Design and Implementation of a Time-Restricted Eating Intervention in a Randomized, Controlled Eating Study
Abstract
1. Introduction
2. Materials and Methods
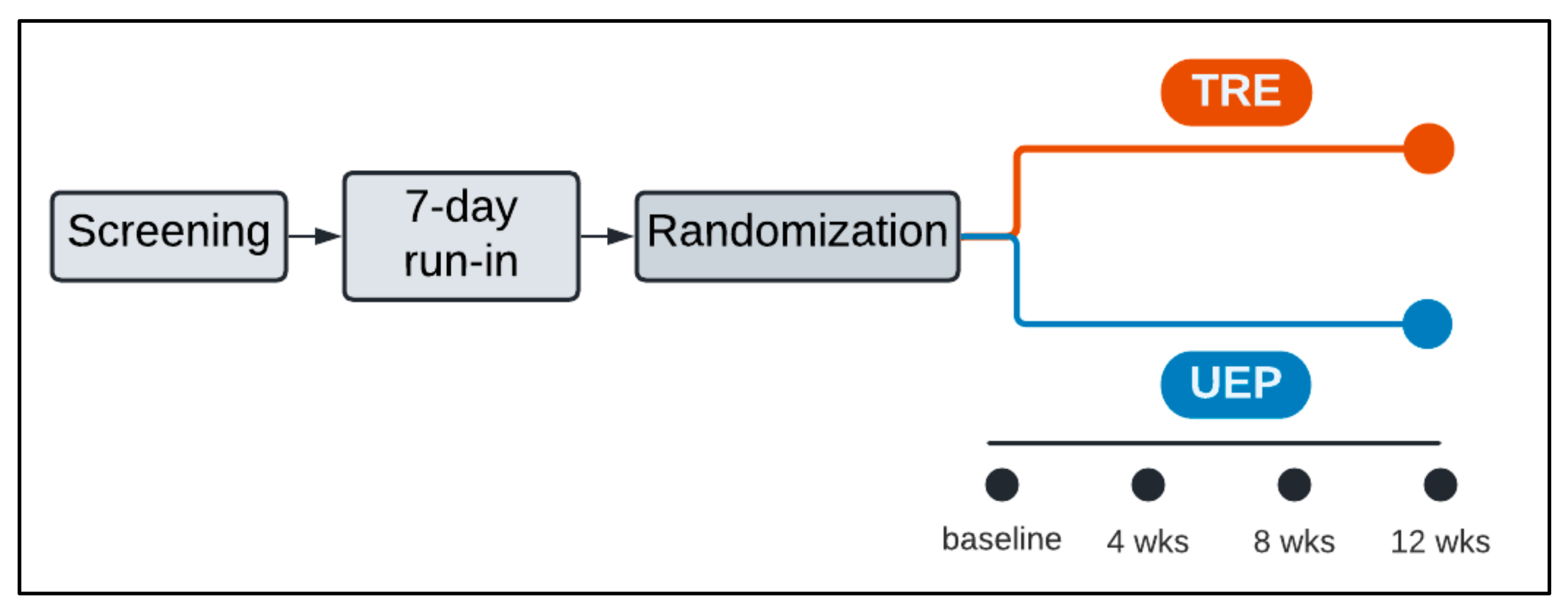
2.1. Overall Design
2.2. Screening and Run-In
2.3. Randomization
2.4. Assessment of Daily Caloric Needs
2.5. Diet Composition and Menu Planning
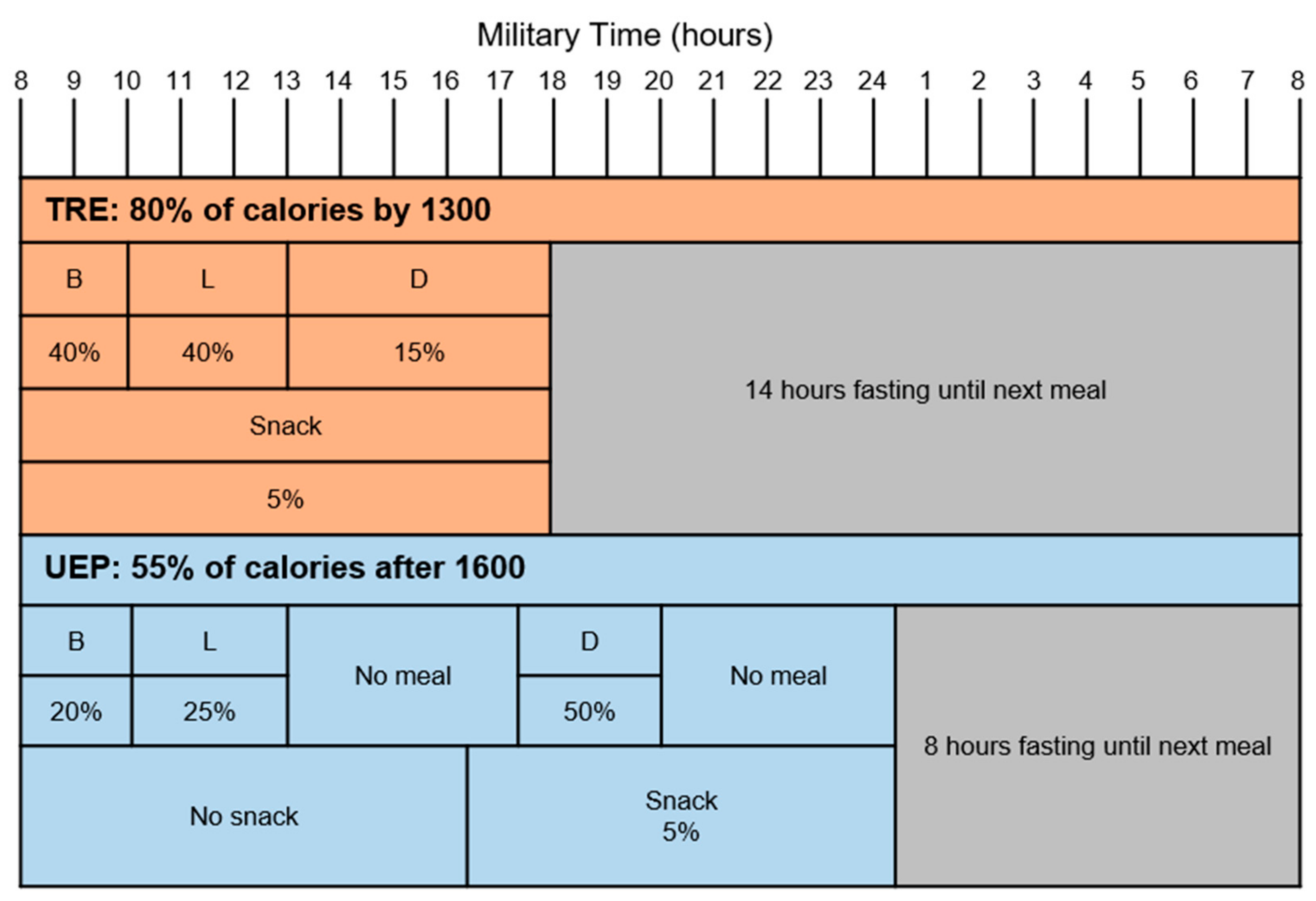
2.6. Timing of Eating
2.7. Controlled Eating
2.8. Staffing
2.9. Methods for Adherence and Retention
2.10. Sample Size
3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hatori, M.; Vollmers, C.; Zarrinpar, A.; DiTacchio, L.; Bushong, E.A.; Gill, S.; Leblanc, M.; Chaix, A.; Joens, M.; Fitzpatrick, J.A.; et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, J.; Hoddy, K.K.; Jambazian, P.; Varady, K.A. Time-restricted feeding and risk of metabolic disease: A review of human and animal studies. Nutr. Rev. 2014, 72, 308–318. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K.; American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; et al. Meal timing and frequency: Implications for cardiovascular disease prevention: A scientific statement from the American Heart Association. Circulation 2017, 135, e96–e121. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Cioffi, I.; Evangelista, A.; Ponzo, V.; Goitre, I.; Ciccone, G.; Ghigo, E.; Bo, S. Effects of time-restricted feeding on body weight and metabolism. A systematic review and meta-analysis. Rev. Endocr. Metab. Disord. 2020, 21, 17–33. [Google Scholar] [CrossRef]
- Moran-Ramos, S.; Baez-Ruiz, A.; Buijs, R.M.; Escobar, C. When to eat? the influence of circadian rhythms on metabolic health: Are animal studies providing the evidence? Nutr. Res. Rev. 2016, 29, 180–193. [Google Scholar] [CrossRef]
- Poggiogalle, E.; Jamshed, H.; Peterson, C.M. Circadian regulation of glucose, lipid, and energy metabolism in humans. Metabolism 2018, 84, 11–27. [Google Scholar] [CrossRef]
- Stote, K.S.; Baer, D.J.; Spears, K.; Paul, D.R.; Harris, G.K.; Rumpler, W.V.; Strycula, P.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am. J. Clin. Nutr. 2007, 85, 981–988. [Google Scholar] [CrossRef]
- Morgan, L.M.; Shi, J.W.; Hampton, S.M.; Frost, G. Effect of meal timing and glycaemic index on glucose control and insulin secretion in healthy volunteers. Br. J. Nutr. 2012, 108, 1286–1291. [Google Scholar] [CrossRef]
- Bandín, C.; Scheer, F.A.; Luque, A.J.; Ávila-Gandía, V.; Zamora, S.; Madrid, J.A.; Gómez-Abellán, P.; Garaulet, M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int. J. Obes. 2015, 39, 828–833. [Google Scholar] [CrossRef]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018, 27, 1212–1221. [Google Scholar] [CrossRef]
- Ravussin, E.; Beyl, R.A.; Poggiogalle, E.; Hsia, D.S.; Peterson, C.M. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity 2019, 27, 1244–1254. [Google Scholar] [CrossRef] [PubMed]
- Jakubowicz, D.; Barnea, M.; Wainstein, J.; Froy, O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity 2013, 21, 2504–2512. [Google Scholar] [CrossRef] [PubMed]
- Moro, T.; Tinsley, G.; Bianco, A.; Marcolin, G.; Pacelli, Q.F.; Battaglia, G.; Palma, A.; Gentil, P.; Neri, M.; Paoli, A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J. Transl. Med. 2016, 14, 290. [Google Scholar] [CrossRef] [PubMed]
- Tinsley, G.M.; Moore, M.L.; Graybeal, A.J.; Paoli, A.; Kim, Y.; Gonzales, J.U.; Harry, J.R.; VanDusseldorp, T.A.; Kennedy, D.N.; Cruz, M.R. Time-restricted feeding plus resistance training in active females: A randomized trial. Am. J. Clin. Nutr. 2019, 110, 628–640. [Google Scholar] [CrossRef]
- Hutchison, A.T.; Regmi, P.; Manoogian, E.N.C.; Fleischer, J.G.; Wittert, G.A.; Panda, S.; Heilbronn, L.K. Time-restricted feeding improves glucose tolerance in men at risk for type 2 diabetes: A randomized crossover trial. Obesity 2019, 27, 724–732. [Google Scholar] [CrossRef]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time-restricted eating effects on body composition and metabolic measures in human, who are overweight: A feasibility study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Ruddick-Collins, L.C.; Morgan, P.J.; Fyfe, C.L.; Filipe, J.A.N.; Horgan, G.W.; Westerterp, K.R.; Johnston, J.D.; Johnstone, A.M. Timing of daily calorie loading affects appetite and hunger responses without changes in energy metabolism in healthy subjects with obesity. Cell Metab. 2022, 34, 1472–1485. [Google Scholar] [CrossRef]
- Jamshed, H.; Steger, F.L.; Bryan, D.R.; Richman, J.S.; Warriner, A.H.; Hanick, C.J.; Martin, C.K.; Salvy, S.; Peterson, C.M. Effectiveness of Early Time-Restricted Eating for Weight Loss, Fat Loss, and Cardiometabolic Health in Adults with Obesity: A Randomized Clinical Trial. JAMA Intern. Med. 2022, 182, 953–962. [Google Scholar] [CrossRef]
- Liu, D.; Huang, Y.; Huang, C.; Yang, S.; Wei, X.; Zhang, P.; Guo, D.; Lin, J.; Xu, B.; Li, C.; et al. Calorie Restriction with or without Time-Restricted Eating in Weight Loss. N. Engl. J. Med. 2022, 386, 1495–1504. [Google Scholar] [CrossRef]
- Thomas, E.A.; Zaman, A.; Sloggett, K.J.; Steinke, S.; Grau, L.; Catenacci, V.A.; Cornier, M.A.; Rynders, C.A. Early time-restricted eating compared with daily caloric restriction: A randomized trial in adults with obesity. Obesity 2022, 30, 1027–1038. [Google Scholar] [CrossRef]
- Andriessen, C.; Fealy, C.E.; Veelen, A.; van Beek, S.M.M.; Roumans, K.H.M.; Connell, N.J.; Mevenkamp, J.; Moonen-Kornips, E.; Havekes, B.; Schrauwen-Hinderling, V.B.; et al. Three weeks of time-restricted eating improves glucose homeostasis in adults with type 2 diabetes but does not improve insulin sensitivity: A randomised crossover trial. Diabetologia 2022, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lowe, D.A.; Wu, N.; Rohdin-Bibby, L.; Moore, A.H.; Kelly, N.; Liu, Y.E.; Philip, E.; Vittinghoff, E.; Heymsfield, S.B.; Olgin, J.E.; et al. Effects of Time-Restricted Eating on Weight Loss and Other Metabolic Parameters in Women and Men with Overweight and Obesity: The TREAT Randomized Clinical Trial. JAMA Intern. Med. 2020, 180, 1491–1499. [Google Scholar] [CrossRef]
- Allison, K.C.; Hopkins, C.M.; Ruggieri, M.; Spaeth, A.M.; Ahima, R.S.; Zhang, Z.; Taylor, D.M.; Goel, N. Prolonged, Controlled Daytime versus Delayed Eating Impacts Weight and Metabolism. Curr. Biol. 2021, 31, 650–657. [Google Scholar] [CrossRef]
- Appel, L.J.; Moore, T.J.; Obarzanek, E.; Vollmer, W.M.; Svetkey, L.P.; Sacks, F.M.; Bray, G.A.; Vogt, T.M.; Cutler, J.A.; Windhauser, M.M.; et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH collaborative research group. N. Engl. J. Med. 1997, 336, 1117–1124. [Google Scholar] [CrossRef]
- Yun, J.S.; Ko, S.H. Current trends in epidemiology of cardiovascular disease and cardiovascular risk management in type 2 diabetes. Metabolism 2021, 123, 154838. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- De Saint Jeor, S.; Bryan, G.T. Clinical research diets: Definition of terms. J. Am. Diet. Assoc. 1973, 62, 47–51. [Google Scholar] [CrossRef]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- The IPAQ Group. International Physical Activity Questionnaire. Available online: https://sites.google.com/site/theipaq/ (accessed on 23 February 2021).
- Cheng, H.L. A Simple, Easy-to-Use Spreadsheet for Automatic Scoring of the International Physical Activity Questionnaire (IPAQ) Short Form; ResearchGate: Berlin, Germany, 2016. [Google Scholar] [CrossRef]
- Appel, L.J.; Sacks, F.M.; Carey, V.J.; Obarzanek, E.; Swain, J.F.; Miller, E.R., 3rd; Conlin, P.R.; Erlinger, T.P.; Rosner, B.A.; Laranjo, N.M.; et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: Results of the OmniHeart randomized trial. JAMA 2005, 294, 2455–2464. [Google Scholar] [CrossRef]
- Sacks, F.M.; Carey, V.J.; Anderson, C.A.; Miller, E.R., 3rd; Copeland, T.; Charleston, J.; Harshfield, B.J.; Laranjo, N.; McCarron, P.; Swain, J.; et al. Effects of high vs low glycemic index of dietary carbohydrate on cardiovascular disease risk factors and insulin sensitivity: The OmniCarb randomized clinical trial. JAMA 2014, 312, 2531–2541. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.A.; Cobb, L.K.; Miller, E.R., 3rd; Woodward, M.; Hottenstein, A.; Chang, A.R.; Mongraw-Chaffin, M.; White, K.; Charleston, J.; Tanaka, T.; et al. Effects of a behavioral intervention that emphasizes spices and herbs on adherence to recommended sodium intake: Results of the SPICE randomized clinical trial. Am. J. Clin. Nutr. 2015, 102, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Windhauser, M.M.; Plaisted, C.S.; Hoben, K.P.; McCullough, M.L.; Obarzanek, E. The linear index model for establishing nutrient goals in the dietary approaches to stop hypertension trial. DASH collaborative research group. J. Am. Diet. Assoc. 1999, 99 (Suppl. S8), 40. [Google Scholar] [CrossRef] [PubMed]
- Roseland, J.M.; Nguyen, Q.A.; Williams, J.R.; Patterson, K.Y.; Showell, B.; Pehrsson, P.R. USDA Table of Cooking Yields for Meat and Poultry; USDA Agricultural Research Service: Washington, DC, USA, 2017. [Google Scholar] [CrossRef]
- Gill, S.; Panda, S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015, 22, 789–798. [Google Scholar] [CrossRef]
- Kant, A.K.; Graubard, B.I. Within-person comparison of eating behaviors, time of eating, and dietary intake on days with and without breakfast: NHANES 2005–2010. Am. J. Clin. Nutr. 2015, 102, 661–670. [Google Scholar] [CrossRef]
- Wu, B.; White, K.; Maw, M.T.T.; Charleston, J.; Zhao, D.; Guallar, E.; Appel, L.J.; Clark, J.M.; Maruthur, N.M.; Pilla, S.J. Adherence to Diet and Meal Timing in a Randomized Controlled Feeding Study of Time-Restricted Feeding. Nutrients 2022, 14, 2283. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R., 3rd; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the dietary approaches to stop hypertension (DASH) diet. DASH-sodium collaborative research group. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar] [CrossRef]
- Committee on Diet and Health, National Research Council. Diet and Health: Implications for Reducing Chronic Disease Risk. 1989; ISBN 0-309-58831-6. Available online: https://www.ncbi.nlm.nih.gov/books/NBK218743/pdf/Bookshelf_NBK218743.pdf (accessed on 14 February 2021).
| 1600 kcal | 2000 kcal | 2500 kcal | 3000 kcal | 3500 kcal | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diet Component | Target | Mean (SD) | Target | Mean (SD) | Target | Mean (SD) | Target | Mean (SD) | Target | Mean (SD) |
| Calories, kcal | 1600 | 1616.2 (13.4) | 2000 | 2010.3 (18.5) | 2500 | 2507.2 (20.3) | 3000 | 3001.4 (30.7) | 3500 | 3499.0 (9.3) |
| Protein, %kcal | 15–18 | 17.3 (1.0) | 15–18 | 16.6 (0.4) | 15–18 | 17.7 (1.2) | 15–18 | 16.8 (1.0) | 15–18 | 17.2 (0.6) |
| Carbohydrate, %kcal | 45–50 | 45.3 (1.2) | 45–50 | 46.1 (1.4) | 45–50 | 45.1 (1.3) | 45–50 | 46.1 (1.3) | 45–50 | 46.5 (1.3) |
| Fat, %kcal | 32–37 | 37.3 (0.9) | 32–37 | 37.3 (1.4) | 32–37 | 37.2 (1.7) | 32–37 | 37.0 (0.9) | 32–37 | 36.3 (1.2) |
| Saturated | <10 | 7.0 (1.6) | <10 | 6.7 (1.3) | <10 | 6.9 (1.7) | <10 | 6.8 (1.6) | <10 | 6.8 (1.3) |
| Calcium, mg/d | 560–800 | 764.8 (137.1) | 700–1000 | 896.1 (135.7) | 875–1250 | 1200.6 (210.2) | 1050–1500 | 1392.0 (222.6) | 1225–1750 | 1513.8 (251.8) |
| Potassium, mg/d | 2000–2800 | 2637.3 (137.2) | 2500–3500 | 3179.9 (203.4) | 3125–4375 | 4015.2 (167.4) | 3750–5250 | 4686.9 (290.5) | 4375–6125 | 5509.4 (350.9) |
| Sodium, mg/d | 1840 | 1854.4 (19.2) | 2300 | 2241.8 (34.7) | 2875 | 2711.2 (92.4) | 3450 | 3146.3 (93.0) | 4025 | 3889.3 (143.7) |
| Fiber, g/d | >20 | 23.9 (3.7) | >25 | 29.9 (3.7) | >30 | 36.1 (4.6) | >38 | 43.9 (6.8) | >44 | 49.5 (5.6) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, K.; Wu, B.; Pilla, S.J.; Charleston, J.; Maw, M.T.T.; Appel, L.J.; Clark, J.M.; Maruthur, N.M. Design and Implementation of a Time-Restricted Eating Intervention in a Randomized, Controlled Eating Study. Nutrients 2023, 15, 1978. https://doi.org/10.3390/nu15081978
White K, Wu B, Pilla SJ, Charleston J, Maw MTT, Appel LJ, Clark JM, Maruthur NM. Design and Implementation of a Time-Restricted Eating Intervention in a Randomized, Controlled Eating Study. Nutrients. 2023; 15(8):1978. https://doi.org/10.3390/nu15081978
Chicago/Turabian StyleWhite, Karen, Beiwen Wu, Scott J. Pilla, Jeanne Charleston, May Thu Thu Maw, Lawrence J. Appel, Jeanne M. Clark, and Nisa M. Maruthur. 2023. "Design and Implementation of a Time-Restricted Eating Intervention in a Randomized, Controlled Eating Study" Nutrients 15, no. 8: 1978. https://doi.org/10.3390/nu15081978
APA StyleWhite, K., Wu, B., Pilla, S. J., Charleston, J., Maw, M. T. T., Appel, L. J., Clark, J. M., & Maruthur, N. M. (2023). Design and Implementation of a Time-Restricted Eating Intervention in a Randomized, Controlled Eating Study. Nutrients, 15(8), 1978. https://doi.org/10.3390/nu15081978







