Effect of Lacticaseibacillus paracasei N1115 on Immunomodulatory and Gut Microbial Composition in Young Children: A Randomized, Placebo-Controlled Study
Abstract
:1. Introduction
2. Materials and Methods
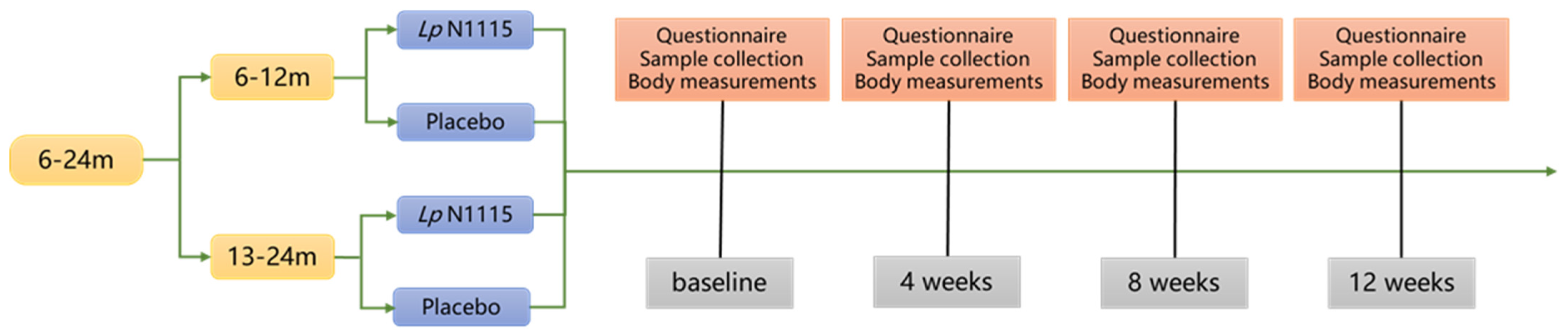
2.1. Study Design
2.2. Participants
2.3. Biological Sample Collection and Detection
2.3.1. Saliva Sample Collection
2.3.2. Fecal Sample Collection
2.4. Sample Measurement and Sequencing
2.5. Statistical Analysis
3. Results
3.1. Study Population
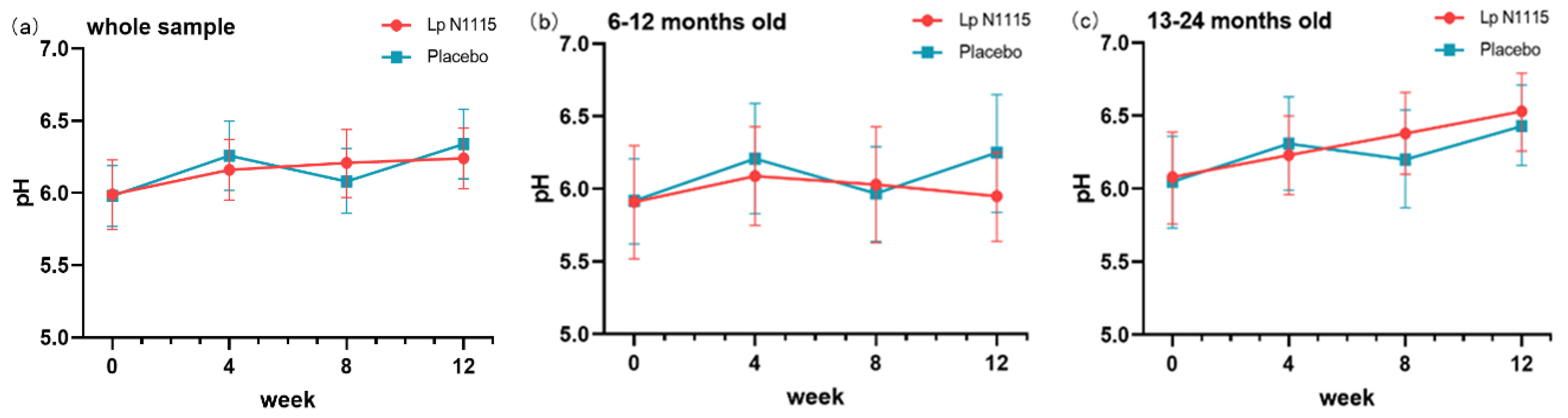
3.2. Fecal pH
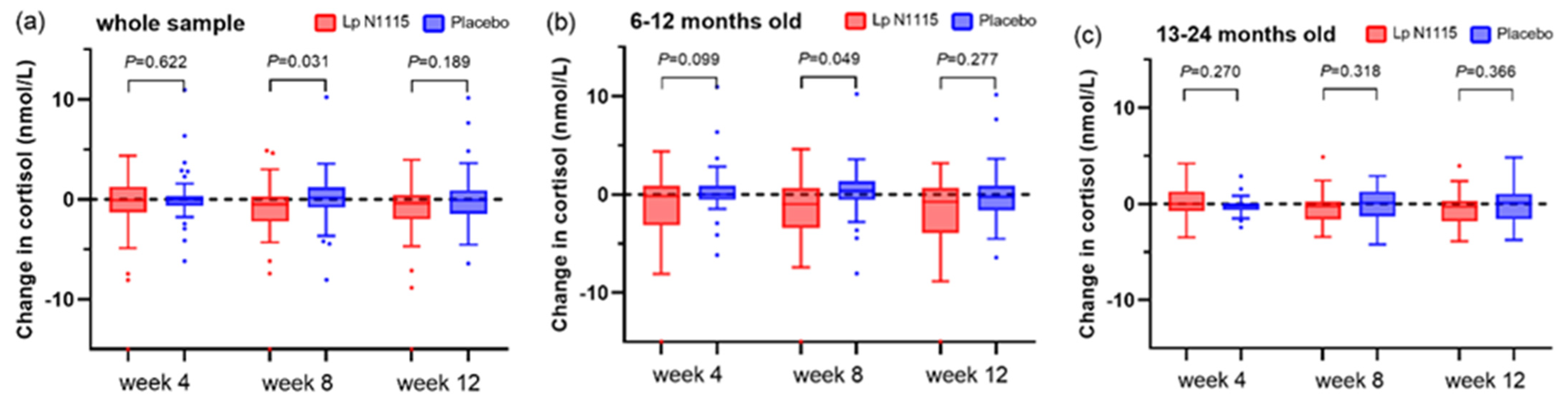
3.3. Saliva Cortisol
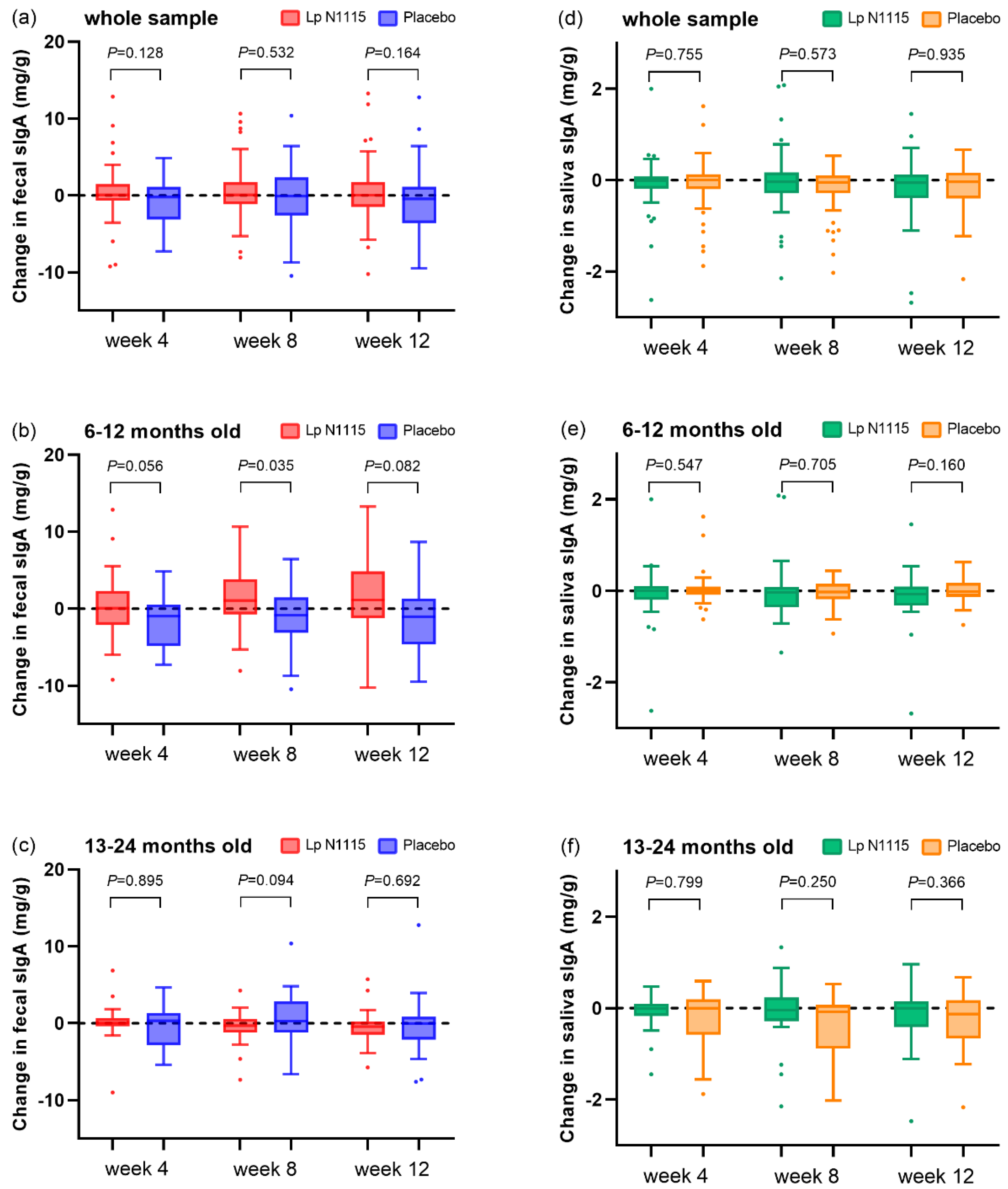
3.4. sIgA
3.5. FC and AAT
3.6. Gut Microbiota
3.6.1. Species Distribution
3.6.2. α and β Diversity
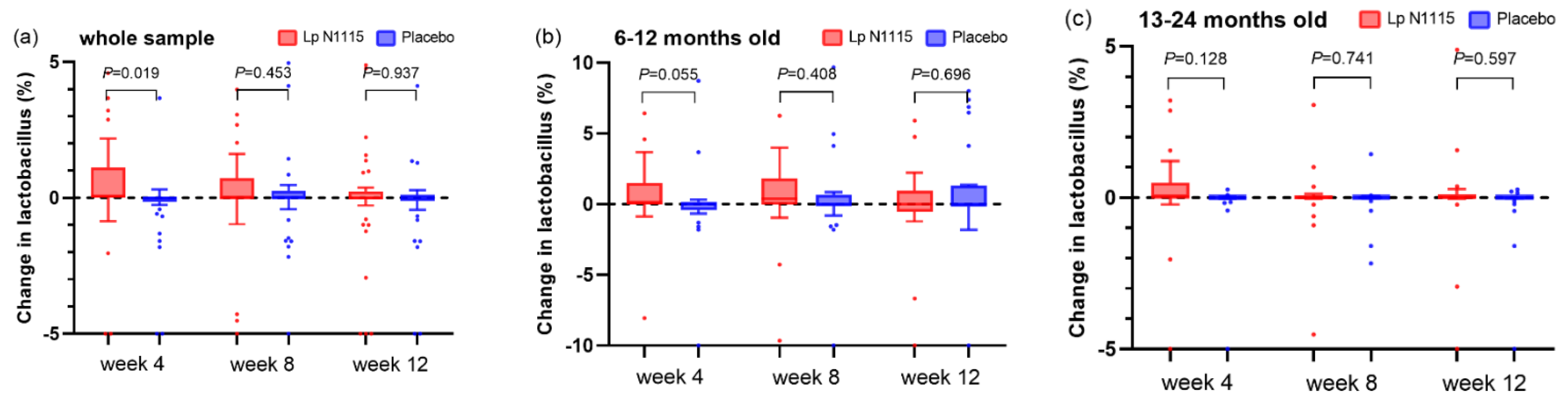
3.6.3. Fecal Lactobacillus
3.7. Correlation between Fecal Lactobacillus and Biochemical Indexes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, H.T.; Hellerstein, S.; Zhou, Y.B.; Liu, J.M.; Blustein, J. Trends in Cesarean Delivery Rates in China, 2008–2018. JAMA 2020, 323, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Gut instincts: Microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 2017, 595, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.E.; Goldacre, R.; Moore, H.C.; Zeltzer, J.; Knight, M.; Morris, C.; Nowell, S.; Wood, R.; Carter, K.W.; Fathima, P.; et al. Mode of birth and risk of infection-related hospitalisation in childhood: A population cohort study of 7.17 million births from 4 high-income countries. PLoS Med. 2020, 17, e1003429. [Google Scholar] [CrossRef]
- Bekem, Ö.; Günay, İ.; Çelik, F.; Apa, H. Interaction of functional gastrointestinal disorders with postpartum conditions related to mother and baby. Turk. J. Pediatr. 2021, 63, 461–470. [Google Scholar] [CrossRef]
- Pan, K.; Zhang, C.; Tian, J. The Effects of Different Modes of Delivery on the Structure and Predicted Function of Intestinal Microbiota in Neonates and Early Infants. Pol. J. Microbiol. 2021, 70, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Moya-Pérez, A.; Luczynski, P.; Renes, I.B.; Wang, S.; Borre, Y.; Anthony Ryan, C.; Knol, J.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Intervention strategies for cesarean section-induced alterations in the microbiota-gut-brain axis. Nutr. Rev. 2017, 75, 225–240. [Google Scholar] [CrossRef]
- Hoang, D.M.; Levy, E.I.; Vandenplas, Y. The impact of Caesarean section on the infant gut microbiome. Acta Paediatr. 2021, 110, 60–67. [Google Scholar] [CrossRef]
- Korpela, K.; de Vos, W.M. Early life colonization of the human gut: Microbes matter everywhere. Curr. Opin. Microbiol. 2018, 44, 70–78. [Google Scholar] [CrossRef]
- Cheng, J.; Ringel-Kulka, T.; Heikamp-de Jong, I.; Ringel, Y.; Carroll, I.; de Vos, W.M.; Salojärvi, J.; Satokari, R. Discordant temporal development of bacterial phyla and the emergence of core in the fecal microbiota of young children. ISME J. 2016, 10, 1002–1014. [Google Scholar] [CrossRef]
- Francavilla, R.; Cristofori, F.; Tripaldi, M.E.; Indrio, F. Intervention for Dysbiosis in Children Born by C-Section. Ann. Nutr. Metab. 2018, 73 (Suppl. S3), 33–39. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, A.; Villa, C.R.; Comelli, E.M. Probiotics in early life: A preventative and treatment approach. Food Funct. 2016, 7, 1752–1768. [Google Scholar] [CrossRef] [PubMed]
- Delacour, D.; Salomon, J.; Robine, S.; Louvard, D. Plasticity of the brush border—The yin and yang of intestinal homeostasis. Nat. Review. Gastroenterol. Hepatol. 2016, 13, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Ali, S.A. Probiotics and gut microbiota: Mechanistic insights into gut immune homeostasis through TLR pathway regulation. Food Funct. 2022, 13, 7423–7447. [Google Scholar] [CrossRef] [PubMed]
- Sakai, F.; Hosoya, T.; Ono-Ohmachi, A.; Ukibe, K.; Ogawa, A.; Moriya, T.; Kadooka, Y.; Shiozaki, T.; Nakagawa, H.; Nakayama, Y.; et al. Lactobacillus gasseri SBT2055 induces TGF-β expression in dendritic cells and activates TLR2 signal to produce IgA in the small intestine. PLoS ONE 2014, 9, e105370. [Google Scholar] [CrossRef] [PubMed]
- Deputy, M.; Devanaboina, R.; Al Bakir, I.; Burns, E.; Faiz, O. The role of faecal calprotectin in the diagnosis of inflammatory bowel disease. BMJ 2023, 380, e068947. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, Y.; Zhao, W.; Chen, J.; Maas, K.; Hussain, N.; Henderson, W.A.; Cong, X. Trends of fecal calprotectin levels and associations with early life experience in preterm infants. Interdiscip. Nurs. Res. 2022, 1, 36–42. [Google Scholar] [CrossRef]
- Zhang, X.; Ostrov, D.A.; Tian, H. Alpha-1 antitrypsin: A novel biomarker and potential therapeutic approach for metabolic diseases. Clin. Chim. Acta; Int. J. Clin. Chem. 2022, 534, 71–76. [Google Scholar] [CrossRef]
- Castanet, M.; Costalos, C.; Haiden, N.; Hascoet, J.M.; Berger, B.; Sprenger, N.; Grathwohl, D.; Brüssow, H.; De Groot, N.; Steenhout, P.; et al. Early Effect of Supplemented Infant Formulae on Intestinal Biomarkers and Microbiota: A Randomized Clinical Trial. Nutrients 2020, 12, 1484. [Google Scholar] [CrossRef]
- Viljanen, M.; Kuitunen, M.; Haahtela, T.; Juntunen-Backman, K.; Korpela, R.; Savilahti, E. Probiotic effects on faecal inflammatory markers and on faecal IgA in food allergic atopic eczema/dermatitis syndrome infants. Pediatr. Allergy Immunol. Off. Publ. Eur. Soc. Pediatr. Allergy Immunol. 2005, 16, 65–71. [Google Scholar] [CrossRef]
- Fatheree, N.Y.; Liu, Y.; Ferris, M.; Van Arsdall, M.; McMurtry, V.; Zozaya, M.; Cai, C.; Rahbar, M.H.; Hessabi, M.; Vu, T.; et al. Hypoallergenic formula with Lactobacillus rhamnosus GG for babies with colic: A pilot study of recruitment, retention, and fecal biomarkers. World J. Gastrointest. Pathophysiol. 2016, 7, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Łubiech, K.; Twarużek, M. Lactobacillus Bacteria in Breast Milk. Nutrients 2020, 12, 3783. [Google Scholar] [CrossRef] [PubMed]
- Szajewska, H.; Kołodziej, M.; Gieruszczak-Białek, D.; Skórka, A.; Ruszczyński, M.; Shamir, R. Systematic review with meta-analysis: Lactobacillus rhamnosus GG for treating acute gastroenteritis in children—A 2019 update. Aliment. Pharmacol. Ther. 2019, 49, 1376–1384. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, H.; He, F.; Luo, Y.; Kang, Z.; Lu, C.; Feng, L.; Lu, X.; Xue, Y.; Wang, H. Whole Genome Sequence of the Probiotic Strain Lactobacillus paracasei N1115, Isolated from Traditional Chinese Fermented Milk. Genome Announc. 2014, 2, e00059-14. [Google Scholar] [CrossRef]
- Xun, Y.; Yan, F.; Zhu, H.; Feng, L.; Zhang, D.; Xue, Y.; He, F.; Wang, S. Oral administration of Lactobacillus paracasei N1115 on neonatal mice prevents the intestinal inflammation in adulthood. Lett. Appl. Microbiol. 2022, 75, 330–337. [Google Scholar] [CrossRef]
- Dang, C.; Zhao, K.; Xun, Y.; Feng, L.; Zhang, D.; Cui, L.; Cui, Y.; Jia, X.; Wang, S. In vitro Intervention of Lactobacillus paracasei N1115 Can Alter Fecal Microbiota and Their SCFAs Metabolism of Pregnant Women with Constipation and Diarrhea. Curr. Microbiol. 2022, 79, 212. [Google Scholar] [CrossRef]
- Pu, F.; Guo, Y.; Li, M.; Zhu, H.; Wang, S.; Shen, X.; He, M.; Huang, C.; He, F. Yogurt supplemented with probiotics can protect the healthy elderly from respiratory infections: A randomized controlled open-label trial. Clin. Interv. Aging 2017, 12, 1223–1231. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xun, Y.; Ahern, G.J.; Feng, L.; Zhang, D.; Xue, Y.; Ross, R.P.; Doolan, A.M.; Stanton, C.; Zhu, H. A randomized, double blind, parallel, placebo-controlled study to investigate the efficacy of Lactobacillus paracasei N1115 in gut development of young children. Food Sci. Nutr. 2021, 9, 6020–6030. [Google Scholar] [CrossRef]
- Ren, Z.; Zhao, A.; Zhang, J.; Yang, C.; Zhong, W.; Mao, S.; Wang, S.; Yuan, Q.; Wang, P.; Zhang, Y. Safety and tolerance of Lacticaseibacillus paracasei N1115 in caesarean-born young children: A randomised, placebo-controlled trial. Benef. Microbes 2022, 13, 205–219. [Google Scholar] [CrossRef]
- Mohan, R.; Koebnick, C.; Schildt, J.; Mueller, M.; Radke, M.; Blaut, M. Effects of Bifidobacterium lactis Bb12 supplementation on body weight, fecal pH, acetate, lactate, calprotectin, and IgA in preterm infants. Pediatr. Res. 2008, 64, 418–422. [Google Scholar] [CrossRef]
- Holscher, H.D.; Czerkies, L.A.; Cekola, P.; Litov, R.; Benbow, M.; Santema, S.; Alexander, D.D.; Perez, V.; Sun, S.; Saavedra, J.M.; et al. Bifidobacterium lactis Bb12 enhances intestinal antibody response in formula-fed infants: A randomized, double-blind, controlled trial. JPEN. J. Parenter. Enter. Nutr. 2012, 36, 106s–117s. [Google Scholar] [CrossRef] [PubMed]
- Abildgaard, A.; Elfving, B.; Hokland, M.; Wegener, G.; Lund, S. Probiotic treatment reduces depressive-like behaviour in rats independently of diet. Psychoneuroendocrinology 2017, 79, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.T.; Jiang, Y.; Zilioli, S. Momentary emotions and salivary cortisol: A systematic review and meta-analysis of ecological momentary assessment studies. Neurosci. Biobehav. Rev. 2021, 125, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Brett, B.E.; Koko, B.K.; Doumbia, H.O.Y.; Koffi, F.K.; Assa, S.E.; Zahé, K.; Faye-Ketté, H.; Kati-Coulibaly, S.; Kort, R.; Sybesma, W.; et al. Salivary biomarkers of stress and inflammation in first graders in Côte d’Ivoire: Effects of a probiotic food intervention. Psychoneuroendocrinology 2021, 129, 105255. [Google Scholar] [CrossRef]
- Brandtzaeg, P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Ann. N. Y. Acad. Sci. 2007, 1098, 288–311. [Google Scholar] [CrossRef]
- Gleeson, M.; Cripps, A.W. Development of mucosal immunity in the first year of life and relationship to sudden infant death syndrome. FEMS Immunol. Med. Microbiol. 2004, 42, 21–33. [Google Scholar] [CrossRef]
- Xiao, L.; Gong, C.; Ding, Y.; Ding, G.; Xu, X.; Deng, C.; Ze, X.; Malard, P.; Ben, X. Probiotics maintain intestinal secretory immunoglobulin A levels in healthy formula-fed infants: A randomised, double-blind, placebo-controlled study. Benef. Microbes 2019, 10, 729–739. [Google Scholar] [CrossRef]
- Baglatzi, L.; Gavrili, S.; Stamouli, K.; Zachaki, S.; Favre, L.; Pecquet, S.; Benyacoub, J.; Costalos, C. Effect of Infant Formula Containing a Low Dose of the Probiotic Bifidobacterium lactis CNCM I-3446 on Immune and Gut Functions in C-Section Delivered Babies: A Pilot Study. Clin. Med. Insights. Pediatr. 2016, 10, 11–19. [Google Scholar] [CrossRef]
- Grech, A.; Collins, C.E.; Holmes, A.; Lal, R.; Duncanson, K.; Taylor, R.; Gordon, A. Maternal exposures and the infant gut microbiome: A systematic review with meta-analysis. Gut Microbes 2021, 13, 1897210. [Google Scholar] [CrossRef]
- Davis, E.C.; Dinsmoor, A.M.; Wang, M.; Donovan, S.M. Microbiome Composition in Pediatric Populations from Birth to Adolescence: Impact of Diet and Prebiotic and Probiotic Interventions. Dig. Dis. Sci. 2020, 65, 706–722. [Google Scholar] [CrossRef]
- Korpela, K.; Salonen, A.; Vepsäläinen, O.; Suomalainen, M.; Kolmeder, C.; Varjosalo, M.; Miettinen, S.; Kukkonen, K.; Savilahti, E.; Kuitunen, M.; et al. Probiotic supplementation restores normal microbiota composition and function in antibiotic-treated and in caesarean-born infants. Microbiome 2018, 6, 182. [Google Scholar] [CrossRef] [PubMed]
- Martín-Peláez, S.; Cano-Ibáñez, N.; Pinto-Gallardo, M.; Amezcua-Prieto, C. The Impact of Probiotics, Prebiotics, and Synbiotics during Pregnancy or Lactation on the Intestinal Microbiota of Children Born by Cesarean Section: A Systematic Review. Nutrients 2022, 14, 341. [Google Scholar] [CrossRef] [PubMed]

| Lp N1115 (n = 51) | Placebo (n = 50) | |
|---|---|---|
| Gender, n (%) | ||
| Male | 24 (47.1) | 24 (48.0) |
| Female | 27 (52.9) | 26 (52.0) |
| Age at enrolment, n (%) | ||
| 6–12 months | 25 (49.0) | 25 (50.0) |
| 13–24 months | 26 (51.0) | 25 (50.0) |
| Solid food intake, n (%) | ||
| Yes | 48 (94.1) | 45 (90.0) |
| No | 3 (5.9) | 5 (10.0) |
| Feeding type within 6 months after birth, n (%) | ||
| Exclusive breastfeeding | 29 (56.9) | 26 (52.0) |
| Mixed feeding | 21 (41.2) | 23 (46.0) |
| Formula feeding | 1 (2.0) | 1 (2.0) |
| Body measurements, mean ± SD | ||
| HAZ | 0.30 ± 0.85 | 0.32 ± 1.02 |
| WAZ | 0.32 ± 1.46 | −0.01 ± 1.24 |
| HCZ | −0.37 ± 1.34 | −0.44 ± 1.27 |
| Fecal pH, mean ± SD | 6.16 ± 0.75 | 6.26 ± 0.84 |
| Biochemical indicators at baseline, median (25th, 75th) | ||
| Saliva cortisol, nmol/L | 2.12 (1.14, 3.44) | 1.54 (1.02, 2.64) |
| Saliva sIgA, mg/g | 0.39 (0.24, 0.74) | 0.35 (0.13, 0.78) |
| Fecal sIgA, mg/g | 2.93 (1.42, 5.65) | 3.36 (1.33, 7.31) |
| Calprotectin, mg/g | 0.03 (0.02, 0.10) | 0.02 (0.01, 0.09) |
| α1-antitrypsin, mg/g | 0.34 (0.27, 0.46) | 0.33 (0.25, 0.40) |
| Species | Weeks | Lp N1115 Group, Median (25th, 75th) | Control Group, Median (25th, 75th) | p a |
|---|---|---|---|---|
| Firmicutes | 0 | 42.4 (30.0, 57.6) | 49.9 (35.4, 66.1) | 0.120 |
| 4 | 47.5 (30.8, 60.1) | 55.3 (34.6, 68.6) | 0.092 | |
| 8 | 41.6 (30.7, 55.1) | 51.2 (40.4, 68.1) | 0.010 | |
| 12 | 44.0 (31.9, 58.7) | 49.5 (39.3, 62.8) | 0.150 | |
| Bacteroidetes | 0 | 21.4 (0.03, 35.1) | 13.3 (0.1, 31.6) | 0.984 |
| 4 | 16.5 (0.03, 37.6) | 12.0 (30.0, 57.6) | 0.994 | |
| 8 | 20.0 (0.05, 45.3) | 23.5 (0.9, 36.0) | 0.676 | |
| 12 | 23.1 (0.13, 40.1) | 26.8 (3.5, 40.7) | 0.534 | |
| F/B | 0 | 2.4 (1.2, 528.2) | 5.7 (1.5, 396.6) | 0.753 |
| 4 | 3.1 (1.1, 363.0) | 5.2 (1.5, 197.9) | 0.726 | |
| 8 | 2.7 (0.8, 554.4) | 2.2 (1.2, 74.6) | 0.699 | |
| 12 | 2.0 (1.1, 147.7) | 2.0 (0.9, 18.2) | 0.632 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Ren, Z.; Zhou, J.; Zhao, A.; Wang, S.; Xun, Y.; Jiang, H.; Wang, P.; Yuan, Q.; Zhang, Y. Effect of Lacticaseibacillus paracasei N1115 on Immunomodulatory and Gut Microbial Composition in Young Children: A Randomized, Placebo-Controlled Study. Nutrients 2023, 15, 1970. https://doi.org/10.3390/nu15081970
Li P, Ren Z, Zhou J, Zhao A, Wang S, Xun Y, Jiang H, Wang P, Yuan Q, Zhang Y. Effect of Lacticaseibacillus paracasei N1115 on Immunomodulatory and Gut Microbial Composition in Young Children: A Randomized, Placebo-Controlled Study. Nutrients. 2023; 15(8):1970. https://doi.org/10.3390/nu15081970
Chicago/Turabian StyleLi, Pin, Zhongxia Ren, Junxiu Zhou, Ai Zhao, Shijie Wang, Yiping Xun, Hua Jiang, Peiyu Wang, Qingbin Yuan, and Yumei Zhang. 2023. "Effect of Lacticaseibacillus paracasei N1115 on Immunomodulatory and Gut Microbial Composition in Young Children: A Randomized, Placebo-Controlled Study" Nutrients 15, no. 8: 1970. https://doi.org/10.3390/nu15081970
APA StyleLi, P., Ren, Z., Zhou, J., Zhao, A., Wang, S., Xun, Y., Jiang, H., Wang, P., Yuan, Q., & Zhang, Y. (2023). Effect of Lacticaseibacillus paracasei N1115 on Immunomodulatory and Gut Microbial Composition in Young Children: A Randomized, Placebo-Controlled Study. Nutrients, 15(8), 1970. https://doi.org/10.3390/nu15081970






