A Tailored and Engaging mHealth Gamified Framework for Nutritional Behaviour Change
Abstract
:1. Background
Related Work
2. Materials and Methods
- It considers dietary guidelines regarding the consumption of key food groups.
- It contains a gamified system based on custom dietary missions.
- It has a pool of motivational recommendations for each mission.
- It is enhanced with a recommender system based on advanced AI techniques.
- It is based on the HAPA Model, a psychosocial theory of health behaviour change that describes how to persuade people to adopt new behaviours.
- It has a user interface properly designed to minimise the actions to be carried out by the user for the introduction of information.
- It is designed and implemented by an interdisciplinary team including nutritionists, data analysts, designers, and developers.
2.1. Behaviour Change Methodology
2.2. Dietary Assessment and User Profiling
2.3. Design of a Gamified Environment
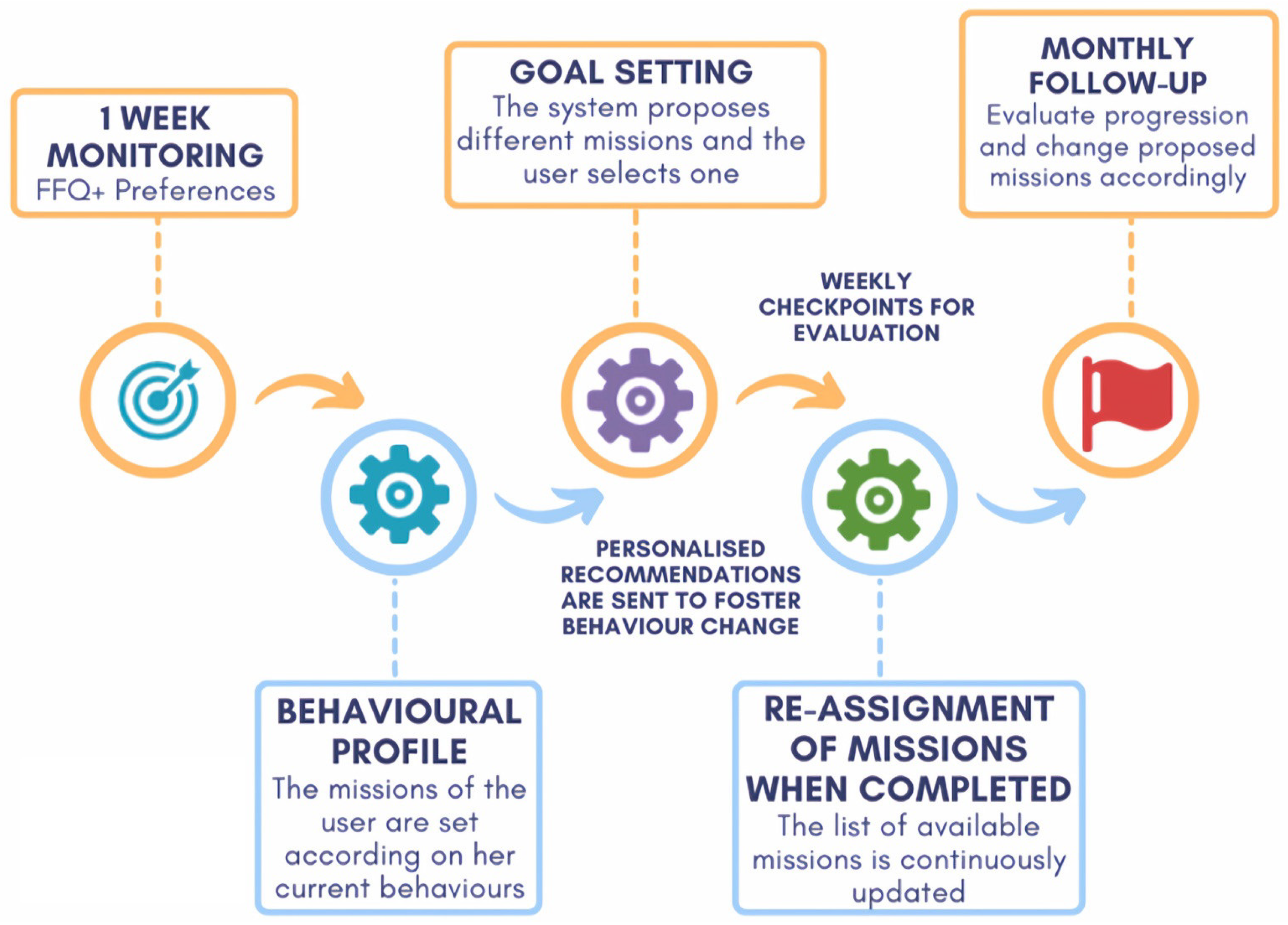
Mission Monitoring
2.4. The Nutritional Recommender System
2.4.1. Scoring System and Mission Selection
- a working memory (WM), which contains the objects representing the user profile derived from the analysis of the questionnaires;
- a rule base (RB) conformed to by all the rules needed to represent the expert’s knowledge and the actions to be triggered; and
- an inference engine (IE), which is a component used to deduce new knowledge from existing knowledge and rules.
2.4.2. The Nutritional Motivational Repository
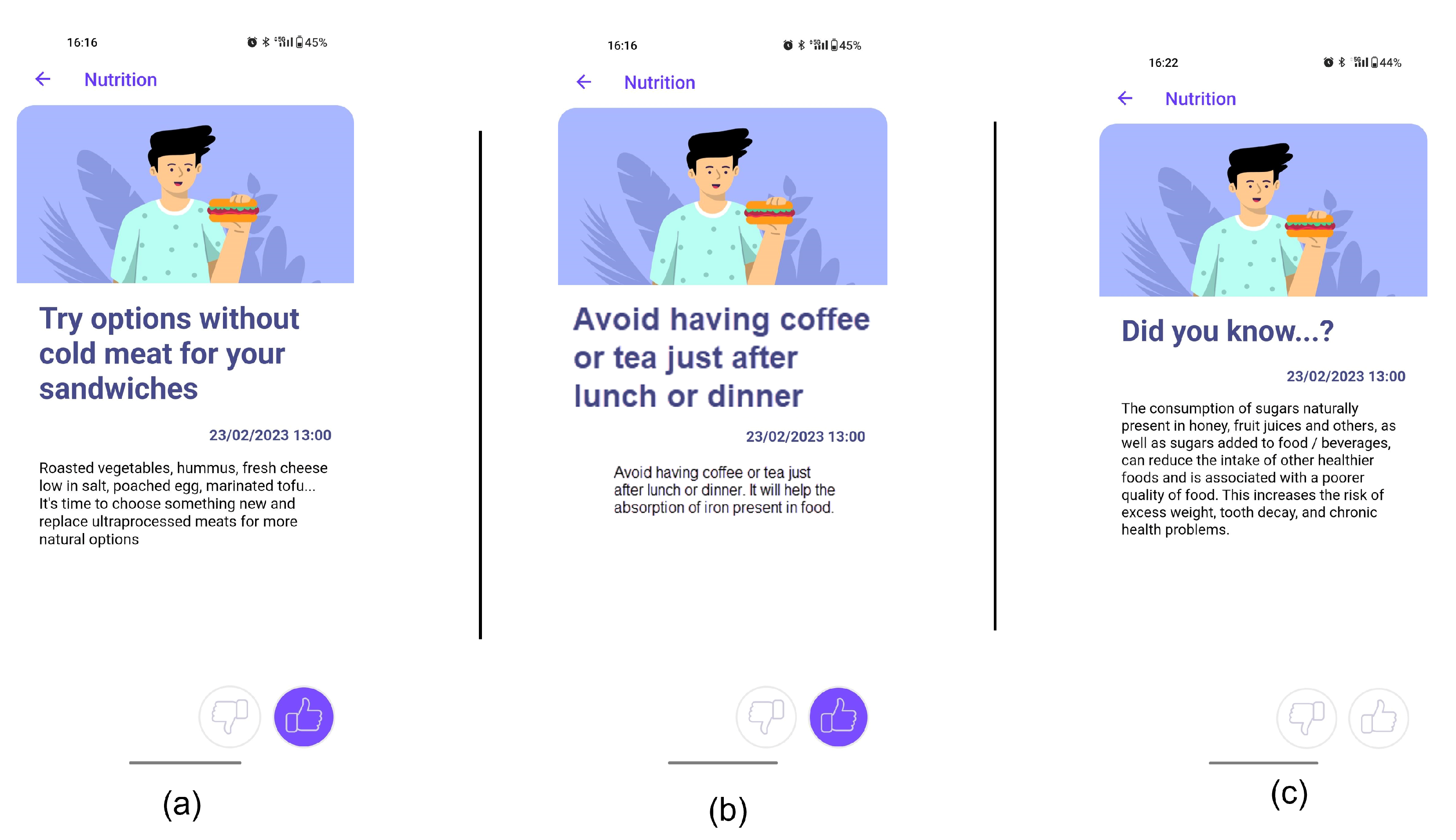
- Did you know…? tips: Some statements providing motivations for healthy eating were developed, including information about nutritional and health benefits or detriments associated with the consumption of food within each food group. This type of recommendation that enhances education in behavioural change is crucial for promoting long-term behaviour change. By providing individuals with clear and actionable information about the benefits and drawbacks of specific behaviours, they are better equipped to make informed decisions and adopt healthier habits. Furthermore, this type of education can help to dispel myths or misconceptions about healthy behaviours, which might have previously discouraged individuals from making positive changes.
- General recommendations for healthy eating: The recommender system also offers general advice on healthy dietary habits not associated with the missions. By providing tips and guidance on healthy cooking techniques, meal planning, and mindful eating, the system helps users to build skills and increase their knowledge about healthy behaviours. This can enhance motivation by providing users with a deeper understanding of the benefits of healthy eating to subsequently make healthy choices a regular part of their daily routine.
- Recommendations linked to missions: To help and motivate the user achieve the missions, a set of dietary and culinary recommendations was developed for each mission. Recommendations included tips, practical ideas, suggestions, and recipes (some of them linked to specific websites). In this context, a total of 390 recommendations were generated. All the recommendations were tagged according to their suitability to the different dietary preferences (vegan, gluten-free…), while some specific recommendations for each dietary preference were also included. Each of the recommendations was provided with an identifier, a general title, a sentence, and the associated food group and mission. Moreover, when relevant, some recommendations were assigned to a specific time.
3. Results
3.1. Design and Development of the User-Centred Mobile Application
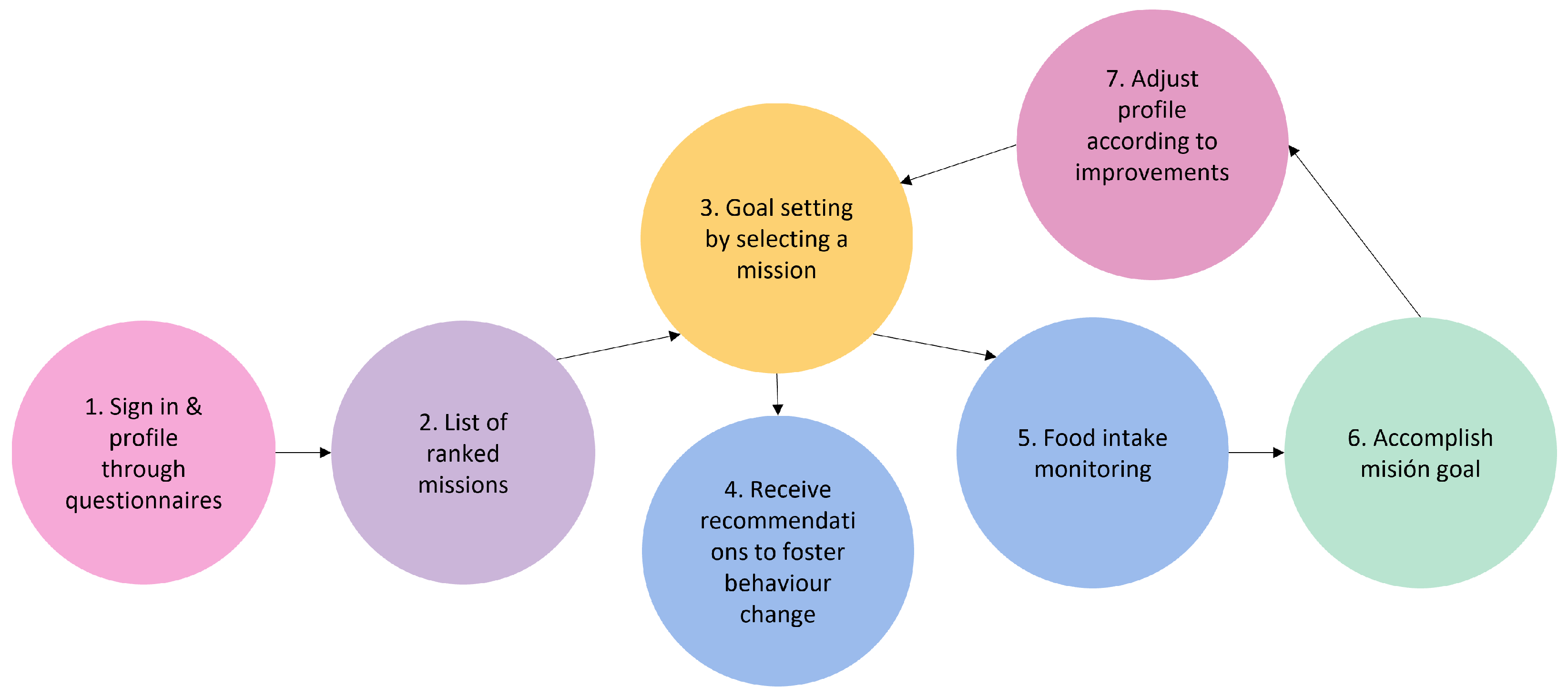
- 1.
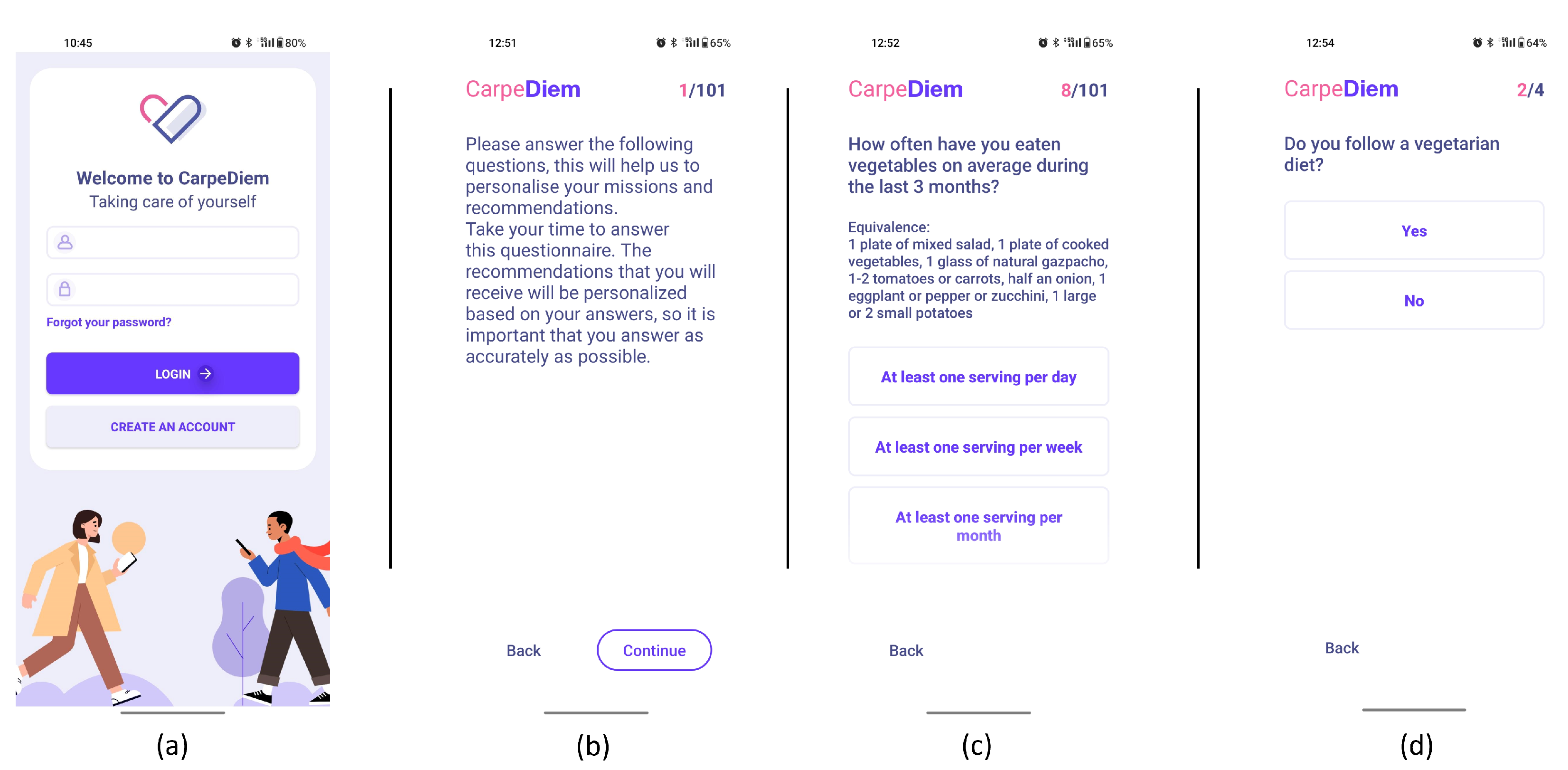
- Sign-in and profiling through questionnaires. After th uses signs in using the interface depicted in Figure 3-screenshot (a) and screen (b) appear to explain the value of responding to the following questionnaires. The user is then prompted to complete the modified FFQ (screenshot (c)) and dietary preference (screenshot (d)) questionnaires.
- 2.
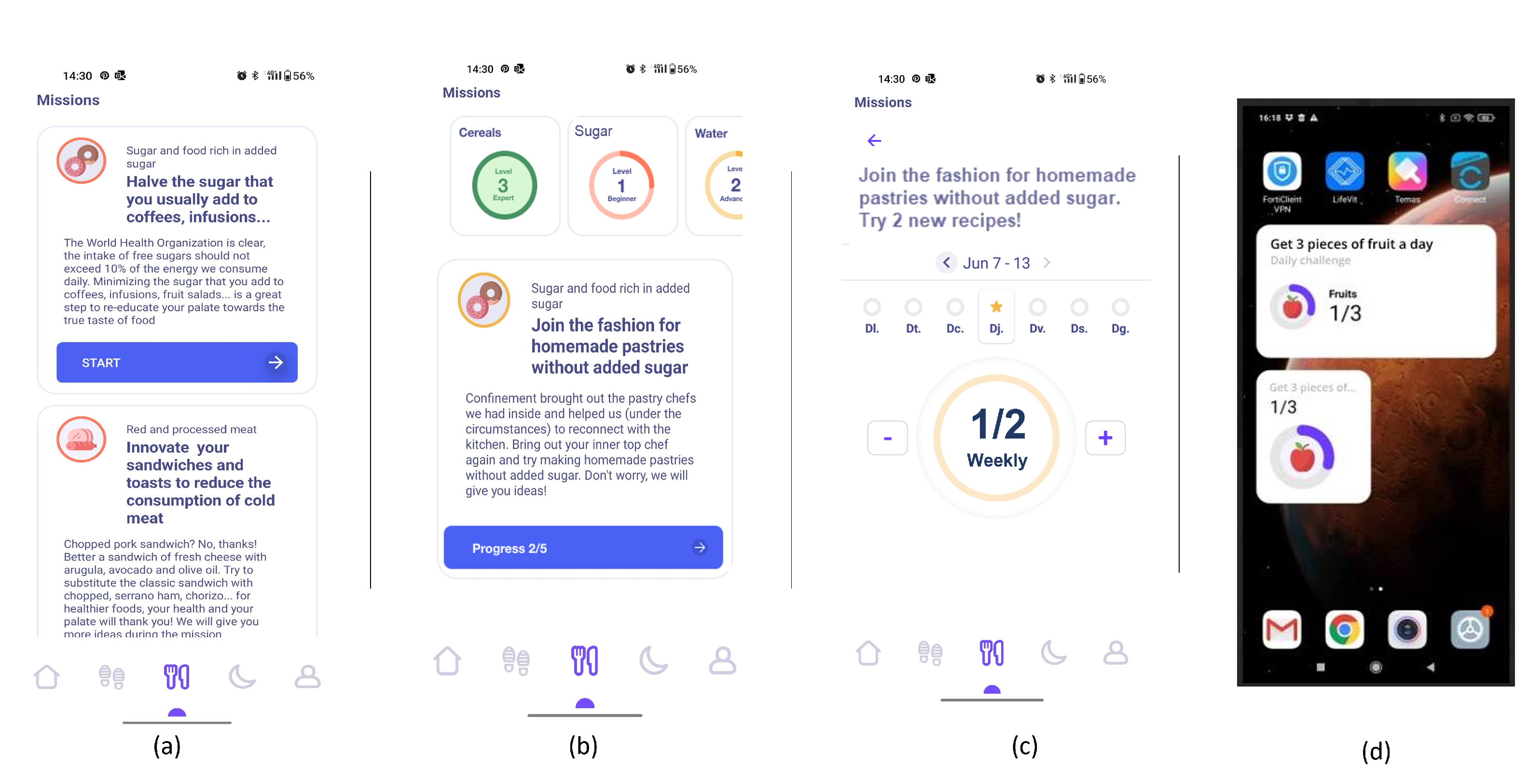
- List of ranked missions. The responses of the questionnaires are used by the NRS to propose to the user a rank of missions and the level that the user should work on. In Figure 4-screenshot (a) and (b), we show three missions that have been proposed to a user.
- 3.
- Goal setting by selecting a mission. When the user decides which mission he or she wants to start working on, the user presses the blue button shown in Figure 4-screenshot (a), and the intervention begins. If the user becomes blocked, bored, or simply wishes to change missions, this can be done at any time by just choosing another one.
- 4.
- Receipt of recommendations to foster behaviour change. Once there is a selected mission, the NRS starts sending recommendations to change the behaviour of the user. The most suitable recommendation for the user is selected from the Nutritional Motivational Repository. In Figure 5, we show the interface of three different types of recommendations. The recommendations, delivered to the user in the form of push notifications are helpful in reminding the user without being overly invasive. The number of recommendations received throughout the week was 3–4.
- 5.
- Food group intake monitoring. In Figure 4-screenshot (c), we show the interface used to log the food intake linked to the mission. In the example, the user just needs to press the + button when trying one new home-made pastry recipe proposed in the recommendations of the mission. By using widgets added to the main screen of their mobile phone (see Figure 4-screenshot (d)), the user can conveniently track their missions, minimising the number of actions they need to take and thus facilitating the monitoring of the mission.
- 6.
- Accomplish mission goal. When a mission is completed, the user is given the option to level up. It means that a higher-level mission from the same food group is proposed. If the use has already completed the highest level mission, the NRS suggests another mission from a different food group based on the results of the scoring system.
- 7.
- Profile adjustment according to improvements. Once missions are completed, the profile is modified in accordance with the improvements, resulting in tailored recommendations.
3.2. HAPA Phases and Strategy Implementation
3.2.1. Motivational Phase
- Risk perception: The system provides informative nutrition recommendations of the “did you know…?” type. Such recommendations enhance education by providing individuals with clear and actionable information about the benefits, drawbacks, and risks of specific behaviours, so they are better equipped to make informed decisions, increase awareness, and adopt healthier habits.
- Outcome expectancies: The system provides general recommendations not associated with the missions. This can enhance motivation by providing users with a deeper understanding of the benefits of healthy eating (healthy cooking techniques, meal planning, and mindful eating) to subsequently make healthy choices a regular part of their daily routine.
- Self-efficacy: When users starts a mission, the system sends several recommendations in a specific order to help them build the skills and knowledge required to achieve their behaviour change goal. This approach ensures that users have the necessary support and guidance to successfully make the desired change, which in turn can increase their confidence in their ability to modify their behaviour for the better.
- Intention formation: During a defined timeframe of one to two weeks, our system encourages users to focus solely on one food group at a time. By centring their efforts on modifying one specific aspect of their diet, users are less likely to feel overwhelmed. This approach allows individuals to make gradual and sustainable changes to their eating habits, without feeling like they have to overhaul their entire diet all at once.
3.2.2. Volitional Phase
- Planning: The system categorizes the user into one of three mission levels (beginner, advanced, or expert), each with a set of realistically achievable goals that progressively increase in complexity towards the final objective. This approach allows users to start with simpler goals that are appropriate for their skill level and gradually work toward more challenging ones.
- Coping planning: The system provides users with feedback in the form of notifications if they are not making progress toward completing their mission. These reminders are designed to help users stay on track and motivated to achieve their behaviour change goals. Additionally, the system recommends alternative missions and provides them with options to choose from. This approach allows users to select a mission that is better suited to their preferences and abilities, which can increase their chances of successfully achieving their desired behaviour change.
- Action control: The system monitors both adherence to the mission and objective adherence to changes in all food groups using a monthly adapted FFQ. By assessing changes in other food groups that may be indirectly affected by improvements in healthy habits, a more accurate track of the overall impact of behaviour change on an individual’s diet is achieved. This approach allows for the acquisition of a comprehensive understanding of how changes in one area of diet and behaviour can have a ripple effect on other areas, which can inform future recommendations and interventions.
- Feedback: The mission screen displays the user’s current level for each food group. Whenever the user completes a mission, the level for that particular food group changes accordingly. To acknowledge user progress, the user receives a congratulatory message upon levelling up.
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Wang, J. Modelling and prediction of global non-communicable diseases. BMC Public Health 2020, 20, 822. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diseases Fact Sheets. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 22 February 2023).
- Fritz, H.; Hu, Y.L.; Gahman, K.; Almacen, C.; Ottolini, J. Intervention to modify habits: A scoping review. OTJR Occup. Particip. Health 2020, 40, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Simeon, R.; Dewidar, O.; Trawin, J.; Duench, S.; Manson, H.; Pardo Pardo, J.; Petkovic, J.; Hatcher Roberts, J.; Tugwell, P.; Yoganathan, M.; et al. Behavior change techniques included in reports of social media interventions for promoting health behaviors in adults: Content analysis within a systematic review. J. Med. Internet Res. 2020, 22, e16002. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, M.; Rahimi, A.; Zare-Farashbandi, F.; Alavi-Naeini, A.M.; Daei, A. Transtheoretical model of health behavioral change: A systematic review. Iran. J. Nurs. Midwifery Res. 2019, 24, 83. [Google Scholar] [PubMed]
- Bandura, A. Health promotion by social cognitive means. Health Educ. Behav. 2004, 31, 143–164. [Google Scholar] [CrossRef] [PubMed]
- Green, E.C.; Murphy, E.M.; Gryboski, K. The health belief model. In The Wiley Encyclopedia of Health Psychology; Jossey-Bass: Hoboken, NJ, USA, 2020; pp. 211–214. [Google Scholar]
- Schwarzer, R.; Lippke, S.; Luszczynska, A. Mechanisms of health behavior change in persons with chronic illness or disability: The Health Action Process Approach (HAPA). Rehabil. Psychol. 2011, 56, 161. [Google Scholar] [CrossRef]
- Dugas, M.; Gao, G.; Agarwal, R. Unpacking mHealth interventions: A systematic review of behavior change techniques used in randomized controlled trials assessing mHealth effectiveness. Digit. Health 2020, 6, 2055207620905411. [Google Scholar] [CrossRef]
- Rowland, S.P.; Fitzgerald, J.E.; Holme, T.; Powell, J.; McGregor, A. What is the clinical value of mHealth for patients? NPJ Digit. Med. 2020, 3, 4. [Google Scholar] [CrossRef]
- Villinger, K.; Wahl, D.R.; Boeing, H.; Schupp, H.T.; Renner, B. The effectiveness of app-based mobile interventions on nutrition behaviours and nutrition-related health outcomes: A systematic review and meta-analysis. Obes. Rev. 2019, 20, 1465–1484. [Google Scholar] [CrossRef]
- Scarry, A.; Rice, J.; O’Connor, E.M.; Tierney, A.C. Usage of Mobile Applications or Mobile Health Technology to Improve Diet Quality in Adults. Nutrients 2022, 14, 2437. [Google Scholar] [CrossRef]
- Cheung, K.L.; Durusu, D.; Sui, X.; de Vries, H. How recommender systems could support and enhance computer-tailored digital health programs: A scoping review. Digit. Health 2019, 5, 2055207618824727. [Google Scholar] [CrossRef] [PubMed]
- Woolford, S.J.; Clark, S.J.; Strecher, V.J.; Resnicow, K. Tailored mobile phone text messages as an adjunct to obesity treatment for adolescents. J. Telemed. Telecare 2010, 16, 458–461. [Google Scholar] [CrossRef]
- Gómez-Martínez, M.; Orte, S.; Ros-Freixedes, L.; Seif, K.; Vargiu, E. Empowering the Citizen in the Main Pillars of Health by Using IoT. In Proceedings of the International Conference on Wearables in Healthcare, Virtual, 10–11 December 2020; Springer: Berlin/Heidelberg, Germany, 2020; pp. 37–53. [Google Scholar]
- Orte, S.; Migliorelli, C.; Sistach-Bosch, L.; Subías-Beltrán, P.; Fritzsche, P.C.; Galofré, M.; Gómez-Martínez, M.; Miralles, F.; Marí, D.; Ribas, V. BECOME: A Modular Recommender System for Coaching and Promoting Empowerment in Healthcare; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Canuto, R.; Garcez, A.; de Souza, R.V.; Kac, G.; Olinto, M.T.A. Nutritional intervention strategies for the management of overweight and obesity in primary health care: A systematic review with meta-analysis. Obes. Rev. 2021, 22, e13143. [Google Scholar] [CrossRef] [PubMed]
- Duncanson, K.; Shrewsbury, V.; Burrows, T.; Chai, L.; Ashton, L.; Taylor, R.; Gow, M.; Ho, M.; Ells, L.; Stewart, L.; et al. Impact of weight management nutrition interventions on dietary outcomes in children and adolescents with overweight or obesity: A systematic review with meta-analysis. J. Hum. Nutr. Diet. 2021, 34, 147–177. [Google Scholar] [CrossRef] [PubMed]
- Hood, K.K.; Hilliard, M.; Piatt, G.; Ievers-Landis, C.E. Effective strategies for encouraging behavior change in people with diabetes. Diabetes Manag. 2015, 5, 499. [Google Scholar] [CrossRef]
- Whelan, M.E.; Denton, F.; Bourne, C.L.; Kingsnorth, A.P.; Sherar, L.B.; Orme, M.W.; Esliger, D.W. A digital lifestyle behaviour change intervention for the prevention of type 2 diabetes: A qualitative study exploring intuitive engagement with real-time glucose and physical activity feedback. BMC Public Health 2021, 21, 130. [Google Scholar] [CrossRef]
- Taylor, S.R.; Falcone, J.N.; Cantley, L.C.; Goncalves, M.D. Developing dietary interventions as therapy for cancer. Nat. Rev. Cancer 2022, 22, 452–466. [Google Scholar] [CrossRef]
- Freire, R. Scientific evidence of diets for weight loss: Different macronutrient composition, intermittent fasting, and popular diets. Nutrition 2020, 69, 110549. [Google Scholar] [CrossRef]
- Wei, S.; Li, C.; Wang, Z.; Chen, Y. Nutritional strategies for intervention of diabetes and improvement of β-cell function. Biosci. Rep. 2023, 43, BSR20222151. [Google Scholar] [CrossRef]
- Parr, E.B.; Devlin, B.L.; Lim, K.H.; Moresi, L.N.; Geils, C.; Brennan, L.; Hawley, J.A. Time-restricted eating as a nutrition strategy for individuals with type 2 diabetes: A feasibility study. Nutrients 2020, 12, 3228. [Google Scholar] [CrossRef]
- Ungersboeck, M.; Tang, X.; Neeff, V.; Steele, D.; Grimm, P.; Fenech, M. Personalised Nutritional Recommendations Based on Individual Post-Prandial Glycaemic Responses Improve Glycaemic Metrics and PROMs in Patients with Type 2 Diabetes: A Real-World Assessment. Nutrients 2022, 14, 2123. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.; Harnack, L.; Pereira, M.A. Assessment of the accuracy of nutrient calculations of five popular nutrition tracking applications. Public Health Nutr. 2018, 21, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Messer, M.; McClure, Z.; Norton, B.; Smart, M.; Linardon, J. Using an app to count calories: Motives, perceptions, and connections to thinness-and muscularity-oriented disordered eating. Eat. Behav. 2021, 43, 101568. [Google Scholar] [CrossRef] [PubMed]
- McCaig, D.; Elliott, M.T.; Prnjak, K.; Walasek, L.; Meyer, C. Engagement with MyFitnessPal in eating disorders: Qualitative insights from online forums. Int. J. Eat. Disord. 2020, 53, 404–411. [Google Scholar] [CrossRef]
- Höchsmann, C.; Martin, C.K. Review of the validity and feasibility of image-assisted methods for dietary assessment. Int. J. Obes. 2020, 44, 2358–2371. [Google Scholar] [CrossRef]
- Tahir, G.A.; Loo, C.K. A Comprehensive Survey of Image-Based Food Recognition and Volume Estimation Methods for Dietary Assessment. Healthcare 2021, 9, 1676. [Google Scholar] [CrossRef]
- Del Caño, R.; Saha, T.; Moonla, C.; De la Paz, E.; Wang, J. Ketone bodies detection: Wearable and mobile sensors for personalized medicine and nutrition. TrAC Trends Anal. Chem. 2023, 159, 116938. [Google Scholar] [CrossRef]
- Sempionatto, J.R.; Montiel, V.R.V.; Vargas, E.; Teymourian, H.; Wang, J. Wearable and mobile sensors for personalized nutrition. ACS Sensors 2021, 6, 1745–1760. [Google Scholar] [CrossRef]
- de Catalunya, G. “Small Changes to Eat Better”. 2020. Available online: https://canalsalut.gencat.cat/ca/vida-saludable/alimentacio/petits-canvis-menjar-millor/index.html#googtrans(ca|en) (accessed on 22 February 2023).
- Schwarzer, R. Health action process approach (HAPA). In Gesundheitspsychologie von A bis Z; Hogrefe: Gottingen, Germany, 2002; pp. 241–245. [Google Scholar]
- Afshin, A.; Sur, P.J.; Fay, K.A.; Cornaby, L.; Ferrara, G.; Salama, J.S.; Mullany, E.C.; Abate, K.H.; Abbafati, C.; Abebe, Z.; et al. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Larncet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Bentham, J.; Di Cesare, M.; BIlano, V.; Boddy, L.M. Worldwide trends in children’s and adolescents’ body mass index, underweight and obesity, in comparison with adults, from 1975 to 2016: A pooled analysis of 2,416 population-based measurement studies with 128.9 million participants. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef]
- Mills, K.T.; Stefanescu, A.; He, J. The global epidemiology of hypertension. Nat. Rev. Nephrol. 2020, 16, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, A.G.; Boateng, E.B. Beyond 2020: Modelling obesity and diabetes prevalence. Diabetes Res. Clin. Pract. 2020, 167, 108362. [Google Scholar] [CrossRef] [PubMed]
- Pineda, E.; Sanchez-Romero, L.M.; Brown, M.; Jaccard, A.; Jewell, J.; Galea, G.; Webber, L.; Breda, J. Forecasting future trends in obesity across Europe: The value of improving surveillance. Obes. Facts 2018, 11, 360–371. [Google Scholar] [CrossRef]
- Cockwell, P.; Fisher, L.A. The global burden of chronic kidney disease. Lancet 2020, 395, 662–664. [Google Scholar] [CrossRef] [PubMed]
- Batko, K.; Ślęzak, A. The use of Big Data Analytics in healthcare. J. Big Data 2022, 9, 3. [Google Scholar] [CrossRef] [PubMed]
- Rising, C.J.; Jensen, R.E.; Moser, R.P.; Oh, A. Characterizing the US population by patterns of mobile health use for health and behavioral tracking: Analysis of the National Cancer Institute’s health information national trends survey data. J. Med. Internet Res. 2020, 22, e16299. [Google Scholar] [CrossRef]
- Odone, A.; Buttigieg, S.; Ricciardi, W.; Azzopardi-Muscat, N.; Staines, A. Public health digitalization in Europe: EUPHA vision, action and role in digital public health. Eur. J. Public Health 2019, 29, 28–35. [Google Scholar] [CrossRef]
- Benjamins, J.; Haveman-Nies, A.; Gunnink, M.; Goudkuil, A.; De Vet, E. How the use of a patient-accessible health record contributes to patient-centered care: Scoping review. J. Med. Internet Res. 2021, 23, e17655. [Google Scholar] [CrossRef]
- Clark, N.; Becker, M.; Janz, N.; Lorig, K.; Rakowski, W.; Anderson, L. Self-management of chronic disease: A review and questions for research. J. Aging Health 1991, 3, 27. [Google Scholar] [CrossRef]
- Adams, R.J. Improving health outcomes with better patient understanding and education. In Risk Management and Healthcare Policy; Taylor & Francis: Abingdon, UK, 2010; pp. 61–72. [Google Scholar]
- Grimani, A.; Aboagye, E.; Kwak, L. The effectiveness of workplace nutrition and physical activity interventions in improving productivity, work performance and workability: A systematic review. BMC Public Health 2019, 19, 1676. [Google Scholar] [CrossRef]
- Wang, Z.; Pang, Y.; Liu, J.; Wang, J.; Xie, Z.; Huang, T. Association of healthy lifestyle with cognitive function among Chinese older adults. Eur. J. Clin. Nutr. 2021, 75, 325–334. [Google Scholar] [CrossRef] [PubMed]
| Mission Name | Description | Type of Users |
|---|---|---|
| Nuts Level 1: Fill your pantry with nuts | They are an ideal snack and still go unnoticed by most people. Nuts, such as walnuts, almonds, hazelnuts, pistachios, and cashews, will surprise you with their beneficial properties. Fibre, healthy fats, proteins, vitamins, minerals, and other protective substances make up this great food | Users who consume 0/1/2 servings of nuts per week |
| Nuts Level 2: Incorporate nuts into your diet at least 3 days a week | Did you know that despite the fact that nuts are rich in calories, their consumption has not been associated with weight gain? Therefore, you should not worry and incorporate them as part of a daily balanced diet. A daily handful is a very good place to start. You can add them in the bowl of yogurt, put them in salads, and eat them as an afternoon snack or in the middle of the morning; the possibilities are endless! | Users who consume 0/1/2 servings of nuts per week and have already completed Nuts Level 1 |
| Nuts Level 3: Be creative and try new recipes that incorporate nuts | It’s time to go a little further and test your culinary abilities. Try experimenting with new culinary preparations if you’re bored of having a handful of nuts with yogurt or as a mid-morning snack. Your creativity is the only limit! | Users who consume more than 3 servings of nuts per week |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orte, S.; Migliorelli, C.; Sistach-Bosch, L.; Gómez-Martínez, M.; Boqué, N. A Tailored and Engaging mHealth Gamified Framework for Nutritional Behaviour Change. Nutrients 2023, 15, 1950. https://doi.org/10.3390/nu15081950
Orte S, Migliorelli C, Sistach-Bosch L, Gómez-Martínez M, Boqué N. A Tailored and Engaging mHealth Gamified Framework for Nutritional Behaviour Change. Nutrients. 2023; 15(8):1950. https://doi.org/10.3390/nu15081950
Chicago/Turabian StyleOrte, Silvia, Carolina Migliorelli, Laura Sistach-Bosch, Meritxell Gómez-Martínez, and Noemi Boqué. 2023. "A Tailored and Engaging mHealth Gamified Framework for Nutritional Behaviour Change" Nutrients 15, no. 8: 1950. https://doi.org/10.3390/nu15081950
APA StyleOrte, S., Migliorelli, C., Sistach-Bosch, L., Gómez-Martínez, M., & Boqué, N. (2023). A Tailored and Engaging mHealth Gamified Framework for Nutritional Behaviour Change. Nutrients, 15(8), 1950. https://doi.org/10.3390/nu15081950





