (g) | 334.42 (10.56) | 58.60 (17.24) | <0.001 | ALAP | |
| Cholesterol (mg/1000 Kcal) | 144.96 (4.03) | 28.90 (8.45) | <0.001 | ||
| Thiamine (B1) (mg) | 2.02 (0.07) | 4.75 (2.46) | 0.056 | 0.1 mg/MJ | 1.4 mg |
| Riboflavin (B2) (mg) | 2.55 (0.09) 2.40 (1.70, 3.20) 1 | 2.95 (0.98) 1.70 (1.20, 2.80) 1 | 0.024 | 2.0 | 1.6 |
| Niacin (B3) (mg) | 43.21 (1.09) | 31.24 (1.75) | <0.001 | 1.6 mg/MJ e | 17 mg e |
| Pantothenic acid (B5) (mg) | 7.93 (0.31) | 12.28 (4.94) | 0.551 | 7 * | 7 * |
| Pyridoxine (B6) (mg) | 2.91 (0.10) | 3.40 (0.60) | 0.997 | 1.7 | 2 |
| Biotin (B7) (μg) | 51.60 (2.94) | 63.86 (12.69) | 0.779 | 45 * | 35 * |
| Folate food + folic acid (B9) (μg) | 473.22 (20.87) | 668.15 (46.65) | <0.001 | 500 f | 500 g |
| Cobalamin (B12) (μg) | 6.92 (0.28) | 258.40 (53.44) | 0.096 | 5 * | 2.8 |
| Vitamin C (mg) | 178.41 (8.87) | 211.86 (18.33) | 0.088 | 155 | <19y: 115 |
| ≥19y: 120 | |||||
| Vitamin A (μg) | 1430.37 (111.15) | 1357.94 (108.26) | 0.704 | 1300 h | <19y: 1200 i |
| ≥19y: 1300 i | |||||
| Vitamin D (μg) | 5.61 (0.47) | 10.78 (4.28) | 0.204 | 15 *j | 15 jk |
| Vitamin E (μg) | 17.16 (0.84) | 20.65 (1.49) | 0.029 | 11 *l | 19 |
| Iodine (μg) | 245.33 (11.76) | 259.58 (47.15) | 0.536 | 200 * | 290 |
| Calcium (mg) | 1148.32 (35.12) | 910.92 (70.48) | 0.002 | 18–24y:1000 | <19y: 1300 |
| ≥25y: 950 | ≥19y: 1000 | ||||
| Phosphorus (mg) | 1677.19 (38.38) | 1436.13 (79.71) | 0.007 | 550 * | <19y: 1250 |
| ≥19y: 700 | |||||
| Iron (mg) | 25.34 (2.17) | 31.33 (5.23) | 0.028 | 16 | <19y: 10 |
| ≥19y: 9 | |||||
| Zinc (mg) | 14.40 (0.52) | 12.07 (0.83) | 0.077 | 10.4–15.6 m | <19y: 13 |
| ≥19y: 12 | |||||
| Selenium (μg) | 118.98 (3.38) | 100.80 (6.66) | 0.010 | 85 * | 70 |
| Nutrient | H-AR * [50] | Donors (n = 92), n (%) | Veg (n = 20), n (%) | p Value |
|---|---|---|---|---|
| Thiamine (B1), mg | 1.2 | 5 (5.4%) | 0 (0.0%) | 0.286 |
| Riboflavin (B2), mg | 1.7 | 13 (14.1%) | 9 (45%) | 0.002 |
| Niacin (B3), mg | 13 | 0 (0.0%) | 0 (0.0%) | - |
| Pantothenic acid (B5), mg | 5.6 | 22 (23.9%) | 6 (30%) | 0.568 |
| Pyridoxine (B6), mg | 1.4 | 1 (1.1%) | 0 (0.0%) | 0.640 |
| Biotin (B7), μg | 36 | 34 (37.0%) | 7 (35.0%) | 0.869 |
| Folate food + folic acid (B9), μg | 380 (DFE) | 36 (39.1%) | 0 (0.0%) | <0.001 |
| Cobalamin (B12), μg | 2.4 | 0 (0.0%) | 5 (25.0%) | <0.001 |
| Vitamin C, mg | 145 | 34 (37.0%) | 4 (20.0%) | 0.147 |
| Vitamin A, μg RAE | 1020 | 32 (34.8%) | 6 (30.0%) | 0.682 |
| Vitamin D, μg | 10 | 81 (88.04) | 15 (75.00) | 0.131 |
| Vitamin E, mg | 16 | 45 (48.9%) | 5 (25.0%) | 0.051 |
| Iodine, μg | 209 | 40 (43.5%) | 8 (40.0%) | 0.776 |
| Calcium, mg | 860 (19–30 y) 750 (31–50 y) | 6 (6.5%) | 9 (45.0%) | <0.001 |
| Phosphorous, mg | 580 | 0 (0.0%) | 0 (0.0%) | - |
| Selenium, μg | 59 | 1 (1.1%) | 0 (0.0%) | 0.640 |
| Donors (n = 92) | Veg (n = 20) | p Value | Recommendations [24] a | |
|---|---|---|---|---|
| Servings per day: | ||||
| Dairy | 2.58 (0.13) | 0.58 (0.28) | <0.001 | ≥4 b |
| Grains, legumes, and nuts | 6.12 (0.20) | 8.11 (0.60) | 0.003 | ≥7 |
| Vegetables and greens | 3.40 (0.14) | 5.11 (0.33) | <0.001 | ≥4 |
| Fruits | 1.85 (0.13) | 1.87 (0.22) | 0.654 | ≥3 |
| Eggs, meat, and fish | 2.94 (0.11) | 0.28 (0.05) | <0.001 | 2–3 c |
| HEI | 63.26 (0.92) | 61.69 (1.50) | 0.478 | |
| Supplement intake: yes, n (%) | 51 (55.4%) | 17 (85%) | 0.028 | |
| Iodized salt intake: yes, n (%) | 62 (67.4%) | 14 (70.0%) | 0.359 | |
| Salt (g/day) | 1.10 (0.06) | 1.89 (0.26) | <0.001 |
| Donors (n = 92) | Veg (n = 20) | p Value | Serving Size [17] a | |
|---|---|---|---|---|
| Milk (servings/day) | 1.48 (0.11) | 0.26 (0.17) | <0.001 | 200–250 mL |
| Other dairy products (servings/day) | 1.06 (0.10) | 0.21 (0.15) | <0.001 | Yogurt 200–250 g Fresh cheese 80–125 g Cured cheese 40–60 g |
| Meats and derivatives (servings/day) | 0.73 (0.06) | 0.00 (0.00) | <0.001 | 100–125 g |
| Fish (servings/week) | 2.19 (0.14) | 0.01 (0.01) | <0.001 | 125–150 g |
| Eggs (servings/week) | 2.92 (0.18) | 1.07 (0.40) | <0.001 | 60 g |
| Fruits (servings/day) | 2.22 (0.16) | 2.87 (0.44) | 0.146 | 120–200 g |
| Raw vegetables (servings/day) | 0.64 (0.06) | 1.04 (0.15) | 0.002 | 150–200 g |
| Cooked vegetables (servings/day) | 0.80 (0.05) | 1.43 (0.15) | <0.001 | 150–200 g |
| Legumes (servings/week) | 1.39 (0.09) | 5.03 (0.55) | <0.001 | 60–80 g |
| Bread (servings/day) | 1.79 (0.18) | 1.73 (0.34) | 0.959 | 40–60 g |
| Pasta, rice, other grains (servings/week) | 3.31 (0.35) | 7.28 (1.20) | <0.001 | 60–80 g |
| Nuts (servings/week) | 4.14 (0.68) | 8.32 (1.15) | 0.003 | 25 g |
| Oils and fats (servings/day) | 2.84 (0.30) | 2.27 (0.33) | 0.765 | 10 g |
| Sweets (grams/week) | 285.83 (44.06) | 124.36 (22.34) | 0.053 |
| Fatty Acid (%) | Common Name | Donors (n = 92) | Veg (n = 20) | p Value |
|---|---|---|---|---|
| ERYTHROCYTES | ||||
| Saturated Fatty Acids (SFAs) | ||||
| C14:0 | Myristic | 0.12 (0.00) | 0.10 (0.01) | 0.041 |
| DMA C16:0 | Dimethylacetal C16:0 | 2.20 (0.02) | 1.64 (0.05) | <0.001 |
| C16:0 | Palmitic | 21.20 (0.21) | 19.97 (0.40) | 0.022 |
| DMA C18:0 | Dimethylacetal C18:0 | 3.46 (0.03) | 2.92 (0.13) | <0.001 |
| C18:0 | Stearic | 20.25 (0.15) | 19.40 (0.34) | 0.066 |
| C24:0 | Lignoceric | 2.40 (0.09) | 3.65 (0.22) | <0.001 |
| Monounsaturated Fatty Acids (MUFAs) | ||||
| C17:1 | Margaroleic | 0.35 (0.01) | 0.52 (0.04) | <0.001 |
| C18:1 cis-11 (n7) | Cis vaccenic | 0.24 (0.01) | 0.32 (0.03) | 0.001 |
| C18:1 cis-9 (n9) | Oleic | 12.62 (0.15) | 13.40 (0.38) | 0.007 |
| n-6 Polyunsaturated Fatty Acids (n-6 PUFAs) | ||||
| C18:2 (n6) | Linoleic | 8.12 (0.14) | 9.24 (0.31) | 0.002 |
| C20:3 (n6) | Dihomo-γ-linolenic | 0.99 (0.05) | 1.57 (0.14) | <0.001 |
| C20:4 (n6) | Arachidonic | 24.30 (0.32) | 24.91 (0.75) | 0.130 |
| n-3 Polyunsaturated Fatty Acids (n-3 PUFAs) | ||||
| C20:5 (n3) | Eicosapentaenoic | 0.12 (0.02) | 0.00 (0.00) | 0.009 |
| C22:5 (n3) | Docosapentaenoic | 0.73 (0.03) | 0.66 (0.04) | 0.151 |
| C22:6 (n3) | Docosahexaenoic | 2.90 (0.12) | 1.72 (0.32) | <0.001 |
| Fatty Acid Families | ||||
| DMAs | 5.66 (0.05) | 4.55 (0.16) | <0.001 | |
| SFAs | 43.98 (0.28) | 43.12 (0.49) | 0.347 | |
| MUFAs | 13.20 (0.15) | 14.23 (0.44) | 0.003 | |
| PUFAs | 37.09 (0.39) | 38.10 (0.65) | 0.308 | |
| MCFAs (C8-C15) | 0.12 (0.00) | 0.10 (0.01) | 0.041 | |
| LCFAs (C16-C18) | 62.78 (0.46) | 62.85 (0.73) | 0.924 | |
| VLCFAs (C20-C24) | 29.04 (0.39) | 28.85 (0.76) | 0.992 | |
| n-6 PUFAs | 33.41 (0.30) | 35.72 (0.59) | 0.007 | |
| n-3 PUFAs | 3.75 (0.16) | 2.38 (0.35) | <0.001 | |
| n-6 PUFAs/n-3 PUFAs | 10.83 (0.59) | 21.09 (2.49) | <0.001 | |
| PLASMA | ||||
| Saturated Fatty Acids (SFAs) | ||||
| C14:0 | Myristic | 0.28 (0.01) | 0.24 (0.03) | 0.062 |
| C15:0 | Pentadecylic | 0.05 (0.00) | 0.03 (0.00) | 0.003 |
| DMA C16:0 | Dimethylacetal C16:0 | 0.20 (0.01) | 0.12 (0.01) | <0.001 |
| C16:0 | Palmitic | 21.17 (0.17) | 19.10 (0.49) | <0.001 |
| DMA C18:0 | Dimethylacetal C18:0 | 0.08 (0.00) | 0.05 (0.01) | <0.001 |
| C18:0 | Stearic | 6.26 (0.07) | 5.86 (0.15) | 0.033 |
| Monounsaturated Fatty Acids (MUFAs) | ||||
| C16:1 cis-9 (n7) | Palmitoleic | 0.43 (0.02) | 0.42 (0.08) | 0.010 |
| C18:1 cis-11 (n7) | Cis vaccenic | 0.44 (0.01) | 0.55 (0.04) | 0.002 |
| C18:1 cis-9 (n9) | Oleic | 18.40 (0.26) | 19.92 (0.99) | 0.411 |
| n-6 Polyunsaturated Fatty Acids (n-6 PUFAs) | ||||
| C18:2 (n6) | Linoleic | 39.20 (0.46) | 41.28 (1.42) | 0.390 |
| C20:3 (n6) | Dihomo-γ-linolenic | 1.11 (0.06) | 1.43 (0.18) | 0.057 |
| C20:4 (n6) | Arachidonic (ARA) | 11.30 (0.33) | 10.56 (0.71) | 0.533 |
| n-3 Polyunsaturated Fatty Acids (n-3 PUFAs) | ||||
| C20:5 (n3) | Eicosapentaenoic (EPA) | 0.24 (0.03) | 0.08 (0.02) | 0.012 |
| C22:6 (n3) | Docosahexaenoic (DHA) | 0.83 (0.06) | 0.37 (0.07) | <0.001 |
| Fatty Acid Families | ||||
| DMAs | 0.29 (0.01) | 0.17 (0.01) | <0.001 | |
| SFAs | 27.76 (0.19) | 25.23 (0.48) | <0.001 | |
| MUFAs | 19.27 (0.26) | 20.89 (1.06) | 0.454 | |
| PUFAs | 52.68 (0.34) | 53.71 (1.11) | 0.309 | |
| MCFA (C8-C15) | 0.33 (0.02) | 0.27 (0.03) | 0.031 | |
| LCFA (C16-C18) | 85.91(0.40) | 87.12 (0.75) | 0.361 | |
| VLCFA (C20-C24) | 13.48 (0.39) | 12.44 (0.74) | 0.456 | |
| n-6 PUFAs | 51.61 (0.34) | 53.26 (1.10) | 0.173 | |
| n-3 PUFAs | 1.07 (0.08) | 0.45 (0.09) | <0.001 | |
| n-6 PUFAs/n-3 PUFAs | 82.35 (6.70) | 173.23 (26.35) | <0.001 | |
| Variable 1 | Donors (n = 92) | Veg (n = 20) | p Value | Comments about the Reference Values or Studies in which the Corresponding Vitamers are Determined 3 | ||
|---|---|---|---|---|---|---|
| n | Value 2 | n | Value 2 | |||
| ERYTHROCYTES | ||||||
| Hemoglobin (Drabkin colorimetric method) | ||||||
| g/dL | 92 | 25.57 (0.38) | 20 | 23.33 (0.84) | 0.011 | |
| EGRAC (assay kit) | 74 | 7 | ||||
| 1.25 (0.03) | 1.53 (0.12) | 0.029 | ||||
| Riboflavin insufficiency/deficiency (EGRAC ≥ 1.4) [26,51,52] | 22 (29.7%) | 4 (57.1%) | 0.224 | |||
| Marginal riboflavin status (EGRAC 1.2 to <1.4) [51,52] | 20 (27.0%) | 2 (28.6%) | ||||
| Acceptable riboflavin status (EGRAC < 1.2) [51,52] | 32 (43.2%) | 1 (14.3%) | ||||
| Riboflavin, B2 (UPLC-MS/MS) | 92 | 20 |
| |||
| ng/L | 767.92 (37.71) | 932.02 (119.42) | 0.277 | |||
| nM | 2.04 (0.10) | 2.48 (0.32) | ||||
| ng/g Hb | 3.09 (0.16) | 4.12 (0.55) | 0.115 | |||
| Riboflavin deficiency (<170 nM) [51] | 92 (100%) | 20 (100%) | - | |||
| Nicotinamide, B3 (UPLC-MS/MS) |
| |||||
| mcg/L | 4718.23 (298.58) | 5039.97 (636.03) | 0.659 | |||
| mcM | 38.64 (2.44) | 41.27 (5.21) | ||||
| mcg/g Hb | 18.51 (1.28) | 22.41 (3.06) | 0.327 | |||
| Pantothenic acid, B5 (UPLC-MS/MS) | 92 | 20 |
| |||
| mcg/L | 58.00 (9.32) | 111.85 (35.59) | 0.849 | |||
| nM | 264.56 (42.51) | 510.19 (162.34) | ||||
| mg/g Hb | 233.36 (42.00) | 489.65 (154.86) | 0.964 | |||
| Pyridoxamine, B6 (UPLC-MS/MS) | 92 | 20 |
| |||
| mcg/L | 519.43 (23.59) | 612.83 (33.22) | 0.044 | |||
| mcM | 3.07 (0.14) | 3.62 (0.20) | ||||
| mcg/g Hb | 2.06 (0.11) | 2.73 (0.20) | 0.006 | |||
| PLASMA | ||||||
| Thiamin, B1 (UPLC-MS/MS) | 92 | 20 |
| |||
| mcg/L | 0.42 (0.03) | 0.49 (0.10) | ||||
| nM | 1.24 (0.09) | 1.45 (0.30) | ||||
| Riboflavin, B2 (UPLC-MS/MS) | 92 | 20 |
| |||
| mcg/L | 23.01 (1.86) | 14.79 (1.89) | 0.006 | |||
| nM | 61.14 (4.94) | 39.30 (5.02) | ||||
| Riboflavin < 6.7 nM [64] | 0 (0.0%) | 0 (0.0%) | ||||
| Nicotinamide, B3 (UPLC-MS/MS) | 92 | 20 |
| |||
| mcg/L | 4.77 (0.36) | 3.46 (0.27) | 0.052 | |||
| nM | 39.06 (2.95) | 28.33 (2.21) | ||||
| Pantothenic acid, B5 (UPLC-MS/MS) | 92 | 20 | ||||
| mcg/L | 175.24 (52.12) | 170.05 (35.48) | 0.410 | |||
| nM | 799.30 (237.90) | 775.60 (161.80) | ||||
| Pyridoxine, B6 (UPLC-MS/MS) | 92 | 20 |
| |||
| mcg/L | 139.29 (3.27) | 144.12 (7.21) | 0.548 | |||
| nM | 823.32 (19.3) | 851.87 (42.62) | ||||
| Pyridoxamine, B6 (UPLC-MS/MS) | 92 | 20 |
| |||
| mcg/L | 264.26 (4.88) | 285.48 (7.30) | 0.006 | |||
| nM | 1571.14 (28.88) | 1691.36 (43.40) | ||||
| Folate, B9 (UPLC-MS/MS) | 91 | 20 |
| |||
| mcg/L | 3.11 (0.45) | 5.20 (2.42) | 0.758 | |||
| nM | 7.05 (1.02) | 11.55 (5.48) | ||||
| Folate status undetermined (6.8–13.4 nM) [71] | 26 (28.6%) | 5 (25.0%) | 0.739 | |||
| Folate deficiency (<6.8 nM) [71] | 60 (65.9%) | 13 (65.0%) | ||||
| Cobalamin, B12 (Competitive immunoassay). | 92 | 20 |
| |||
| pM | 557.30 (22.46) | 519.80 (61.17) | 0.276 | |||
| B12 depletion (148–221 pM) [72] | 1 (1.1%) | 2 (10.0%) | 0.025 | |||
| B12 deficiency (<148 pM) [72] | 0 (0.0%) | 0 (0.0%) | ||||
| Severe B12 deficiency (<75 pM) [72] | 0 (0.0%) | 0 (0.0%) | ||||
| Holotranscobalamin II (Immunoassay ELISA kit) | 92 | 20 |
| |||
| pM | 191.52 (7.61) | 185.04 (21.41) | 0.384 | |||
| B12 depletion (Holo-TC II < 35 pM) [73] | 0 (0.0%) | 0 (0.0%) | - | |||
| Homocysteine (enzymatic assay) | 92 | 20 | ||||
| mcM | 10.37 (0.37) | 8.30 (0.78) | 0.006 | |||
| Homocysteine elevated (>13 mcM) [71,74] | 21 (22.8%) | 2 (10.0%) | 0.198 | |||
| Ascorbic acid (HPLC-DAD) | 92 | 20 |
| |||
| mcM | 51.88 (3.23) | 62.14 (3.80) | 0.012 | |||
| <11 mcM: scurvy [27] | 1 (1.1%) | 0 (0.0%) | 0.640 | |||
| Retinol (HPLC with fluorescence and UV detector) | 91 | 20 | ||||
| mcg/dL | 51.35 (1.61) | 44.53 (3.74) | 0.056 | |||
| mcM | 1.79 (0.06) | 1.55 (0.13) | ||||
| Vit A deficiency (Retinol <20 mcg/dL, <0.7 mcM) [76,77] | 0 (0.0%) | 0 (0.0%) | - | |||
| 25(OH), D (UPLC-electrospray ionization/tandem MS) | 92 | 20 | ||||
| ng/mL | 7.37 (0.42) | 9.22 (1.29) | 0.264 | |||
| nM | 18.40 (1.05) | 23.01 (3.22) | ||||
| Risk for vit D inadequacy (25(OH)D 12- < 20 ng/mL; 30- < 50 nM) [30,78,79] | 11 (12.0%) | 4 (20.0%) | 0.044 | |||
| Risk for vitamin deficiency (25(OH)D < 12 ng/mL; <30 nM) [30,78,79] | 80 (87.0%) | 14 (70%) | ||||
| 1,25(OH)2D (UPLC-electrospray ionization/tandem MS) | 92 | 20 | ||||
| pg/mL | 146.50 (15.29) | 271.41 (35.70) | <0.001 | |||
| pM | 351.65 (36.70) | 651.49 (85.69) | ||||
| α-tocopherol (HPLC with fluorescence and UV detector) | 90 | 20 |
| |||
| mcg/dL | 287.63 (24.42) | 311.37 (56.64) | 0.941 | |||
| mcM | 6.68 (0.57) | 7.23 (1.31) | ||||
| Vit E deficiency (<500 mcg/dL; <0.5 mg/dL; <11.6 mcM) [27] | 80 (89.9%) | 16 (80.0%) | 0.218 | |||
| Severe vit E deficiency (<5.8 mcM) [81] | 56 (62.9%) | 9 (45.0%) | 0.140 | |||
| α-tocopherol:total lipids (cholesterol+triacylglycerols) | 89 | 20 |
| |||
| (mcmol:mmol) | 1.32 (0.12) | 1.45 (0.29) | 0.196 | |||
| Ratio < 1.6 mcmol:mmol [81] | 70 (78.7%) | 13 (65.0%) | ||||
| α-tocopherol:cholesterol (mcmol:mmol) | 89 | 1.47 (0.13) | 20 | 1.63 (0.34) | 0.457 |
|
| Ratio < 2.2 mcmol:mmol [81] | 77 (86.5%) | 16 (80.0%) | ||||
| γ-tocopherol (HPLC with fluorescence and UV detector) | 88 | 20 | ||||
| mcg/dL | 37.62 (2.16) | 52.66 (4.98) | 0.003 | |||
| Total cholesterol (enzymatic assay) | 92 | 20 | ||||
| mg/dL | 183.01 (3.51) | 182.01 (6.03) | 0.840 | |||
| mM | 4.74 (0.09) | 4.71 (0.16) | ||||
| Hypercholesterolemia (>240 mg/dL) [79] | 6 (6.5%) | 1 (5.0%) | 0.799 | |||
| Triacylglycerols (enzymatic assay) | 92 | 20 | ||||
| mg/dL | 48.93 (1.90) | 43.26 (2.43) | 0.336 | |||
| mM | 0.55 (0.02) | 0.49 (0.03) | ||||
| Hypertriglyceridemia (>200 mg/dL) [79] | 0 (0.0%) | 0 (0.0%) | - | |||
| HDL (enzymatic assay) | 92 | 20 | ||||
| mg/dL | 62.32 (1.08) | 69.49 (2.37) | 0.008 | |||
| mM | 1.61 (0.03) | 1.80 (0.06) | ||||
| Low HDL levels (<40 mg/dL) [79] | 1 (1.1%) | 0 (0.0%) | 0.640 | |||
| LDL (enzymatic assay) | 92 | 20 | ||||
| mg/dL | 103.92 (2.70) | 105.26 (6.29) | 0.790 | |||
| mM | 2.69 (0.07) | 2.73 (0.16) | ||||
| High LDL levels (>160 mg/dL) [79] | 3 (3.3%) | 0 (0.0%) | 0.413 | |||
| URINE | ||||||
| Cr (Jaffé colorimetric kinetic method) | 92 | 121.17 (5.67) | 20 | 121.22 (13.23) | 0.776 | |
| mg/dL | ||||||
| Methylmalonic acid (UPLC-MS/MS) | 91 | 20 |
| |||
| mg/L | 6.17 (0.32) | 5.97 (0.79) | 0.618 | |||
| mcg/mg Cr | 5.28 (0.27) | 5.38 (0.81) | 0.613 | |||
| mcmol/mmol Cr | 5.06 (0.26) | 5.15 (0.78) | 0.613 |
| ||
| B12 deficiency marker (Methylmalonic acid/Cr >4 mcg/mg; >3.8 mmol/mol) [73] | 55 (61.1%) | 12 (60.0%) | 0.927 | |||
| Iodine (mcg/L) (ICP-MS) | 92 | 20 | ||||
| Mean (SE) mcg/L | 127.71 (7.57) | 112.00 (21.47) | 0.061 |
| ||
| Median (p25, p75) mcg/L | 109.64 (75.80, 159.54) * | 66.28 (50.26, 139.36) * | ||||
| mcg/mg Cr | 0.11 (0.01) | 0.10 (0.02) | 0.082 | |||
| mcg/g Cr | 110 (10) | 100 (20) |
| |||
| Sodium (ICP-MS) | 92 | 20 | ||||
| mg/L | 3365.51 (139.32) | 3693.99 (454.86) | 0.704 | |||
| M | 0.15 (0.01) | 0.16 (0.02) | ||||
| mg/mg Cr | 3.07 (0.15) | 3.38 (0.38) | 0.528 | |||
| mmol/g Cr | 133.48 (6.52) | 146.93 (16.52) |
| |||
| Calcium (ICP-MS) | 92 | 20 | ||||
| mg/L | 105.00 (9.28) | 72.02 (14.68) | 0.082 | |||
| mg/mg Cr | 0.09 (0.01) | 0.06 (0.01) | 0.024 |
| ||
| Phosphorus (ICP-MS) | 92 | 20 | ||||
| mg/L | 1053.17 (52.08) | 840.20 (89.73) | ||||
| mg/mg Cr | 0.88 (0.03) | 0.74 (0.06) |
|
| Nutrient | Donors | Veg | p Value | ||
|---|---|---|---|---|---|
| n | Mean (SE) | n | Mean (SE) | ||
| Macronutrients (g/100 mL milk) | |||||
| Lipids | 84 | 3.13 (0.17) | 18 | 3.14 (0.34) | 0.958 |
| Carbohydrates | 7.73 (0.03) | 7.76 (0.06) | 0.779 | ||
| Proteins | 1.17 (0.03) | 1.16 (0.05) | 0.533 | ||
| Lipid classes (g/100 g fat) | |||||
| Triacylglycerols | 19 | 95.56 (0.55) | 18 | 95.33 (0.66) | 0.832 |
| Diacylglycerols | 3.97 (0.49) | 4.18 (0.61) | 0.808 | ||
| Monoacylglycerols | 0.04 (0.01) | 0.03 (0.01) | 0.586 | ||
| Free fatty acids + cholesterol | 0.38 (0.05) | 0.41 (0.05) | 0.543 | ||
| Polar lipids | 0.05 (0.00) | 0.06 (0.00) | 0.047 | ||
| Triacylglycerols (g/100 g fat) | |||||
| CN24 | 19 | 0.01 (0.00) | 18 | 0.01 (0.00) | 0.428 |
| CN26 | 0.10 (0.01) | 0.11 (0.01) | 0.713 | ||
| CN28 | 0.09 (0.01) | 0.06 (0.01) | 0.038 | ||
| CN30 | 0.21 (0.03) | 0.13 (0.02) | 0.024 | ||
| CN32 | 0.31 (0.05) | 0.26 (0.04) | 0.543 | ||
| CN34 | 0.36 (0.07) | 0.22 (0.03) | 0.301 | ||
| CN36 | 0.40 (0.05) | 0.38 (0.05) | 0.915 | ||
| CN38 | 1.61 (0.16) | 1.16 (0.10) | 0.022 | ||
| CN40 | 1.98 (0.12) | 2.09 (0.13) | 0.533 | ||
| CN42 | 2.65 (0.21) | 2.72 (0.26) | 0.671 | ||
| CN44 | 4.95 (0.32) | 4.47 (0.37) | 0.627 | ||
| CN46 | 7.45 (0.35) | 6.60 (0.45) | 0.346 | ||
| CN48 | 10.68 (0.34) | 10.62 (0.62) | 0.574 | ||
| CN50 | 14.83 (0.49) | 12.18 (0.52) | 0.001 | ||
| CN52 | 37.14 (1.11) | 32.37 (1.34) | 0.011 | ||
| CN54 | 17.23 (1.20) | 26.64 (1.47) | <0.001 | ||
| Phospholipids (g/100 g polar lipids) | |||||
| Phosphatidylethanolamine | 19 | 24.62 (1.86) | 18 | 30.72 (1.88) | 0.048 |
| Phosphatidylcholine | 30.55 (1.10) | 26.88 (0.67) | 0.018 | ||
| Sphingomyelin | 44.83 (2.58) | 42.40 (1.82) | 0.429 | ||
| Fatty Acid (%) | Common Name | Donors (n = 88) | Veg (n = 20) | p Value | Reference Values | |
|---|---|---|---|---|---|---|
| European [88] 1 | World [89] 2 | |||||
| Saturated Fatty Acids (SFAs) | ||||||
| C6:0 | Caproic | 0.11 (0.00) | 0.10 (0.00) | 0.631 | 0.08 ± 0.02 | 0.13 ± 0.47 |
| C8:0 | Caprylic | 0.18 (0.00) | 0.17 (0.01) | 0.095 | 0.22 ± 0.06 | 0.21 ± 0.22 |
| C10:0 | Capric | 1.18 (0.03) | 1.05 (0.06) | 0.018 | 1.44 ± 0.34 | 1.37 ± 0.86 |
| C12:0 | Lauric | 5.38 (0.17) | 5.14 (0.36) | 0.719 | 5.46 ± 1.84 | 5.7 ± 2.81 |
| C14:0 | Myristic | 6.50 (0.30) | 5.45 (0.44) | 0.255 | 6.19 ± 1.93 | 6.56 ± 3.05 |
| C15:0 | 0.19 (0.01) | 0.08 (0.01) | <0.001 | |||
| C15:0 ai | C15:0 anteiso | 0.03 (0.00) | 0.02 (0.00) | <0.001 | ||
| C15:0 i | C15:0 iso | 0.04 (0.00) | 0.01 (0.00) | <0.001 | ||
| C16:0 i | C16:0 iso | 0.02 (0.00) | 0.01 (0.01) | <0.001 | ||
| C16:0 | Palmitic | 19.64 (0.27) | 15.24 (0.52) | <0.001 | 21.94 ± 2.92 | 21.5 ± 4.82 |
| C17:0 ai | C17:0 anteiso | 0.05 (0.00) | 0.01 (0.00) | <0.001 | ||
| C17:0 i | C17:0 iso | 0.27 (0.01) | 0.30 (0.02) | 0.102 | ||
| C17:0 | Margaric | 0.20 (0.01) | 0.09 (0.01 | <0.001 | 0.31 ± 0.15 | |
| C18:0 | Stearic | 5.83 (0.14) | 4.06 (0.22) | <0.001 | 6.68 ± 1.59 | 6.36 ± 2.07 |
| C20:0 | Arachidic | 0.16 (0.01) | 0.17 (0.03) | 0.733 | 0.17 ± 0.04 | 0.23 ± 0.17 |
| Monounsaturated Fatty Acids (MUFAs) | ||||||
| C14:1 cis-9 (n5) | Myristoleic | 0.08 (0.00) | 0.03 (0.01) | <0.001 | ||
| C16:1 cis-9 (n7) | Palmitoleic | 1.55 (0.05) | 1.32 (0.11) | <0.001 | 2.21 ± 0.64 | 2.3 ± 0.92 |
| C17:1 | Margaroleic | 0.07 (0.00) | 0.04 (0.00) | <0.001 | ||
| ∑ C18:1 trans | 0.27 (0.02) | 0.07 (0.03) | <0.001 | 0.66 ± 0.35 | ||
| C18:1 cis-9 (n9) | Oleic | 38.31 (0.53) | 41.38 (1.22) | 0.025 | 35.59 ± 4.17 | 32.6 ± 5.84 |
| C18:1 cis-11 (n7) | Cis vaccenic | 1.59 (0.03) | 1.66 (0.05) | 0.282 | 2.38 ± 0.53 | |
| C20:1 (n9) | Gondoic | 0.69 (0.06) | 1.42 (0.21) | <0.001 | 0.38 ± 0.12 | 0.46 ± 0.28 |
| n-6 Polyunsaturated Fatty Acids (n-6 PUFAs) | ||||||
| C18:2 (n6) | Linoleic (LA) | 15.29 (0.39) | 20.02 (1.15) | <0.001 | 14.00 ± 4.95 | 15.7 ± 7.15 |
| C20:2 (n6) | Eicosadienoic | 0.27 (0.01) | 0.32 (0.02) | 0.063 | 0.26 ± 0.07 | 0.37 ± 0.19 |
| C20:3 (n6) | Dihomo-γ-linolenic | 0.33 (0.01) | 0.36 (0.04) | 0.652 | 0.31 ± 0.09 | 0.37 ± 0.18 |
| C20:4 (n6) | Arachidonic (AA) | 0.55 (0.02) | 0.46 (0.03) | 0.012 | 0.44 ± 0.12 | 0.50 ± 0.25 |
| n-3 Polyunsaturated Fatty Acids (n-3 PUFAs) | ||||||
| C18:3 (n3) | Linolenic (ALA) | 0.52 (0.02) | 0.61 (0.05) | 0.044 | 0.94 ± 0.55 | 1.11 ± 1.05 |
| C22:5 (n3) | Docosapentaenoic (DPA) | 0.08 (0.01) | 0.04 (0.01) | <0.001 | ||
| C22:6 (n3) | Docosahexaenoic (DHA) | 0.33 (0.02) | 0.15 (0.04) | <0.001 | 0.34 ± 0.35 | 0.37 ± 0.31 |
| n-7 Polyunsaturated Fatty Acids (n-7 PUFAs) | ||||||
| C18:2 c9, t11 (n7) | Rumenic | 0.09 (0.01) | 0.04 (0.02) | |||
| Fatty Acid Families | ||||||
| Not identified | 0.21 (0.02) | 0.18 (0.02) | 0.297 | |||
| SFAs | 39.78 (0.54) | 31.90 (0.91) | <0.001 | 42.23 ± 5.29 | 42.2 ± 7.73 | |
| MUFAs | 42.55 (0.56) | 45.91 (1.17) | 0.017 | 41.34 ± 4.48 | 36.3 ± 6.46 | |
| PUFAs | 17.46 (0.39) | 22.00 (1.15) | <0.001 | 16.43 ± 5.07 | 21.2 ± 8.18 | |
| SCFAs | 0.11 (0.00) | 0.10 (0.00) | 0.654 | |||
| MCFAs (C8-C15) | 13.59 (0.47) | 11.94 (0.82) | 0.317 | |||
| LCFAs (C16-C18) | 83.60 (0.48) | 84.81 (0.93) | 0.509 | |||
| VLCFAs (C20-C24) | 2.50 (0.09) | 2.96 (0.24) | 0.071 | |||
| n-6 PUFAs | 16.44 (0.39) | 21.16 (1.16) | <0.001 | 17.8 ± 7.51 | ||
| n-3 PUFAs | 0.93 (0.04) | 0.80 (0.06) | 0.104 | 1.88 ± 2.63 | ||
| n-6 PUFAs/n-3 PUFAs | 20.90 (1.20) | 29.32 (2.49) | 0.001 | |||
| LA/ALA ratio | 33.61 (1.64) | 36.46 (3.25) | 0.272 | |||
| ARA/DHA ratio | 2.47 (0.19) | 6.68 (1.05) | <0.001 | 1.68 ± 0.89 | ||
| Nutrient 1 | Mature Milk Nutrient Concentration Reference | Donors | (n = 92) | Veg | (n = 20) | p Value |
|---|---|---|---|---|---|---|
| n (o) | Mean (SE)/Median (RIQ) | n (o) | Mean (SE)/Median (RIQ) | |||
| Free thiamin, B1 (UPLC-MS/MS) mcg/L | Free thiamin 18.5 [90] Total thiamin 180 [91] | 92 (362) | 22.01 (0.92)/17.45 (0.50, 30.40) | 20 (80) | 24.75 (2.35)/22.50 (7.70, 33.95) | 0.560 |
| Free riboflavin, B2 (UPLC-MS/MS) mcg/L | Free riboflavin 11.2 [90] Total riboflavin 364 [92] | 92 (362) | 78.72 (4.93)/43.05 (22.10, 104.50) | 20 (80) | 89.49 (22.16)/15.70 (8.80, 60.20) | <0.001 |
| Nicotinamide, B3 (UPLC-MS/MS) mcg/L | Nicotinamide 275 [90] Total niacin 2100 [93] | 92 (362) | 59.43 (2.62)/44.55 (23.60, 79.30) | 20 (80) | 33.11 (2.51)/27.10 (13.80, 49.10) | <0.001 |
| Pantothenic acid, B5 (UPLC-MS/MS) mcg/L | 2500 [94] | 92 (362) | 2176.31 (25.85)/2230.70 (1763.80, 2538.30) | 20 (80) | 2305.05 (62.94)/2370.00 (1929.55, 2718.70) | 0.034 |
| Pyridoxal, B6 (UPLC-MS/MS) mcg/L | Pyridoxal 96 [90] B6 130 [95] | 92 (362) | 40.91 (1.12)/37.05 (26.40, 51.70) | 20 (80) | 48.46 (3.87)/43.35 (23.70, 59.90) | 0.233 |
| Folate, B9 (UPLC-MS/MS) mcg/L | 80 [95] | 92 (362) | 19.82 (0.40)/18.95 (14.60, 25.10) | 20 (80) | 18.79 (1.57)/15.75 (11.25, 21.30) | 0.002 |
| Cobalamin, B12 (Competitive immunoassay) | 92 (358) | 20 (79) | 0.007 | |||
| pM | 482.89 (4.11)/479.70 (435.70–527.80) | 545.69 (20.49)/510.90 (440.70–566.80) | ||||
| mcg/L | 0.5 [96] | 0.654 (0.01)/0.650 (0.591, 0.715) | 0.740 (0.03)/0.692 (0.597, 0.768) | |||
| Vitamin C * (HPLC-DAD) | 91 (358) | 20 (80) | 0.247 | |||
| mg/dL | 6.33 (0.08)/6.20 (5.45, 7.08) | 6.50 (0.16)/6.40 (5.63, 7.77) | ||||
| mg/L | 35–90 [75] | 63.30 (0.80)/62.00 (54.50, 70.80) | 65.00 (1.60)/64.00 (56.30, 77.70) | |||
| Retinol (HPLC with fluorescence and UV detector) | 91 (357) | 19 (76) | <0.001 | |||
| mcg/dL | 1130.67 (243.99)/38.80 (24.10, 68.60) | 1123.11 (400.04)/200.60 (40.60, 249.35) | ||||
| mcg/L | 530 [97] | 11306.7 (2439.9)/388.0 (241.0, 686.0) | 11231.1 (4000.4)/2000.6 (406.0, 2493.5) | |||
| Vitamin D3 (UPLC–electrospray ionization/tandem MS) | 92 (362) | 20 (80) | <0.001 | |||
| pg/mL | 3433.84 (291.29)/1141.75 (340.15, 4345.90) | 1102.01 (224.89)/336.05 (123.55, 1345.55) | ||||
| mcg/L | 0.25–2 [98] | 3.43 (0.29)/1.14 (0.34, 4.35) | 1.10 (0.22)/0.34 (0.12, 1.35) | |||
| 25(OH)D (UPLC–electrospray ionization/tandem MS) | 92 (362) | 20 (80) | 0.084 | |||
| pg/mL | 82.66 (5.57)/46.50 (23.35, 102.60) | 100.36 (11.14)/62.70 (30.70, 131.45) | ||||
| mcg/L | 0.083 (0.006)/0.047 (0.023, 0.103) | 0.104 (0.011)/0.063 (0.031, 0.131) | ||||
| α-tocopherol (HPLC with fluorescence and UV detector) | 91 (357) | 19 (76) | 0.206 | |||
| mcg/dL | 465.80 (10.85)/433.00 (309.80, 579.70) | 432.34 (23.01)/407.25 (279.35, 558.40) | ||||
| mg/L | 4.6 [99] | 4.65 (0.10)/4.33 (3.10, 5.80) | 4.32 (0.23)/4.07 (2.79, 5.58) | |||
| γ-tocopherol (HPLC with fluorescence and UV detector) | 91 (357) | 19 (76) | <0.001 | |||
| mcg/dL | 53.86 (1.36)/47.20 (35.10, 69.39) | 77.48 (4.45)/68.45 (47.10, 104.40) | ||||
| mg/L | 0.45 [90] | 0.539 (0.014)/0.472 (0.351, 0.694) | 0.775 (0.045)/0.685 (0.471, 0.104) | |||
| Vitamin E (as TE) ** mg/L | 5.2 [90] | 91 (357) | 4.77 (0.11)/4.46 (3.19, 5.94) | 19 (76) | 4.52 (0.24)/4.27 (2.99, 5.93) | 0.330 |
| Iodine (ICP-MS) ppb (mcg/L) | 50–100 [95] 100–200 [100,101] | 92 (366) | 159.22 (5.13)/135.65 (88.30, 197.50) | 20 (80) | 126.42 (13.37)/95.65 (50.25, 145.65) | <0.001 |
| Calcium (ICP-MS) ppm (mg/L) | 200–300 [102] | 92 (366) | 98.93 (2.87)/91.00 (54.80, 135.60) | 20 (80) | 83.54 (3.70)/80.00 (60.40, 102.55) | 0.093 |
| Phosphorous (ICP-MS) ppm (mg/L) | 120–140 [95,103] | 92 (366) | 131.22 (1.49)/128.85 (111.30, 145.80) | 20 (80) | 120.02 (2.25)/120.75 (107.40, 134.20) | 0.002 |
| Selenium (ICP-MS) ppb (mcg/L) | 18 [104] | 92 (366) | 11.72 (0.25)/10.80 (9.10, 13.30) | 20 (80) | 9.90 (0.37)/9.45 (7.75, 11.00) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ureta-Velasco, N.; Keller, K.; Escuder-Vieco, D.; Fontecha, J.; Calvo, M.V.; Megino-Tello, J.; Serrano, J.C.E.; Romero Ferreiro, C.; García-Lara, N.R.; Pallás-Alonso, C.R. Human Milk Composition and Nutritional Status of Omnivore Human Milk Donors Compared with Vegetarian/Vegan Lactating Mothers. Nutrients 2023, 15, 1855. https://doi.org/10.3390/nu15081855
Ureta-Velasco N, Keller K, Escuder-Vieco D, Fontecha J, Calvo MV, Megino-Tello J, Serrano JCE, Romero Ferreiro C, García-Lara NR, Pallás-Alonso CR. Human Milk Composition and Nutritional Status of Omnivore Human Milk Donors Compared with Vegetarian/Vegan Lactating Mothers. Nutrients. 2023; 15(8):1855. https://doi.org/10.3390/nu15081855
Chicago/Turabian StyleUreta-Velasco, Noelia, Kristin Keller, Diana Escuder-Vieco, Javier Fontecha, María V. Calvo, Javier Megino-Tello, José C. E. Serrano, Carmen Romero Ferreiro, Nadia Raquel García-Lara, and Carmen R. Pallás-Alonso. 2023. "Human Milk Composition and Nutritional Status of Omnivore Human Milk Donors Compared with Vegetarian/Vegan Lactating Mothers" Nutrients 15, no. 8: 1855. https://doi.org/10.3390/nu15081855
APA StyleUreta-Velasco, N., Keller, K., Escuder-Vieco, D., Fontecha, J., Calvo, M. V., Megino-Tello, J., Serrano, J. C. E., Romero Ferreiro, C., García-Lara, N. R., & Pallás-Alonso, C. R. (2023). Human Milk Composition and Nutritional Status of Omnivore Human Milk Donors Compared with Vegetarian/Vegan Lactating Mothers. Nutrients, 15(8), 1855. https://doi.org/10.3390/nu15081855








