Impact of Maternal Ketogenic Diet on NLRP3 Inflammasome Response in the Offspring Brain
Abstract
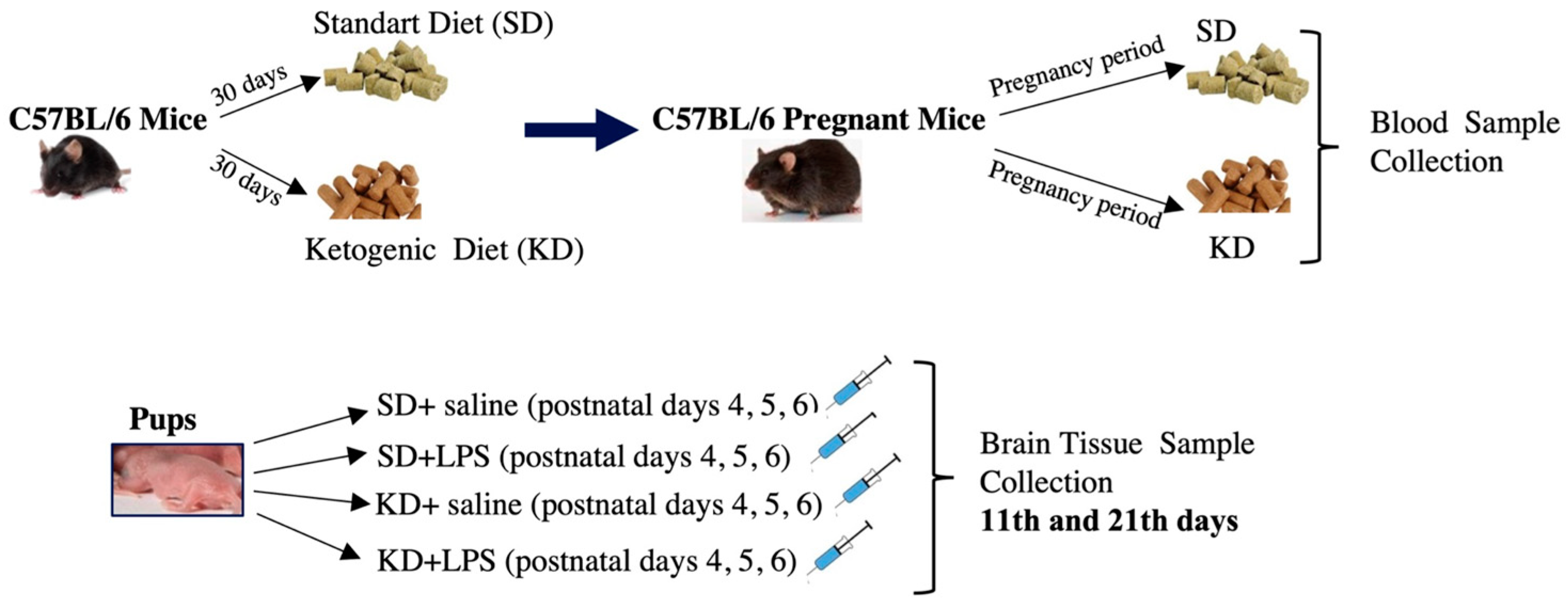
:1. Introduction
2. Materials and Methods
2.1. Animals and Study Design
2.2. Diet
2.3. Histomorphological Evaluation
2.4. Neuronal Density Measurement
2.5. Immunohistochemistry
2.6. Quantification of Immunohistochemical Data
2.7. Statistical Analysis
3. Results
3.1. Maternal Weight Gain
3.2. Maternal Blood Biochemistry
3.3. Litter Numbers, Body and Brain Weights in the Offspring
3.4. Neuronal Density Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Francis, E.; Hinkle, S.N.; Ajjarapu, A.S.; Zhang, C. Preconception and Prenatal Nutrition and Neurodevelopmental Disorders: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Edlow, A.G.; Guedj, F.; Sverdlov, D.; Pennings, J.A.; Bianchi, D.W. Significant Effects of Maternal Diet during Pregnancy on the Murine Fetal Brain Transcriptome and Off-spring Behavior. Front. Neurosci. 2019, 13, 1335. [Google Scholar] [CrossRef] [PubMed]
- Prado, E.L.; Dewey, K.G. Nutrition and brain development in early life. Nutr. Rev. 2014, 72, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Mhanna, A.; Mhanna, M.; Beran, A.; Al-Chalabi, M.; Aladamat, N.; Mahfooz, N. Modified Atkins Diet versus Ketogenic Diet in Children with Drug-Resistant Epilepsy: A Meta-Analysis of Comparative Studies. Clin. Nutr. ESPEN 2022, 51, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Kishk, N.A.; Yousof, H.Z.; Ebraheim, A.M.; Elkholy, T.A.F.A.; Soliman, S.H.; Mohammed, R.A.; Shamloul, R.M. The effect of ketogenic diet escalation in adolescents and adults with drug-resistant epilepsy: A prospective study. Nutr. Neurosci. 2021, 25, 2023–2032. [Google Scholar] [CrossRef]
- Valenzuela, P.L.; Castillo-García, A.; Lucia, A.; Naclerio, F. Effects of Combining a Ketogenic Diet with Resistance Training on Body Composition, Strength, and Mechanical Power in Trained Individuals: A Narrative Review. Nutrients 2021, 13, 3083. [Google Scholar] [CrossRef]
- Sussman, D.; van Eede, M.; Wong, M.D.; Adamson, S.L.; Henkelman, M. Effects of a ketogenic diet during pregnancy on embryonic growth in the mouse. BMC Pregnancy Childbirth 2013, 13, 109–111. [Google Scholar] [CrossRef]
- Maysinger, D.; Zhang, I. Nutritional and Nanotechnological Modulators of Microglia. Front. Immunol. 2016, 7, 270. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Cerezo, A.B.; De Pablos, R.M.; Krisa, S.; Richard, T.; García-Parrilla, M.C.; Troncoso, A.M. Phenolic Compounds Characteristic of the Mediterranean Diet in Mitigating Microglia-Mediated Neuroinflammation. Front. Cell Neurosci. 2018, 12, 373. [Google Scholar] [CrossRef]
- Song, L.; Pei, L.; Yao, S.; Wu, Y.; Shang, Y. NLRP3 Inflammasome in Neurological Diseases, from Functions to Therapies. Front. Cell Neurosci. 2017, 11, 63. [Google Scholar] [CrossRef]
- Ghosh, S.; Castillo, E.; Frias, E.S.; Swanson, R.A. Bioenergetic regulation of microglia. Glia 2017, 66, 1200–1212. [Google Scholar] [CrossRef]
- Shippy, D.C.; Wilhelm, C.; Viharkumar, P.A.; Raife, T.J.; Ulland, T.K. β-Hydroxybutyrate inhibits inflammasome activation to attenuate Alzheimer’s disease pathology. J. Neuroinflamm. 2020, 17, 280. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.l.; Emerson, M.; et al. Reporting animal research: Explanation and elaboration for the ARRIVE guidelines 2.0. PLoS Biol. 2020, 18, e3000411. [Google Scholar] [CrossRef]
- Grochowska, K.; Przeliorz, A. The Effect of the Ketogenic Diet on the Therapy of Neurodegenerative Diseases and Its Impact on Improving Cognitive Functions. Dement. Geriatr. Cogn. Disord. Extra 2022, 12, 100–106. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates: Hard Cover Edition; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Micili, S.C.; Engür, D.; Genc, S.; Ercan, I.; Soy, S.; Baysal, B.; Kumral, A. Oxygen exposure in early life activates NLRP3 inflammasome in mouse brain. Neurosci. Lett. 2020, 738, 135389. [Google Scholar] [CrossRef]
- Micili, S.C.; Goker, A.; Sayin, O.; Akokay, P.; Ergur, B.U. The effect of lipoic acid on wound healing in a full thickness uterine injury model in rats. Histochem. J. 2013, 44, 339–345. [Google Scholar] [CrossRef]
- Bordeleau, M.; de Cossío, L.F.; Chakravarty, M.M.; Tremblay, M. From Maternal Diet to Neurodevelopmental Disorders: A Story of Neuroinflammation. Front. Cell Neurosci. 2021, 14, 612705. [Google Scholar] [CrossRef]
- Xavier, S.; Soch, A.; Younesi, S.; Malik, S.; Spencer, S.J.; Sominsky, L. Maternal diet before and during pregnancy modulates microglial activation and neurogenesis in the post-partum rat brain. Brain Behav. Immun. 2021, 98, 185–197. [Google Scholar] [CrossRef]
- Sussman, D.; Ellegood, J.; Henkelman, M. A gestational ketogenic diet alters maternal metabolic status as well as offspring physiological growth and brain structure in the neonatal mouse. BMC Pregnancy Childbirth 2013, 13, 198. [Google Scholar] [CrossRef]
- Sussman, D.; Germann, J.; Henkelman, M. Gestational ketogenic diet programs brain structure and susceptibility to de-pression & anxiety in the adult mouse offspring. Brain Behav. 2015, 5, e00300. [Google Scholar]
- Kosiek, W.; Rauk, Z.; Szulc, P.; Cichy, A.; Rugieł, M.; Chwiej, J.; Janeczko, K.; Setkowicz, Z. Ketogenic diet impairs neurological development of neonatal rats and affects biochemical composition of maternal brains: Evidence of functional recovery in pups. Anat. Embryol. 2022, 227, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Arqoub, A.M.S.; Flynn, K.G.; Martinez, L.A. Gestational exposure to a ketogenic diet increases sociability in CD-1 mice. Behav. Neurosci. 2020, 134, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Menassa, D.A.; Gomez-Nicola, D. Microglial Dynamics during Human Brain Development. Front. Immunol. 2018, 9, 1014. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.K.; Vidyadaran, S. Role of microglia in embryonic neurogenesis. ExBiol. Med. 2016, 241, 1669–1675. [Google Scholar] [CrossRef]
- Sominsky, L.; De Luca, S.; Spencer, S.J. Microglia: Key players in neurodevelopment and neuronal plasticity. Int. J. Biochem. Cell Biol. 2018, 94, 56–60. [Google Scholar] [CrossRef]
- Wright-Jin, E.C.; Gutmann, D.H. Microglia as Dynamic Cellular Mediators of Brain Function. Trends Mol. Med. 2019, 25, 967–979. [Google Scholar] [CrossRef]
- Boche, D.; Perry, V.H.; Nicoll, J.A.R. Review: Activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18. [Google Scholar] [CrossRef]
- Eren, E.; Tufekci, K.U.; Isci, K.B.; Tastan, B.; Genc, K.; Genc, S. Sulforaphane Inhibits Lipopolysaccharide-Induced Inflammation, Cytotoxicity, Oxidative Stress, and miR-155 Expression and Switches to Mox Phenotype through Activating Extracellular Signal-Regulated Kinase 1/2-Nuclear Factor Erythroid 2-Related Factor 2/Antioxidant Response Element Pathway in Murine Microglial Cells. Front. Immunol. 2018, 9, 36. [Google Scholar]
- Ozaki, K.; Kato, D.; Ikegami, A.; Hashimoto, A.; Sugio, S.; Guo, Z.; Shibushita, M.; Tatematsu, T.; Haruwaka, K.; Moorhouse, A.J.; et al. Maternal immune activation induces sustained changes in fetal microglia motility. Sci. Rep. 2020, 10, 21378. [Google Scholar] [CrossRef]
- Pierre, K.; Pellerin, L. Monocarboxylate transporters in the central nervous system: Distribution, regulation and function. J. Neurochem. 2005, 94, 1–14. [Google Scholar] [CrossRef]
- Huang, C.; Wang, P.; Xu, X.; Zhang, Y.; Gong, Y.; Hu, W.; Gao, M.; Wu, Y.; Ling, Y.; Zhao, X.; et al. The ketone body metabolite β-hydroxybutyrate induces an antidepression-associated ramification of microglia via HDACs inhibition-triggered Akt-small RhoGTPase activation. Glia 2017, 66, 256–278. [Google Scholar] [CrossRef]
- Kong, G.; Liu, J.; Li, R.; Lin, J.; Huang, Z.; Yang, Z.; Wu, X.; Huang, Z.; Zhu, Q.; Wu, X. Ketone Metabolite β-Hydroxybutyrate Ameliorates Inflammation After Spinal Cord Injury by Inhibiting the NLRP3 Inflammasome. Neurochem. Res. 2020, 46, 213–229. [Google Scholar] [CrossRef]
- Arcuri, C.; Mecca, C.; Bianchi, R.; Giambanco, I.; Donato, R. The Pathophysiological Role of Microglia in Dynamic Surveillance, Phagocytosis and Structural Remodeling of the Developing CNS. Front. Mol. Neurosci. 2017, 10, 191. [Google Scholar] [CrossRef]
- Hanslik, K.L.; Ulland, T. The Role of Microglia and the Nlrp3 Inflammasome in Alzheimer’s Disease. Front. Neurol. 2020, 11, 570711. [Google Scholar] [CrossRef]
- Youm, Y.-H.; Nguyen, K.Y.; Grant, R.W.; Goldberg, E.L.; Bodogai, M.; Kim, D.; D’Agostino, D.; Planavsky, N.; Lupfer, C.; Kanneganti, T.-D.; et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome–mediated inflammatory disease. Nat. Med. 2015, 21, 263–269. [Google Scholar] [CrossRef]
- Hattori, Y. The behavior and functions of embryonic microglia. Anat. Sci. Int. 2021, 97, 1–14. [Google Scholar] [CrossRef]
- Tan, Y.-L.; Yuan, Y.; Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 2019, 25, 351–367. [Google Scholar] [CrossRef]





| Body Weight (g) | SD (n = 5) | KD (n = 7) | p | ||
|---|---|---|---|---|---|
| ± M | IQR (25–75%) | ± M | IQR (25–75%) | ||
| GD0 | 22.64 ± 22.6 | 21.2–24.1 | 21.71 ± 21 | 20.6–23 | 0.43 |
| GD10 | 25.52 ± 26.4 | 22.3–28.3 | 24.89 ± 25 | 23–26.8 | 0.75 |
| GD20 | 36.72 ± 39 | 32.8–39.5 | 35.63 ± 36 | 33.8–38.2 | 0.53 |
| PP0 | 26.92 ± 28 | 24–29.3 | 24.97 ± 24.2 | 22.6–27 | 0.34 |
| PP10 | 30.16 ± 30.6 | 28.1–32 | 26.63 ± 26.8 | 25.4–29.2 | 0.04 * |
| PP20 | 29.32 ± 30.6 | 26–32 | 25.51 ± 25.6 | 23.4–27 | 0.07 * |
| BHB (mmol/L) | SD | KD | p | ||
|---|---|---|---|---|---|
| ± M | IQR (25–75%) | ± M | IQR (25–75%) | ||
| GD2 | 0.74 ± 0.8 | 0.55–0.9 | 1.24 ± 1.3 | 1.1–1.3 | 0.003 * |
| GD10 | 0.80 ± 0.8 | 0.7–0.9 | 1.70 ± 1.6 | 1.4–2.1 | 0.003 * |
| GD18 | 0.72 ± 0.7 | 0.6–0.85 | 2.80 ± 2.2 | 2.1–3.3 | 0.003 * |
| Glucose (mg/dL) | SD | KD | p | ||
|---|---|---|---|---|---|
| ± M | IQR (25–75%) | ± M | IQR (25–75%) | ||
| GD2 | 176.0 ± 175 | 166.5–186 | 185.4 ± 189 | 184–192 | 0.149 |
| GD10 | 165.0 ± 170 | 154–173.5 | 159.6 ± 159 | 155–169 | 0.75 |
| GD18 | 170.8 ± 175 | 164.5–175 | 109.6 ± 108 | 98–115 | 0.003 * |
| SD | KD | p | Z | |||
|---|---|---|---|---|---|---|
| ± M | IQR (25–75%) | ± M | IQR (25–75%) | |||
| Total cholesterol (mg/dL) | 71.80 ± 71.0 | 57.5–86.5 | 121.33 ± 118,5 | 57.5–86.5 | 0.006 * | −2.739 |
| Trigyceride (mg/dL) | 59.80 ± 56.0 | 53–68.5 | 109.67 ± 101.50 | 72–121 | 0.003 * | −2.191 |
| Groups | PN2 | PN10 | PN20 | |||
|---|---|---|---|---|---|---|
| ± M | IQR (25–75%) | ± M | IQR (25–75%) | ± M | IQR (25–75%) | |
| SD (1) | 1.58 ± 1.60 (n = 19) | 1.4–2.0 | 4.36 ± 4.6 (n = 19) | 4.0–5.0 | 8.44 ± 8.60 (n = 9) | 7.5–9.0 |
| KD (2) | 1.47 ± 1.60 (n = 25) | 1.2–2.0 | 4.82 ± 5.0 (n = 25) | 4.0–5.6 | 9.56 ± 9.50 (n = 12) | 8.6–10.75 |
| SD + LPS (3) | 1.69 ± 1.60 (n = 15) | 1.4–1.8 | 3.77 ± 3.6 (n = 15) | 2.6–4.2 | 7.20 ± 8.00 (n = 7) | 5.6–8.0 |
| KD + LPS (4) | 1.77 ± 1.8 (n = 14) | 1.6–2.0 | 4.94 ± 5.2 (n = 14) | 4.4–5.8 | 10.49 ± 10.60 (n = 7) | 9.6–11.2 |
| p | 0.05 | 0.000 * | 0.008 * | |||
| (1) > (3), (2) > (3), (4) > (3) | (3) < (4) | |||||
| Groups | PN11 | PN21 | ||
|---|---|---|---|---|
| ± M | IQR (25–75%) | ± M | IQR (25–75%) | |
| SD (1) | 0.27 ± 0.27 (n = 10) | 0.24–0.31 | 0.33 ± 0.32 (n = 9) | 0.30–0.35 |
| KD (2) | 0.28 ± 0.26 (n = 12) | 0.21–0.34 | 0.47 ± 0.49 (n = 12) | 0.41–0.51 |
| SD + LPS (3) | 0.22 ± 0.22 (n = 8) | 0.19–0.25 | 0.32 ± 0.03 (n = 7) | 0.28–0.38 |
| KD + LPS (4) | 0.32 ± 0.33 (n = 7) | 0.29–0.37 | 0.32 ± 0.32 (n = 7) | 0.31–0.34 |
| p | 0.021 * | 0.003 * | ||
| (3) < (4) | (1) < (2), (2) > (3), (2) > (4) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altınöz, S.; Micili, S.C.; Soy, S.; Engür, D.; Baysal, B.; Kumral, A. Impact of Maternal Ketogenic Diet on NLRP3 Inflammasome Response in the Offspring Brain. Nutrients 2023, 15, 1994. https://doi.org/10.3390/nu15081994
Altınöz S, Micili SC, Soy S, Engür D, Baysal B, Kumral A. Impact of Maternal Ketogenic Diet on NLRP3 Inflammasome Response in the Offspring Brain. Nutrients. 2023; 15(8):1994. https://doi.org/10.3390/nu15081994
Chicago/Turabian StyleAltınöz, Sevsen, Serap Cilaker Micili, Sıla Soy, Defne Engür, Bora Baysal, and Abdullah Kumral. 2023. "Impact of Maternal Ketogenic Diet on NLRP3 Inflammasome Response in the Offspring Brain" Nutrients 15, no. 8: 1994. https://doi.org/10.3390/nu15081994
APA StyleAltınöz, S., Micili, S. C., Soy, S., Engür, D., Baysal, B., & Kumral, A. (2023). Impact of Maternal Ketogenic Diet on NLRP3 Inflammasome Response in the Offspring Brain. Nutrients, 15(8), 1994. https://doi.org/10.3390/nu15081994






