Tinnitus at the Junction of Traditional Medicine and Modern Technology
1. Introduction
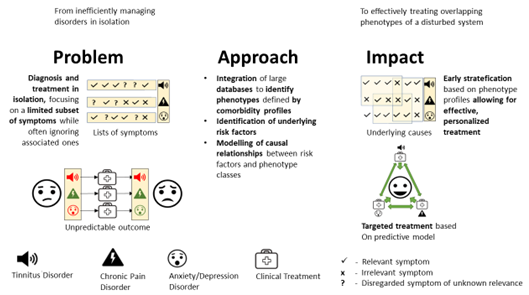
2. The Problem: Comorbid, Chronic Diseases Are Diagnosed and Treated in Isolation, Hampering Efficient Treatment
3. The Solution: Treating Comorbid Diseases as Symptoms of a Disturbed System and Develop Early Risk Predictors for Chronification
4. A Personalised Systems Medicine Approach for Tinnitus
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. World Report on Hearing; 9789240020481 (Electronic Version); World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Mazurek, B.; Hesse, G.; Dobel, C.; Kratzsch, V.; Lahmann, C.; Sattel, H. Chronic Tinnitus. Dtsch. Arztebl. Int. 2022, 119, 219–225. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, D.; Schlee, W.; Vanneste, S.; Londero, A.; Weisz, N.; Kleinjung, T.; Shekhawat, G.S.; Elgoyhen, A.B.; Song, J.J.; Andersson, G.; et al. Tinnitus and tinnitus disorder: Theoretical and operational definitions (an international multidisciplinary proposal). Prog. Brain Res. 2021, 260, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Biswas, R.; Lugo, A.; Akeroyd, M.A.; Schlee, W.; Gallus, S.; Hall, D.A. Tinnitus prevalence in Europe: A multi-country cross-sectional population study. Lancet Reg. Health-Eur. 2022, 12, 100250. [Google Scholar] [CrossRef]
- Gallus, S.; Lugo, A.; Garavello, W.; Bosetti, C.; Santoro, E.; Colombo, P.; Perin, P.; La Vecchia, C.; Langguth, B. Prevalence and Determinants of Tinnitus in the Italian Adult Population. Neuroepidemiology 2015, 45, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Friis, K.; Pedersen, M.H.; Larsen, F.B.; Lasgaard, M. A National Population Study of the Co-Occurrence of Multiple Long-Term Conditions in People With Multimorbidity, Denmark, 2013. Prev. Chronic. Dis. 2016, 13, E12. [Google Scholar] [CrossRef] [Green Version]
- Tziridis, K.; Friedrich, J.; Brueggemann, P.; Mazurek, B.; Schulze, H. Estimation of Tinnitus-Related Socioeconomic Costs in Germany. Int. J. Environ. Res. Public Health 2022, 19, 455. [Google Scholar] [CrossRef]
- Maes, I.H.; Cima, R.F.; Vlaeyen, J.W.; Anteunis, L.J.; Joore, M.A. Tinnitus: A cost study. Ear. Hear. 2013, 34, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivansic, D.; Besteher, B.; Gantner, J.; Guntinas-Lichius, O.; Pantev, C.; Nenadic, I.; Dobel, C. Psychometric assessment of mental health in tinnitus patients, depressive and healthy controls. Psychiatry Res. 2019, 281, 112582. [Google Scholar] [CrossRef]
- Zirke, N.; Seydel, C.; Arsoy, D.; Klapp, B.F.; Haupt, H.; Szczepek, A.J.; Olze, H.; Goebel, G.; Mazurek, B. Analysis of mental disorders in tinnitus patients performed with Composite International Diagnostic Interview. Qual. Life Res. 2013, 22, 2095–2104. [Google Scholar] [CrossRef]
- Mazurek, B.; Boecking, B.; Brueggemann, P. Association Between Stress and Tinnitus-New Aspects. Otol. Neurotol. 2019, 40, e467–e473. [Google Scholar] [CrossRef]
- Ivansic, D.; Guntinas-Lichius, O.; Müller, B.; Volk, G.F.; Schneider, G.; Dobel, C. Impairments of Speech Comprehension in Patients with Tinnitus-A Review. Front. Aging Neurosci. 2017, 9, 224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pergamin-Hight, L.; Naim, R.; Bakermans-Kranenburg, M.J.; van IJzendoorn, M.H.; Bar-Haim, Y. Content specificity of attention bias to threat in anxiety disorders: A meta-analysis. Clin. Psychol. Rev. 2015, 35, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Zorn, J.V.; Schür, R.R.; Boks, M.P.; Kahn, R.S.; Joëls, M.; Vinkers, C.H. Cortisol stress reactivity across psychiatric disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 2017, 77, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Brueggemann, P.; Mebus, W.; Boecking, B.; Amarjargal, N.; Niemann, U.; Spiliopoulou, M.; Dobel, C.; Rose, M.; Mazurek, B. Dimensions of Tinnitus-Related Distress. Brain Sci. 2022, 12, 275. [Google Scholar] [CrossRef]
- Niemann, U.; Brueggemann, P.; Boecking, B.; Mebus, W.; Rose, M.; Spiliopoulou, M.; Mazurek, B. Phenotyping chronic tinnitus patients using self-report questionnaire data: Cluster analysis and visual comparison. Sci. Rep. 2020, 10, 16411. [Google Scholar] [CrossRef]
- Eden, A.S.; Schreiber, J.; Anwander, A.; Keuper, K.; Laeger, I.; Zwanzger, P.; Zwitserlood, P.; Kugel, H.; Dobel, C. Emotion regulation and trait anxiety are predicted by the microstructure of fibers between amygdala and prefrontal cortex. J. Neurosci. 2015, 35, 6020–6027. [Google Scholar] [CrossRef] [Green Version]
- Roesmann, K.; Dellert, T.; Junghoefer, M.; Kissler, J.; Zwitserlood, P.; Zwanzger, P.; Dobel, C. The causal role of prefrontal hemispheric asymmetry in valence processing of words-Insights from a combined cTBS-MEG study. NeuroImage 2019, 191, 367–379. [Google Scholar] [CrossRef]
- Husain, F.T.; Schmidt, S.A. Using resting state functional connectivity to unravel networks of tinnitus. Hear. Res. 2014, 307, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Friston, K.J.; Frith, C.D.; Liddle, P.F.; Frackowiak, R.S. Functional connectivity: The principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab. 1993, 13, 5–14. [Google Scholar] [CrossRef]
- Stein, A.; Engell, A.; Lau, P.; Wunderlich, R.; Junghoefer, M.; Wollbrink, A.; Bruchmann, M.; Rudack, C.; Pantev, C. Enhancing inhibition-induced plasticity in tinnitus--spectral energy contrasts in tailor-made notched music matter. PLoS ONE 2015, 10, e0126494. [Google Scholar] [CrossRef] [Green Version]
- Maudoux, A.; Lefebvre, P.; Cabay, J.E.; Demertzi, A.; Vanhaudenhuyse, A.; Laureys, S.; Soddu, A. Connectivity graph analysis of the auditory resting state network in tinnitus. Brain Res. 2012, 1485, 10–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crippa, A.; Lanting, C.P.; van Dijk, P.; Roerdink, J.B. A diffusion tensor imaging study on the auditory system and tinnitus. Open Neuroimag. J. 2010, 4, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leaver, A.M.; Seydell-Greenwald, A.; Rauschecker, J.P. Auditory-limbic interactions in chronic tinnitus: Challenges for neuroimaging research. Hear. Res. 2016, 334, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.J.; Roberts, L.E. The neuroscience of tinnitus: Understanding abnormal and normal auditory perception. Front. Syst. Neurosci. 2012, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Pantev, C.; Okamoto, H.; Teismann, H. Music-induced cortical plasticity and lateral inhibition in the human auditory cortex as foundations for tonal tinnitus treatment. Front. Syst. Neurosci. 2012, 6, 50. [Google Scholar] [CrossRef] [Green Version]
- De Ridder, D.; Adhia, D.; Langguth, B. Tinnitus and Brain Stimulation. Curr. Top. Behav. Neurosci. 2021, 51, 249–293. [Google Scholar] [CrossRef]
- Mirz, F.; Gjedde, A.; Ishizu, K.; Pedersen, C.B. Cortical networks subserving the perception of tinnitus—A PET study. Acta Otolaryngol. Suppl. 2000, 543, 241–243. [Google Scholar] [CrossRef]
- Tziridis, K.; Schulze, H. Preventive Effects of Ginkgo-Extract EGb 761(®) on Noise Trauma-Induced Cochlear Synaptopathy. Nutrients 2022, 14, 3015. [Google Scholar] [CrossRef]
- Jarach, C.M.; Lugo, A.; Garavello, W.; van den Brandt, P.A.; Odone, A.; Cederroth, C.R.; Bosetti, C.; Gallus, S. The Role of Diet in Tinnitus Onset: A Hospital-Based Case-Control Study from Italy. Nutrients 2023, 15, 621. [Google Scholar] [CrossRef]
- Marcrum, S.C.; Engelke, M.; Goedhart, H.; Langguth, B.; Schlee, W.; Vesala, M.; Simoes, J.P. The Influence of Diet on Tinnitus Severity: Results of a Large-Scale, Online Survey. Nutrients 2022, 14, 5356. [Google Scholar] [CrossRef]
- Boecking, B.; Klasing, S.; Walter, M.; Brueggemann, P.; Nyamaa, A.; Rose, M.; Mazurek, B. Vascular-Metabolic Risk Factors and Psychological Stress in Patients with Chronic Tinnitus. Nutrients 2022, 14, 2256. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, B.; Rose, M.; Schulze, H.; Dobel, C. Systems Medicine Approach for Tinnitus with Comorbid Disorders. Nutrients 2022, 14, 4320. [Google Scholar] [CrossRef] [PubMed]
- Heller, M.F.; Bergman, M. Tinnitus aurium in normally hearing persons. Ann. Otol. Rhinol. Laryngol. 1953, 62, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Schlee, W.; Schoisswohl, S.; Staudinger, S.; Schiller, A.; Lehner, A.; Langguth, B.; Schecklmann, M.; Simoes, J.; Neff, P.; Marcrum, S.C.; et al. Towards a unification of treatments and interventions for tinnitus patients: The EU research and innovation action UNITI. Prog. Brain Res. 2021, 260, 441–451. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurek, B.; Schulze, H.; Schlee, W.; Dobel, C. Tinnitus at the Junction of Traditional Medicine and Modern Technology. Nutrients 2023, 15, 1898. https://doi.org/10.3390/nu15081898
Mazurek B, Schulze H, Schlee W, Dobel C. Tinnitus at the Junction of Traditional Medicine and Modern Technology. Nutrients. 2023; 15(8):1898. https://doi.org/10.3390/nu15081898
Chicago/Turabian StyleMazurek, Birgit, Holger Schulze, Winfried Schlee, and Christian Dobel. 2023. "Tinnitus at the Junction of Traditional Medicine and Modern Technology" Nutrients 15, no. 8: 1898. https://doi.org/10.3390/nu15081898





