The Effect of Maternal Dietary Patterns on Birth Weight for Gestational Age: Findings from the MAMI-MED Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Dietary Assessment
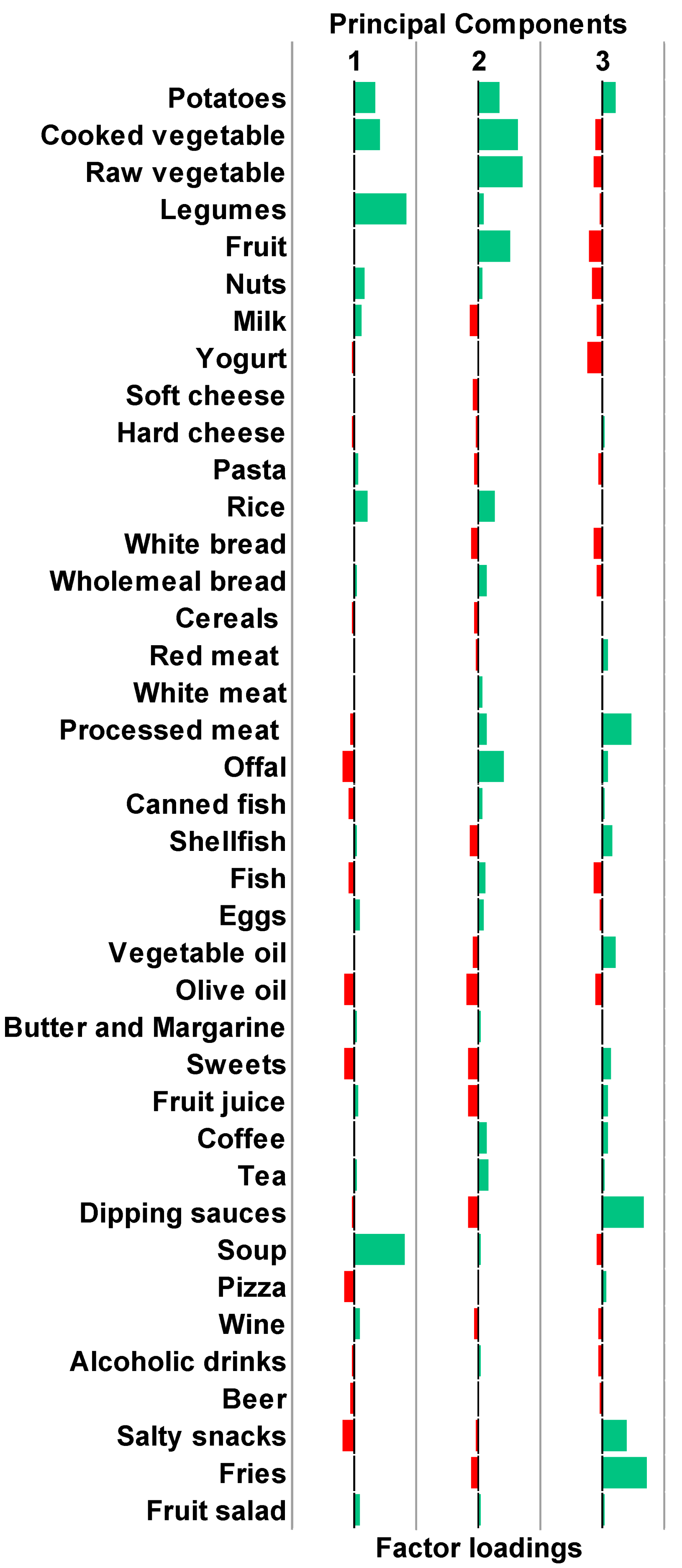
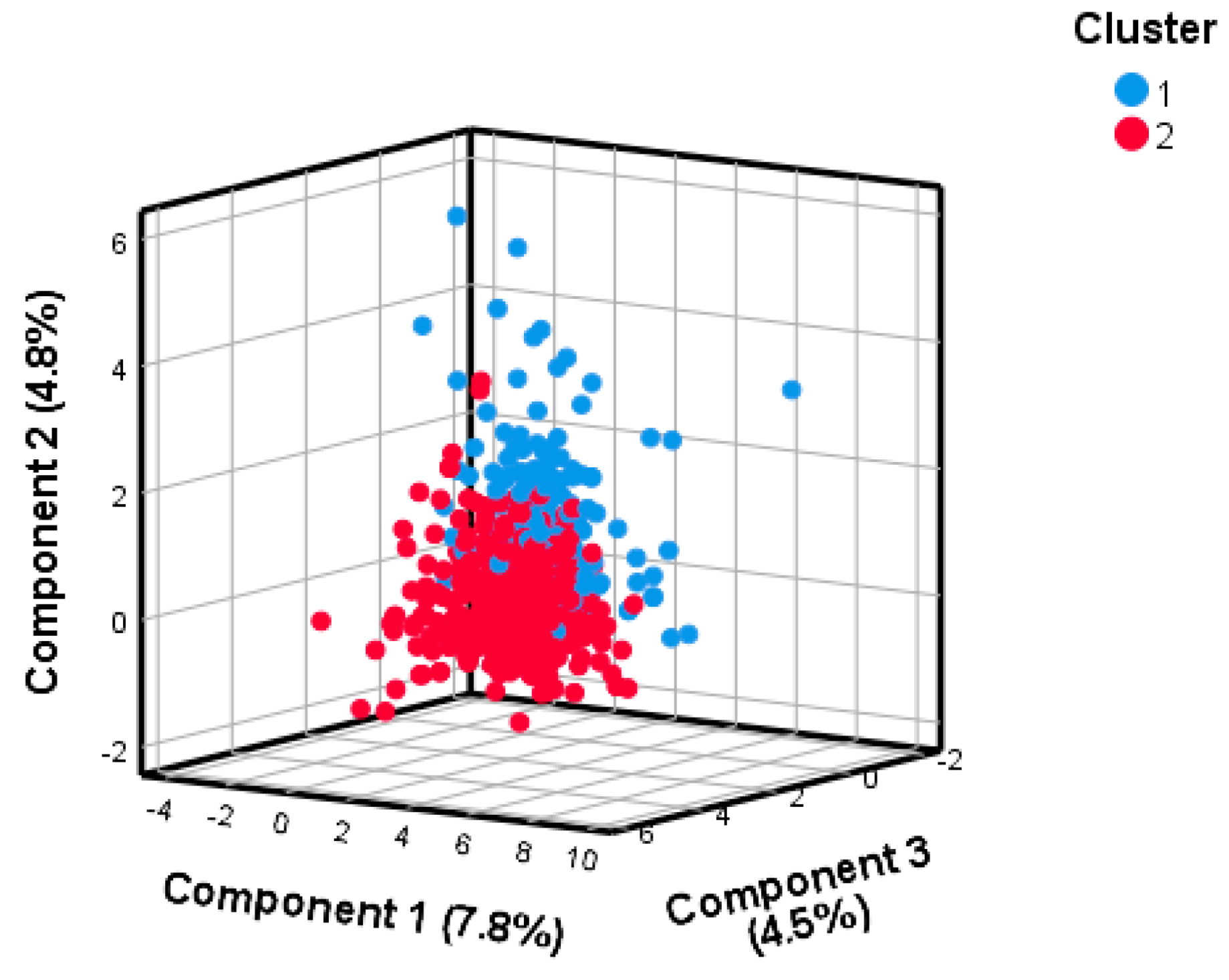
2.4. Clustering on Principal Components
2.5. Statistical Analysis
3. Results
3.1. Study Population
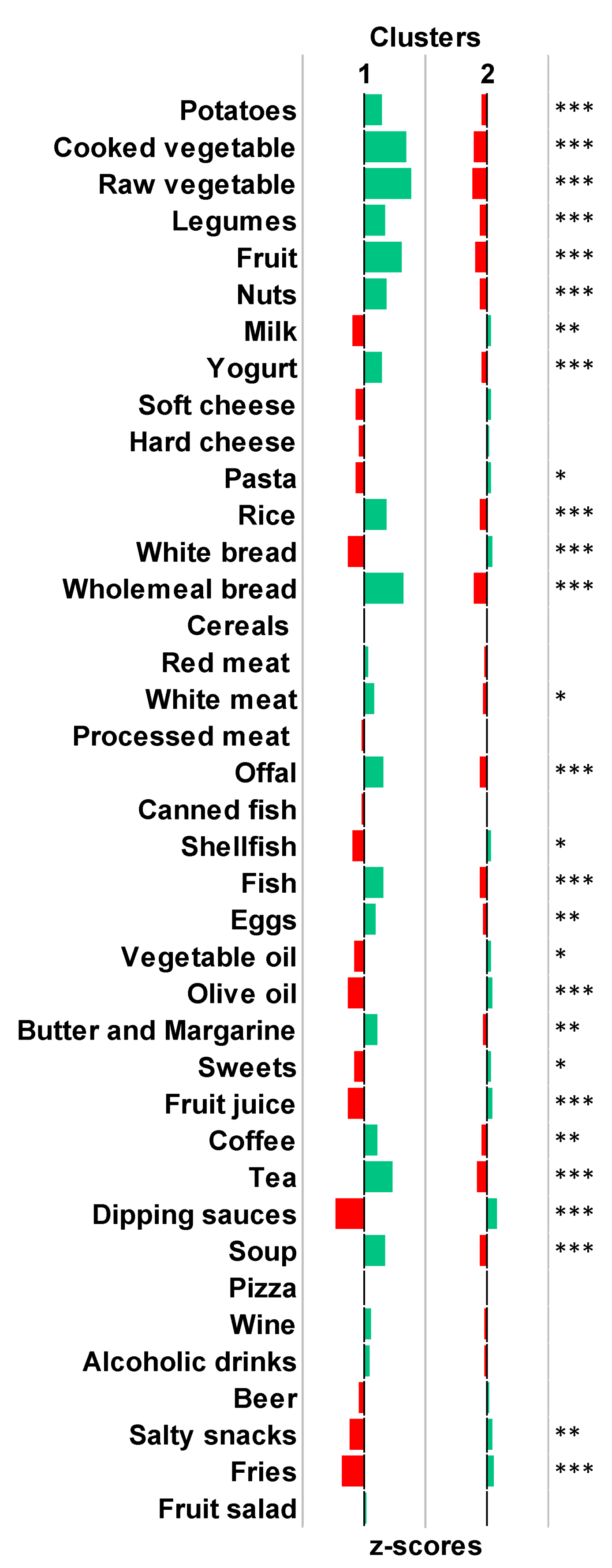
3.2. Derivation of Clusters Reflecting Distinct Dietary Patterns
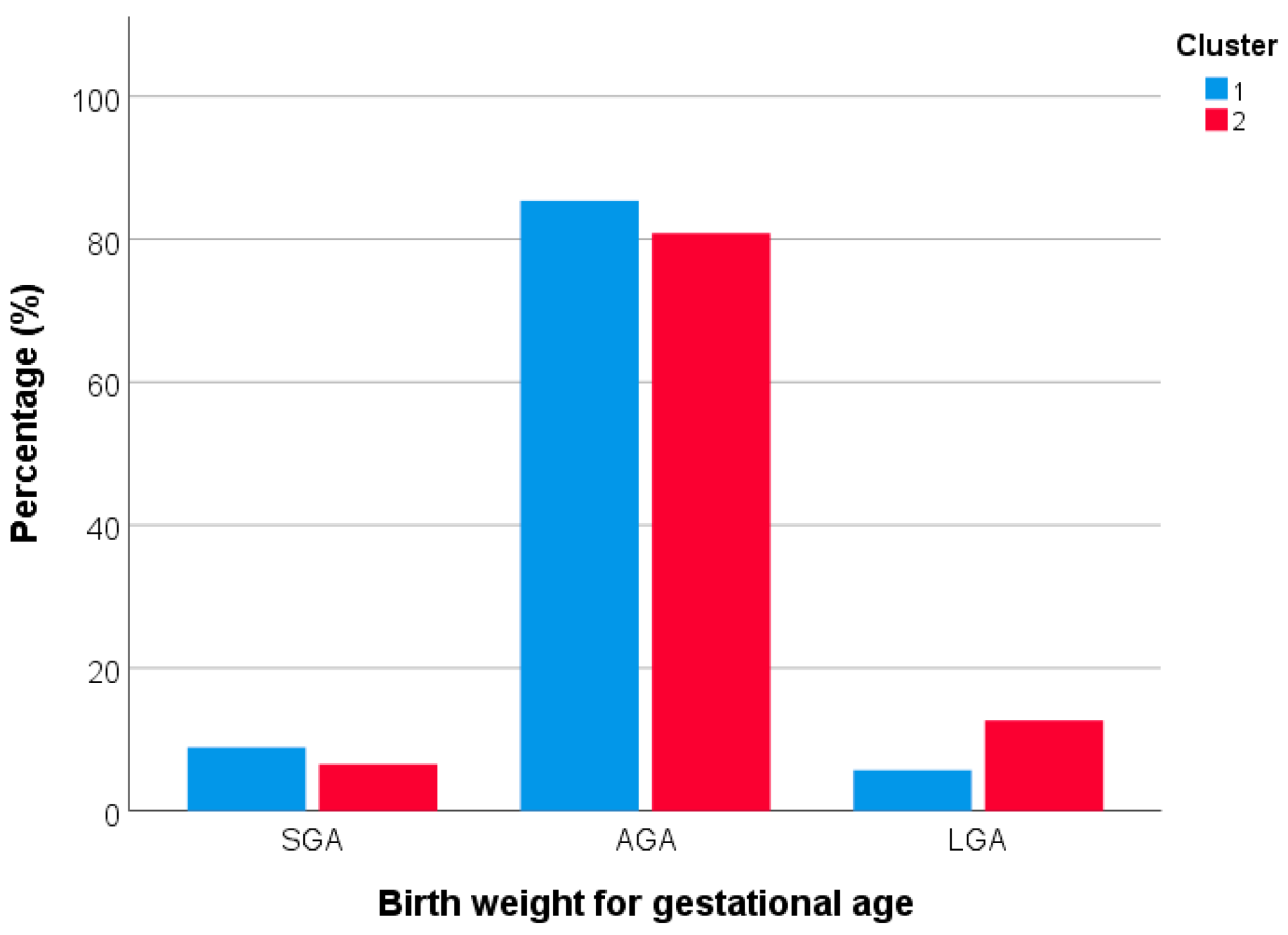
3.3. Differences in Maternal Characteristics and Birth Outcomes according to Dietary Patterns
3.4. Factors Associated with Birth Weight for Gestational Age
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Maternal Health. Available online: https://www.who.int/health-topics/maternal-health#tab=tab_1 (accessed on 20 February 2023).
- Bertini, A.; Gárate, B.; Pardo, F.; Pelicand, J.; Sobrevia, L.; Torres, R.; Chabert, S.; Salas, R. Impact of Remote Monitoring Technologies for Assisting Patients With Gestational Diabetes Mellitus: A Systematic Review. Front. Bioeng. Biotechnol. 2022, 10, 819697. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Lee, C.F.; Huang, J.P.; Hsiung, Y.; Chi, L.K. Effectiveness of a nurse-led mHealth app to prevent excessive gestational weight gain among overweight and obese women: A randomized controlled trial. J. Nurs. Sch. 2022, 55, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.M.T.; Ahmed, S.I.; Khan, M.A.; Sarker, S.A.; Ahmed, T. Achieving Optimal Gestational Weight Gain, Birth Weight, and Perinatal Outcomes Among Pregnant Women at Risk of Hypertension: Protocol for a Pilot Randomized Controlled Trial. JMIR Res. Protoc. 2020, 9, e16676. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ma, Y.; Wang, N.; Lin, W.; Liu, Y.; Wen, D. Maternal body mass index and risk of neonatal adverse outcomes in China: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2019, 19, 105. [Google Scholar] [CrossRef] [PubMed]
- Olson, C.M.; Strawderman, M.S. Modifiable behavioral factors in a biopsychosocial model predict inadequate and excessive gestational weight gain. J. Am. Diet Assoc. 2003, 103, 48–54. [Google Scholar] [CrossRef]
- Tieu, J.; Shepherd, E.; Middleton, P.; Crowther, C.A. Dietary advice interventions in pregnancy for preventing gestational diabetes mellitus. Cochrane Database Syst. Rev. 2017, 1, Cd006674. [Google Scholar] [CrossRef]
- Rosenfeld, T.; Salem, H.; Altarescu, G.; Grisaru-Granovsky, S.; Tevet, A.; Birk, R. Maternal-fetal vitamin D receptor polymorphisms significantly associated with preterm birth. Arch. Gynecol. Obstet. 2017, 296, 215–222. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Grisaru-Granovsky, S.; Reichman, B.; Lerner-Geva, L.; Boyko, V.; Hammerman, C.; Samueloff, A.; Schimmel, M.S.; Network, I.N. Population-based trends in mortality and neonatal morbidities among singleton, very preterm, very low birth weight infants over 16 years. Early Hum. Dev. 2014, 90, 821–827. [Google Scholar] [CrossRef]
- Moutquin, J.M. Classification and heterogeneity of preterm birth. BJOG 2003, 110, 30–33. [Google Scholar] [CrossRef]
- Lin, X.; Lim, I.Y.; Wu, Y.; Teh, A.L.; Chen, L.; Aris, I.M.; Soh, S.E.; Tint, M.T.; MacIsaac, J.L.; Morin, A.M.; et al. Developmental pathways to adiposity begin before birth and are influenced by genotype, prenatal environment and epigenome. BMC Med. 2017, 15, 50. [Google Scholar] [CrossRef]
- Yu, Z.B.; Han, S.P.; Zhu, G.Z.; Zhu, C.; Wang, X.J.; Cao, X.G.; Guo, X.R. Birth weight and subsequent risk of obesity: A systematic review and meta-analysis. Obes. Rev. 2011, 12, 525–542. [Google Scholar] [CrossRef]
- WHO. Biomarkers and Risk Assessment: Concept and Principles; WHO: Geneva, Switzerland, 1993. [Google Scholar]
- Baird, J.; Jacob, C.; Barker, M.; Fall, C.H.; Hanson, M.; Harvey, N.C.; Inskip, H.M.; Kumaran, K.; Cooper, C. Developmental Origins of Health and Disease: A Lifecourse Approach to the Prevention of Non-Communicable Diseases. Healthcare 2017, 5, 14. [Google Scholar] [CrossRef]
- Montagnoli, C.; Santoro, C.B.; Buzzi, T.; Bortolus, R. Maternal periconceptional nutrition matters. A scoping review of the current literature. J. Matern. Fetal. Neonatal Med. 2021, 35, 8123–8140. [Google Scholar] [CrossRef]
- Marangoni, F.; Cetin, I.; Verduci, E.; Canzone, G.; Giovannini, M.; Scollo, P.; Corsello, G.; Poli, A. Maternal Diet and Nutrient Requirements in Pregnancy and Breastfeeding. An Italian Consensus Document. Nutrients 2016, 8, 629. [Google Scholar] [CrossRef]
- Cucó, G.; Arija, V.; Iranzo, R.; Vilà, J.; Prieto, M.T.; Fernández-Ballart, J. Association of maternal protein intake before conception and throughout pregnancy with birth weight. Acta Obstet. Gynecol. Scand. 2006, 85, 413–421. [Google Scholar] [CrossRef]
- Grieger, J.A.; Grzeskowiak, L.E.; Clifton, V.L. Preconception dietary patterns in human pregnancies are associated with preterm delivery. J. Nutr. 2014, 144, 1075–1080. [Google Scholar] [CrossRef]
- Lamers, Y.; MacFarlane, A.J.; O’Connor, D.L.; Fontaine-Bisson, B. Periconceptional intake of folic acid among low-risk women in Canada: Summary of a workshop aiming to align prenatal folic acid supplement composition with current expert guidelines. Am. J. Clin. Nutr. 2018, 108, 1357–1368. [Google Scholar] [CrossRef]
- Pauwels, S.; Ghosh, M.; Duca, R.C.; Bekaert, B.; Freson, K.; Huybrechts, I.; AS Langie, S.; Koppen, G.; Devlieger, R.; Godderis, L. Dietary and supplemental maternal methyl-group donor intake and cord blood DNA methylation. Epigenetics 2017, 12, 1–10. [Google Scholar] [CrossRef]
- Veeranki, S.P.; Gebretsadik, T.; Mitchel, E.F.; Tylavsky, F.A.; Hartert, T.V.; Cooper, W.O.; Dupont, W.D.; Dorris, S.L.; Hartman, T.J.; Carroll, K.N. Maternal Folic Acid Supplementation During Pregnancy and Early Childhood Asthma. Epidemiology 2015, 26, 934–941. [Google Scholar] [CrossRef]
- Wu, Y.; Zeng, Y.; Zhang, Q.; Xiao, X. The Role of Maternal Vitamin D Deficiency in Offspring Obesity: A Narrative Review. Nutrients 2023, 15, 533. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Barchitta, M.; Magnano San Lio, R.; La Rosa, M.C.; La Mastra, C.; Favara, G.; Ferlito, M.; Giunta, G.; Panella, M.; Cianci, A.; et al. The Effect of Alcohol on Telomere Length: A Systematic Review of Epidemiological Evidence and a Pilot Study during Pregnancy. Int. J. Environ. Res. Public Health 2021, 18, 5038. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Favara, G.; La Mastra, C.; La Rosa, M.C.; Agodi, A. Dietary Folate Intake and Folic Acid Supplements among Pregnant Women from Southern Italy: Evidence from the “Mamma & Bambino” Cohort. Int. J. Environ. Res. Public Health 2020, 17, 638. [Google Scholar] [CrossRef] [PubMed]
- Barchitta, M.; Maugeri, A.; La Rosa, M.C.; Magnano San Lio, R.; Favara, G.; Panella, M.; Cianci, A.; Agodi, A. Single Nucleotide Polymorphisms in Vitamin D Receptor Gene Affect Birth Weight and the Risk of Preterm Birth: Results From the "Mamma & Bambino" Cohort and A Meta-Analysis. Nutrients 2018, 10, 1172. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Ribeiro, A.C.; Meireles, M.; Ferro-Lebres, V.; Almeida-de-Souza, J. Olive oil consumption confers protective effects on maternal-fetal outcomes: A systematic review of the evidence. Nutr. Res. 2023, 110, 87–95. [Google Scholar] [CrossRef]
- Liu, L.; Yan, F.; Yan, H.; Wang, Z. Impact of iron supplementation on gestational diabetes mellitus: A literature review. Diabetes Obes. Metab. 2023, 25, 342–353. [Google Scholar] [CrossRef]
- Brough, L.; Rees, G.A.; Crawford, M.A.; Morton, R.H.; Dorman, E.K. Effect of multiple-micronutrient supplementation on maternal nutrient status, infant birth weight and gestational age at birth in a low-income, multi-ethnic population. Br. J. Nutr. 2010, 104, 437–445. [Google Scholar] [CrossRef]
- Håberg, S.E.; London, S.J.; Stigum, H.; Nafstad, P.; Nystad, W. Folic acid supplements in pregnancy and early childhood respiratory health. Arch. Dis. Child. 2009, 94, 180–184. [Google Scholar] [CrossRef]
- Haggarty, P.; Hoad, G.; Campbell, D.M.; Horgan, G.W.; Piyathilake, C.; McNeill, G. Folate in pregnancy and imprinted gene and repeat element methylation in the offspring. Am. J. Clin. Nutr. 2013, 97, 94–99. [Google Scholar] [CrossRef]
- McStay, C.L.; Prescott, S.L.; Bower, C.; Palmer, D.J. Maternal Folic Acid Supplementation during Pregnancy and Childhood Allergic Disease Outcomes: A Question of Timing? Nutrients 2017, 9, 123. [Google Scholar] [CrossRef]
- Agodi, A.; Barchitta, M.; Valenti, G.; Marzagalli, R.; Frontini, V.; Marchese, A.E. Increase in the prevalence of the MTHFR 677 TT polymorphism in women born since 1959: Potential implications for folate requirements. Eur. J. Clin. Nutr. 2011, 65, 1302–1308. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Krohn, M.A.; Simhan, H.N. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J. Nutr. 2009, 139, 1157–1161. [Google Scholar] [CrossRef]
- Delvin, E.E.; Salle, B.L.; Glorieux, F.H.; Adeleine, P.; David, L.S. Vitamin D supplementation during pregnancy: Effect on neonatal calcium homeostasis. J. Pediatr. 1986, 109, 328–334. [Google Scholar] [CrossRef]
- De-Regil, L.M.; Palacios, C.; Lombardo, L.K.; Peña-Rosas, J.P. Vitamin D supplementation for women during pregnancy. Sao Paulo Med. J. 2016, 134, 274–275. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M. A Systematic Review of Ecological Momentary Assessment of Diet: Implications and Perspectives for Nutritional Epidemiology. Nutrients 2019, 11, 2696. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Favara, G.; Magnano San Lio, R.; Riela, P.M.; Guarnera, L.; Battiato, S.; Agodi, A. Development of a Web-App for the Ecological Momentary Assessment of Dietary Habits among College Students: The HEALTHY-UNICT Project. Nutrients 2022, 14, 330. [Google Scholar] [CrossRef]
- Maugeri, A.; Magnano San Lio, R.; Favara, G.; La Rosa, M.C.; La Mastra, C.; Riela, P.M.; Guarnera, L.; Battiato, S.; Barchitta, M.; Agodi, A. Impact of Eating Context on Dietary Choices of College Students: Evidence from the HEALTHY-UNICT Project. Nutrients 2022, 14, 4418. [Google Scholar] [CrossRef]
- Ashurst, J.; van Woerden, I.; Dunton, G.; Todd, M.; Ohri-Vachaspati, P.; Swan, P.; Bruening, M. The Association among Emotions and Food Choices in First-Year College Students Using mobile-Ecological Momentary Assessments. BMC Public Health 2018, 18, 573. [Google Scholar] [CrossRef]
- Berge, J.M.; Tate, A.; Trofholz, A.; Fertig, A.; Crow, S.; Neumark-Sztainer, D.; Miner, M. Examining within- and across-day relationships between transient and chronic stress and parent food-related parenting practices in a racially/ethnically diverse and immigrant population: Stress types and food-related parenting practices. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 7. [Google Scholar] [CrossRef]
- Berkman, E.T.; Giuliani, N.R.; Pruitt, A.K. Comparison of text messaging and paper-and-pencil for ecological momentary assessment of food craving and intake. Appetite 2014, 81, 131–137. [Google Scholar] [CrossRef]
- Bruening, M.; van Woerden, I.; Todd, M.; Brennhofer, S.; Laska, M.N.; Dunton, G. A Mobile Ecological Momentary Assessment Tool (devilSPARC) for Nutrition and Physical Activity Behaviors in College Students: A Validation Study. J. Med. Internet Res. 2016, 18, e209. [Google Scholar] [CrossRef] [PubMed]
- Chmurzynska, A.; Mlodzik-Czyzewska, M.A.; Malinowska, A.M.; Czarnocinska, J.; Wiebe, D. Use of a Smartphone Application Can Improve Assessment of High-Fat Food Consumption in Overweight Individuals. Nutrients 2018, 10, 1692. [Google Scholar] [CrossRef] [PubMed]
- Comulada, W.S.; Swendeman, D.; Koussa, M.K.; Mindry, D.; Medich, M.; Estrin, D.; Mercer, N.; Ramanathan, N. Adherence to self-monitoring healthy lifestyle behaviours through mobile phone-based ecological momentary assessments and photographic food records over 6 months in mostly ethnic minority mothers. Public Health Nutr. 2018, 21, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, E.M.; Hu, F.B. Dietary patterns: From nutritional epidemiologic analysis to national guidelines. Am. J. Clin. Nutr. 2015, 101, 899–900. [Google Scholar] [CrossRef] [PubMed]
- Agodi, A.; Maugeri, A.; Kunzova, S.; Sochor, O.; Bauerova, H.; Kiacova, N.; Barchitta, M.; Vinciguerra, M. Association of Dietary Patterns with Metabolic Syndrome: Results from the Kardiovize Brno 2030 Study. Nutrients 2018, 10, 898. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Quattrocchi, A.; Agrifoglio, O.; Scalisi, A.; Agodi, A. The Association of Dietary Patterns with High-Risk Human Papillomavirus Infection and Cervical Cancer: A Cross-Sectional Study in Italy. Nutrients 2018, 10, 469. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; Favara, G.; La Rosa, M.C.; La Mastra, C.; Quattrocchi, A.; Agodi, A. Dietary Patterns are Associated with Leukocyte LINE-1 Methylation in Women: A Cross-Sectional Study in Southern Italy. Nutrients 2019, 11, 1843. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; La Mastra, C.; Rosa, M.C.; Favara, G.; Lio, R.M.S.; Agodi, A. Dietary Antioxidant Intake and Human Papillomavirus Infection: Evidence from a Cross-Sectional Study in Italy. Nutrients 2020, 12, 1384. [Google Scholar] [CrossRef]
- Maugeri, A.; Kunzova, S.; Medina-Inojosa, J.R.; Agodi, A.; Barchitta, M.; Homolka, M.; Kiacova, N.; Bauerova, H.; Sochor, O.; Lopez-Jimenez, F.; et al. Association between eating time interval and frequency with ideal cardiovascular health: Results from a random sample Czech urban population. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 847–855. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Fiore, V.; Rosta, G.; Favara, G.; La Mastra, C.; La Rosa, M.C.; Magnano San Lio, R.; Agodi, A. Determinants of Adherence to the Mediterranean Diet: Findings from a Cross-Sectional Study in Women from Southern Italy. Int. J. Environ. Res. Public Health 2019, 16, 2963. [Google Scholar] [CrossRef]
- Maugeri, A.; Hruskova, J.; Jakubik, J.; Hlinomaz, O.; Medina-Inojosa, J.R.; Barchitta, M.; Agodi, A.; Vinciguerra, M. How dietary patterns affect left ventricular structure, function and remodelling: Evidence from the Kardiovize Brno 2030 study. Sci. Rep. 2019, 9, 19154. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Magnano San Lio, R.; Favara, G.; La Rosa, M.C.; La Mastra, C.; Basile, G.; Agodi, A. Adherence to the Mediterranean diet partially mediates socioeconomic differences in leukocyte LINE-1 methylation: Evidence from a cross-sectional study in Italian women. Sci. Rep. 2020, 10, 14360. [Google Scholar] [CrossRef]
- Maugeri, A.; Barchitta, M.; Kunzova, S.; Bauerova, H.; Agodi, A.; Vinciguerra, M. The association of social and behavioral factors with dietary risks in adults: Evidence from the Kardiovize Brno 2030 study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 896–906. [Google Scholar] [CrossRef]
- Arkkola, T.; Uusitalo, U.; Kronberg-Kippilä, C.; Männistö, S.; Virtanen, M.; Kenward, M.G.; Veijola, R.; Knip, M.; Ovaskainen, M.L.; Virtanen, S.M. Seven distinct dietary patterns identified among pregnant Finnish women--associations with nutrient intake and sociodemographic factors. Public Health Nutr. 2008, 11, 176–182. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, D.; Mao, X.; Xia, Y.; Baker, P.N.; Zhang, H. Maternal Dietary Patterns and Pregnancy Outcome. Nutrients 2016, 8, 351. [Google Scholar] [CrossRef]
- de Castro, M.B.; Freitas Vilela, A.A.; de Oliveira, A.S.; Cabral, M.; de Souza, R.A.; Kac, G.; Sichieri, R. Sociodemographic characteristics determine dietary pattern adherence during pregnancy. Public Health Nutr. 2016, 19, 1245–1251. [Google Scholar] [CrossRef]
- Jarman, M.; Mathe, N.; Ramazani, F.; Pakseresht, M.; Robson, P.J.; Johnson, S.T.; Bell, R.C.; teams, A.a.E.s. Dietary Patterns Prior to Pregnancy and Associations with Pregnancy Complications. Nutrients 2018, 10, 914. [Google Scholar] [CrossRef]
- Raghavan, R.; Dreibelbis, C.; Kingshipp, B.L.; Wong, Y.P.; Abrams, B.; Gernand, A.D.; Rasmussen, K.M.; Siega-Riz, A.M.; Stang, J.; Casavale, K.O.; et al. Dietary patterns before and during pregnancy and birth outcomes: A systematic review. Am. J. Clin. Nutr. 2019, 109, 729s–756s. [Google Scholar] [CrossRef]
- Chia, A.R.; Chen, L.W.; Lai, J.S.; Wong, C.H.; Neelakantan, N.; van Dam, R.M.; Chong, M.F. Maternal Dietary Patterns and Birth Outcomes: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 685–695. [Google Scholar] [CrossRef]
- Klebanoff, M.A.; Yip, R. Influence of maternal birth weight on rate of fetal growth and duration of gestation. J. Pediatr. 1987, 111, 287–292. [Google Scholar] [CrossRef]
- Thompson, J.M.; Wall, C.; Becroft, D.M.; Robinson, E.; Wild, C.J.; Mitchell, E.A. Maternal dietary patterns in pregnancy and the association with small-for-gestational-age infants. Br. J. Nutr. 2010, 103, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Barchitta, M.; Favara, G.; La Rosa, M.C.; La Mastra, C.; Magnano San Lio, R.; Agodi, A. Maternal Dietary Patterns Are Associated with Pre-Pregnancy Body Mass Index and Gestational Weight Gain: Results from the “Mamma & Bambino” Cohort. Nutrients 2019, 11, 1308. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.M.; Reedy, J.; Millen, A.E.; Dixon, L.B.; Newby, P.K.; Tucker, K.L.; Krebs-Smith, S.M.; Guenther, P.M. Dietary patterns: Challenges and opportunities in dietary patterns research an Experimental Biology workshop, April 1, 2006. J. Am. Diet. Assoc. 2007, 107, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ward, M.H.; Graubard, B.I.; Heineman, E.F.; Markin, R.M.; Potischman, N.A.; Russell, R.M.; Weisenburger, D.D.; Tucker, K.L. Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am. J. Clin. Nutr. 2002, 75, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, A.; Barchitta, M.; Favara, G.; La Mastra, C.; La Rosa, M.C.; Magnano San Lio, R.; Agodi, A. The Application of Clustering on Principal Components for Nutritional Epidemiology: A Workflow to Derive Dietary Patterns. Nutrients 2022, 15, 195. [Google Scholar] [CrossRef]
- Maugeri, A.; Magnano San Lio, R.; La Rosa, M.C.; Giunta, G.; Panella, M.; Cianci, A.; Caruso, M.A.T.; Agodi, A.; Barchitta, M. The Relationship between Telomere Length and Gestational Weight Gain: Findings from the Mamma & Bambino Cohort. Biomedicines 2022, 10, 67. [Google Scholar]
- Magnano San Lio, R.; Maugeri, A.; La Rosa, M.C.; Cianci, A.; Panella, M.; Giunta, G.; Agodi, A.; Barchitta, M. The Impact of Socio-Demographic Factors on Breastfeeding: Findings from the “Mamma & Bambino” Cohort. Medicina 2021, 57, 103. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Magnano San Lio, R.; La Rosa, M.C.; La Mastra, C.; Favara, G.; Giunta, G.; Cianci, A.; Agodi, A. Vaccination Status of Mothers and Children from the ‘Mamma & Bambino’ Cohort. Vaccines 2021, 9, 168. [Google Scholar]
- Maugeri, A.; Barchitta, M.; Agrifoglio, O.; Favara, G.; La Mastra, C.; La Rosa, M.C.; Magnano San Lio, R.; Panella, M.; Cianci, A.; Agodi, A. The impact of social determinants and lifestyles on dietary patterns during pregnancy: Evidence from the “Mamma & Bambino” study. Ann. Ig 2019, 31, 81–89. [Google Scholar]
- Eveleth, P.B.; Andres, R.; Chumlea, W.C.; Eiben, O.; Ge, K.; Harris, T.; Heymsfield, S.B.; Launer, L.J.; Rosenberg, I.H.; Solomons, N.W.; et al. Uses and interpretation of anthropometry in the elderly for the assessment of physical status. Report to the Nutrition Unit of the World Health Organization: The Expert Subcommittee on the Use and Interpretation of Anthropometry in the Elderly. J. Nutr. Health Aging 1998, 2, 5–17. [Google Scholar]
- Institute of Medicine (US); National Research Council (US); Committee to Reexamine IOM Pregnancy Weight Guidelines. Weight Gain During Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Moore Simas, T.A.; Waring, M.E.; Sullivan, G.M.; Liao, X.; Rosal, M.C.; Hardy, J.R.; Berry, R.E. Institute of medicine 2009 gestational weight gain guideline knowledge: Survey of obstetrics/gynecology and family medicine residents of the United States. Birth 2013, 40, 237–246. [Google Scholar] [CrossRef]
- Lodato, F.; Araújo, J.; Barros, H.; Lopes, C.; Agodi, A.; Barchitta, M.; Ramos, E. Caffeine intake reduces sleep duration in adolescents. Nutr. Res. 2013, 33, 726–732. [Google Scholar] [CrossRef]
- Agodi, A.; Barchitta, M.; Quattrocchi, A.; Marchese, A.E.; Boffetta, P. Folate deficiency is not associated with increased mitochondrial genomic instability: Results from dietary intake and lymphocytic mtDNA 4977-bp deletion in healthy young women in Italy. Mutagenesis 2014, 29, 101–106. [Google Scholar] [CrossRef]
- Agodi, A.; Barchitta, M.; Quattrocchi, A.; Maugeri, A.; Canto, C.; Marchese, A.E.; Vinciguerra, M. Low fruit consumption and folate deficiency are associated with LINE-1 hypomethylation in women of a cancer-free population. Genes Nutr. 2015, 10, 480. [Google Scholar] [CrossRef]
- Barchitta, M.; Quattrocchi, A.; Adornetto, V.; Marchese, A.E.; Agodi, A. Tumor necrosis factor-alpha -308 G>A polymorphism, adherence to Mediterranean diet, and risk of overweight/obesity in young women. Biomed Res. Int. 2014, 2014, 742620. [Google Scholar] [CrossRef]
- Willet, W.; Stampfer, M.J. Total energy intake: Implications for epidemiologic analyses. Am. J. Epidemiol. 1986, 124, 17–27. [Google Scholar] [CrossRef]
- Magnano San Lio, R.; Barchitta, M.; Maugeri, A.; La Rosa, M.C.; Giunta, G.; Panella, M.; Cianci, A.; Galvani, F.; Pappalardo, E.; Ettore, G.; et al. The Impact of the COVID-19 Pandemic on Dietary Patterns of Pregnant Women: A Comparison between Two Mother-Child Cohorts in Sicily, Italy. Nutrients 2022, 14, 3380. [Google Scholar] [CrossRef]
- Ojeda-Granados, C.; Barchitta, M.; La Rosa, M.C.; La Mastra, C.; Roman, S.; Panduro, A.; Agodi, A.; Maugeri, A. Evaluating Dietary Patterns in Women from Southern Italy and Western Mexico. Nutrients 2022, 14, 1603. [Google Scholar] [CrossRef]
- Barchitta, M.; Maugeri, A.; Quattrocchi, A.; Barone, G.; Mazzoleni, P.; Catalfo, A.; De Guidi, G.; Iemmolo, M.G.; Crimi, N.; Agodi, A. Mediterranean Diet and Particulate Matter Exposure Are Associated With LINE-1 Methylation: Results From a Cross-Sectional Study in Women. Front. Genet. 2018, 9, 514. [Google Scholar] [CrossRef]
- Belahsen, R. Nutrition transition and food sustainability. Proc. Nutr. Soc. 2014, 73, 385–388. [Google Scholar] [CrossRef]
- Costacou, T.; Bamia, C.; Ferrari, P.; Riboli, E.; Trichopoulos, D.; Trichopoulou, A. Tracing the Mediterranean diet through principal components and cluster analyses in the Greek population. Eur. J. Clin. Nutr. 2003, 57, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Warden, B.A.; Paeratakul, S.; Bray, G.A. Healthy Eating Index and obesity. Eur. J. Clin. Nutr. 2004, 58, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Thiele, S.; Mensink, G.B.; Beitz, R. Determinants of diet quality. Public Health Nutr. 2004, 7, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Dallongeville, J.; Marecaux, N.; Cottel, D.; Bingham, A.; Amouyel, P. Association between nutrition knowledge and nutritional intake in middle-aged men from Northern France. Public Health Nutr. 2001, 4, 27–33. [Google Scholar] [CrossRef]
- Irala-Estevez, J.D.; Groth, M.; Johansson, L.; Oltersdorf, U.; Prattala, R.; Martinez-Gonzalez, M.A. A systematic review of socio-economic differences in food habits in Europe: Consumption of fruit and vegetables. Eur. J. Clin. Nutr. 2000, 54, 706–714. [Google Scholar] [CrossRef]
- Khoury, J.; Henriksen, T.; Christophersen, B.; Tonstad, S. Effect of a cholesterol-lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: A randomized clinical trial. Am. J. Obstet. Gynecol. 2005, 193, 1292–1301. [Google Scholar] [CrossRef]
- Shah, P.S. Parity and low birth weight and preterm birth: A systematic review and meta-analyses. Acta Obstet. Gynecol. Scand. 2010, 89, 862–875. [Google Scholar] [CrossRef]
- Fitzsimons, E.; Pongiglione, B. The impact of maternal employment on children’s weight: Evidence from the UK. SSM Popul. Health 2019, 7, 100333. [Google Scholar] [CrossRef]
- Poerksen, A.; Petitti, D.B. Employment and low birth weight in black women. Soc. Sci. Med. 1991, 33, 1281–1286. [Google Scholar] [CrossRef]
| Characteristics | Cluster 1 (n = 158) | Cluster 2 (n = 509) | p-Value a |
|---|---|---|---|
| Age (years) b | 32.0 (5.0) | 30.0 (7.0) | <0.001 |
| High educational level | 29.7% | 23.4% | 0.018 |
| Employed | 55.1% | 49.3% | 0.207 |
| Non-smoker | 94.9% | 89.8% | 0.055 |
| Primiparous | 46.5% | 52.5% | 0.191 |
| Total energy intake (kcal/day) b | 1567 (486) | 1749 (503) | <0.001 |
| Pre-pregnancy BMI (kg/m2) b | 23.5 (5.4) | 23.2 (5.9) | 0.373 |
| Pre-pregnancy BMI classification | |||
| Underweight | 5.7% | 5.3% | 0.965 |
| Normal weight | 58.6% | 60.9% | |
| Overweight | 22.3% | 21.2% | |
| Obese | 13.4% | 12.6% | |
| GWG (kg) b | 11.0 (8.3) | 12.0 (8.0) | 0.272 |
| GWG classification | |||
| Reduced | 42.9% | 37.2% | 0.289 |
| Adequate | 27.9% | 34.3% | |
| Excessive | 29.2% | 28.5% | |
| Gestational week at delivery (weeks) b | 39.0 (2.0) | 39.0 (2.0) | 0.489 |
| Preterm birth | 8.3% | 5.3% | 0.174 |
| Birth weight (kg) b | 3.2 (0.6) | 3.3 (0.6) | 0.171 |
| Birth length (cm) b | 50.0 (2.0) | 50.0 (2.0) | 0.233 |
| Characteristics | SGA | LGA | ||
|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | |
| Cluster 2 vs. Cluster 1 | 0.537 (0.262–1.104) | 0.091 | 2.213 (1.047–4.679) | 0.038 |
| Age (continuous) | 0.965 (0.894–1.041) | 0.356 | 0.955 (0.899–1.014) | 0.132 |
| Pre-pregnancy BMI (continuous) | 1.003 (0.939–1.071) | 0.934 | 1.107 (1.053–1.163) | <0.001 |
| GWG (continuous) | 0.966 (0.928–1.005) | 0.089 | 1.030 (0.997–1.064) | 0.075 |
| High educational level | 1.060 (0.617–1.821) | 0.834 | 1.154 (0.754–1.767) | 0.509 |
| Employed | 0.359 (0.168–0.769) | 0.008 | 0.745 (0.414–1.341) | 0.327 |
| Primiparous | 2.681 (1.293–5.558) | 0.008 | 0.980 (0.563–1.704) | 0.942 |
| Smoker | 1.841 (0.697–4.865) | 0.218 | 0.352 (0.102–1.214) | 0.098 |
| Total energy intake (continuous) | 1.000 (1.000–1.001) | 0.207 | 1.000 (1.000–1.001) | 0.474 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barchitta, M.; Magnano San Lio, R.; La Rosa, M.C.; La Mastra, C.; Favara, G.; Ferrante, G.; Galvani, F.; Pappalardo, E.; Ettore, C.; Ettore, G.; et al. The Effect of Maternal Dietary Patterns on Birth Weight for Gestational Age: Findings from the MAMI-MED Cohort. Nutrients 2023, 15, 1922. https://doi.org/10.3390/nu15081922
Barchitta M, Magnano San Lio R, La Rosa MC, La Mastra C, Favara G, Ferrante G, Galvani F, Pappalardo E, Ettore C, Ettore G, et al. The Effect of Maternal Dietary Patterns on Birth Weight for Gestational Age: Findings from the MAMI-MED Cohort. Nutrients. 2023; 15(8):1922. https://doi.org/10.3390/nu15081922
Chicago/Turabian StyleBarchitta, Martina, Roberta Magnano San Lio, Maria Clara La Rosa, Claudia La Mastra, Giuliana Favara, Giuliana Ferrante, Fabiola Galvani, Elisa Pappalardo, Carla Ettore, Giuseppe Ettore, and et al. 2023. "The Effect of Maternal Dietary Patterns on Birth Weight for Gestational Age: Findings from the MAMI-MED Cohort" Nutrients 15, no. 8: 1922. https://doi.org/10.3390/nu15081922
APA StyleBarchitta, M., Magnano San Lio, R., La Rosa, M. C., La Mastra, C., Favara, G., Ferrante, G., Galvani, F., Pappalardo, E., Ettore, C., Ettore, G., Agodi, A., & Maugeri, A. (2023). The Effect of Maternal Dietary Patterns on Birth Weight for Gestational Age: Findings from the MAMI-MED Cohort. Nutrients, 15(8), 1922. https://doi.org/10.3390/nu15081922







