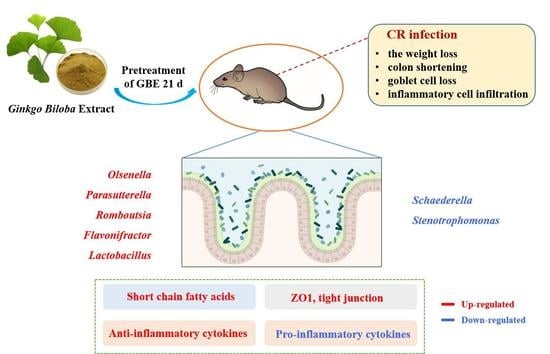
Ginkgo biloba Extract Preventively Intervenes in Citrobacter Rodentium-Induced Colitis in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. GBE Synthesis and Identification
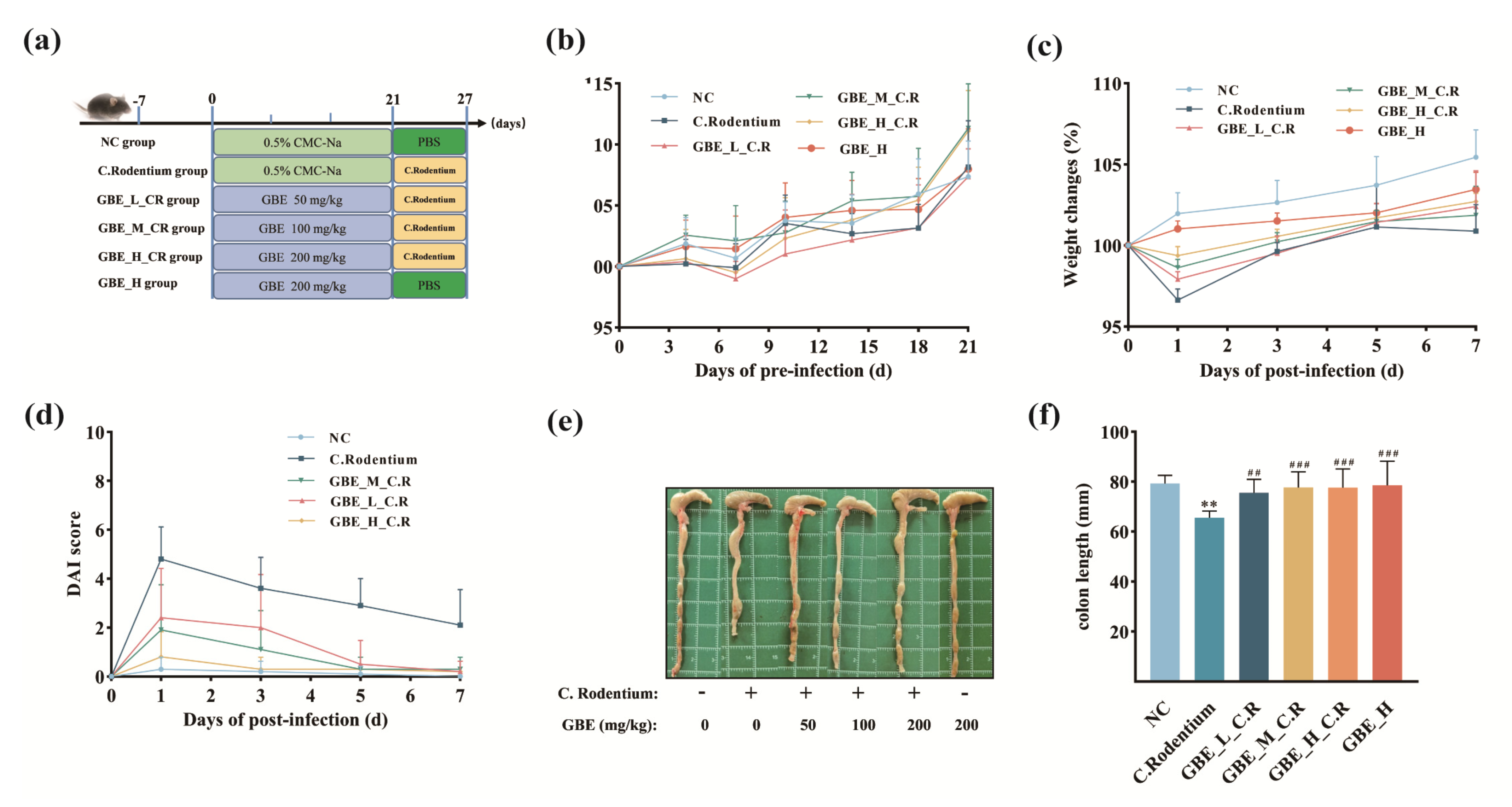
2.2. Animals
2.3. Disease Activity Index (DAI)
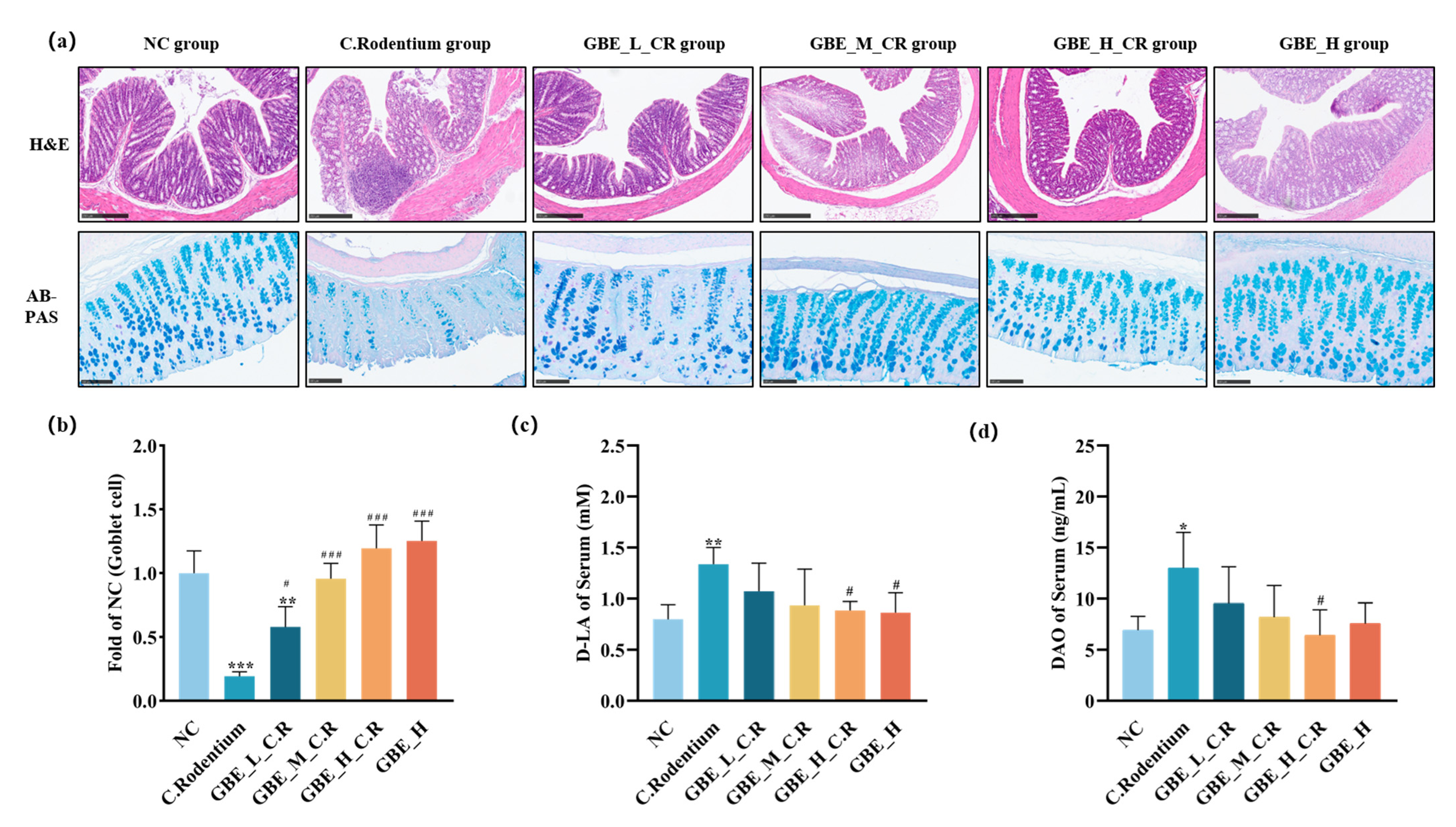
2.4. Histological Examination
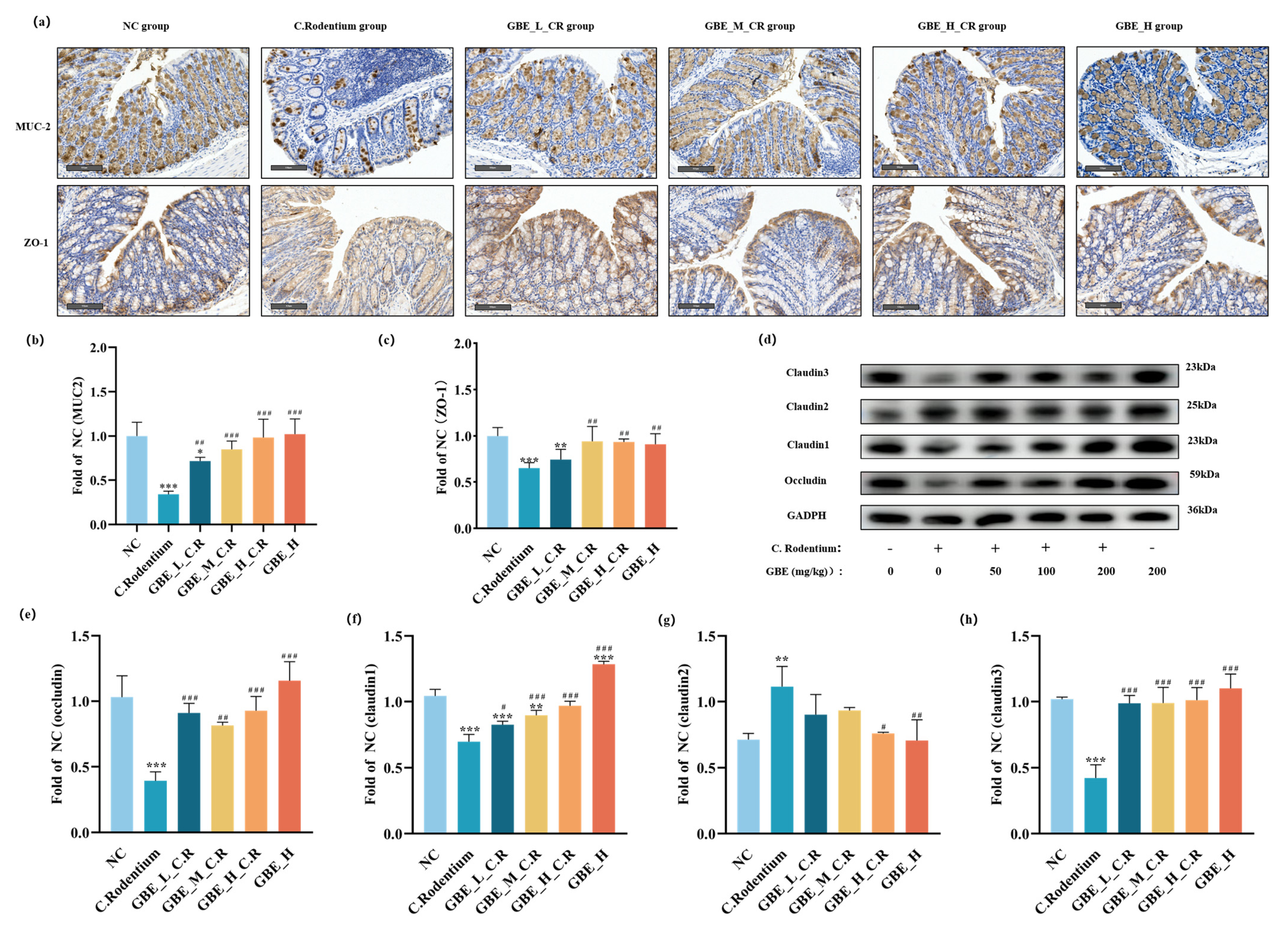
2.5. Immunohistochemistry (IHC)
2.6. Measurement of Cytokines, Diamine Oxidase (DAO), and D-Lactic Acid (D-LA)
2.7. Immunoblotting
2.8. 16S rRNA Gene Sequencing
2.9. Measurement of SCFAs
2.10. Statistical Analyses
3. Results
3.1. Preparation and Identification of GBE
3.2. GBE Ameliorated Symptoms of CR-Induced Colitis in Mice
3.3. GBE Improved Colonic Lesions in Mice
3.4. GBE Suppressed the Pro-Inflammatory Factors and Promoted the Anti-Inflammatory Factors within Mice
3.5. GBE Enhanced Mouse Intestinal Barrier Function
3.6. GBE Modulated Gut Microbiota
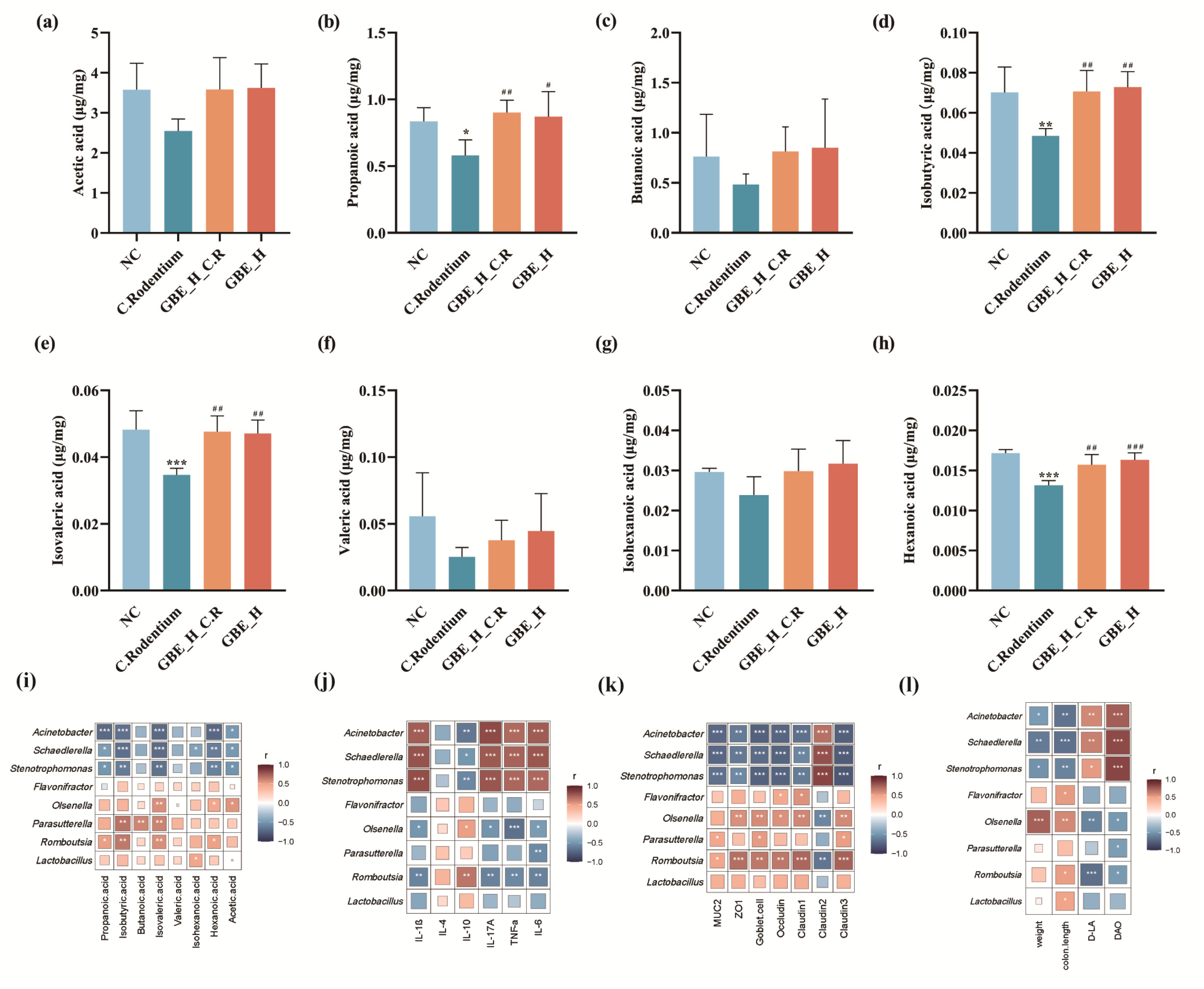
3.7. GBE Promoted Intestinal SCFAs in Mice
3.8. Correlation Analysis of Gut Microbiota and SCFAs, Cytokines, and TJ Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neurath, M.F. Host-microbiota interactions in inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 76–77. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Li, Q.M.; Zha, X.Q.; Luo, J.P. Dendrobium fimbriatum Hook polysaccharide ameliorates dextran-sodium-sulfate-induced colitis in mice via improving intestinal barrier function, modulating intestinal microbiota, and reducing oxidative stress and inflammatory responses. Food Funct. 2022, 13, 143–160. [Google Scholar] [CrossRef] [PubMed]
- Linson, E.A.; Hanauer, S.B. Epidemiology of Colorectal Cancer in Inflammatory Bowel Disease—The Evolving Landscape. Curr. Gastroenterol. Rep. 2021, 23, 16. [Google Scholar] [CrossRef] [PubMed]
- Aniwan, S.; Park, S.H.; Loftus, E.V. Epidemiology, Natural History, and Risk Stratification of Crohn’s Disease. Gastroenterol. Clin. N. 2017, 46, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Nakase, H.; Uchino, M.; Shinzaki, S.; Matsuura, M.; Matsuoka, K.; Kobayashi, T.; Saruta, M.; Hirai, F.; Hata, K.; Hiraoka, S.; et al. Evidence-based clinical practice guidelines for inflammatory bowel disease 2020. J. Gastroenterol. 2021, 56, 489–526. [Google Scholar] [CrossRef]
- Schwartz, D.J.; Langdon, A.E.; Dantas, G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020, 12, 82. [Google Scholar] [CrossRef]
- Alhagamhmad, M.H.; Day, A.S.; Lemberg, D.A.; Leach, S.T. An overview of the bacterial contribution to Crohn disease pathogenesis. J. Med. Microbiol. 2016, 65, 1049–1059. [Google Scholar] [CrossRef]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The Microbiome in Inflammatory Bowel Disease: Current Status and the Future Ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The Human Intestinal Microbiome in Health and Disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, S.G.; Park, J.H.; Yanagisawa, M.; Kim, C.H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 2013, 145, 396–406.e391–310. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Gao, Y.; Davis, B.; Zhu, W.; Zheng, N.; Meng, D.; Walker, W.A. Short-chain fatty acid butyrate, a breast milk metabolite, enhances immature intestinal barrier function genes in response to inflammation in vitro and in vivo. Am. J. Physiol. Gastrointest Liver Physiol. 2021, 320, G521–G530. [Google Scholar] [CrossRef]
- Burger-van Paassen, N.; Vincent, A.; Puiman, P.J.; van der Sluis, M.; Bouma, J.; Boehm, G.; van Goudoever, J.B.; van Seuningen, I.; Renes, I.B. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: Implications for epithelial protection. Biochem. J. 2009, 420, 211–219. [Google Scholar] [CrossRef]
- Hatayama, H.; Iwashita, J.; Kuwajima, A.; Abe, T. The short chain fatty acid, butyrate, stimulates MUC2 mucin production in the human colon cancer cell line, LS174T. Biochem. Biophys. Res. Commun. 2007, 356, 599–603. [Google Scholar] [CrossRef]
- Al-Lahham, S.; Rezaee, F. Propionic acid counteracts the inflammation of human subcutaneous adipose tissue: A new avenue for drug development. Daru 2019, 27, 645–652. [Google Scholar] [CrossRef]
- Aguilar, E.C.; Leonel, A.J.; Teixeira, L.G.; Silva, A.R.; Silva, J.F.; Pelaez, J.M.N.; Capettini, L.S.A.; Lemos, V.S.; Santos, R.A.S.; Alvarez-Leite, J.I. Butyrate impairs atherogenesis by reducing plaque inflammation and vulnerability and decreasing NF kappa B activation. Nutr. Metab. Cardiovasc. 2014, 24, 606–613. [Google Scholar] [CrossRef]
- Mullineaux-Sanders, C.; Sanchez-Garrido, J.; Hopkins, E.G.D.; Shenoy, A.R.; Barry, R.; Frankel, G. Citrobacter rodentium-host-microbiota interactions: Immunity, bioenergetics and metabolism. Nat. Rev. Microbiol. 2019, 17, 701–715. [Google Scholar] [CrossRef]
- Vallance, B.A.; Deng, W.Y.; Jacobson, K.; Finlay, B.B. Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect. Immun. 2003, 71, 3443–3453. [Google Scholar] [CrossRef]
- Itoh, K.; Matsui, T.; Tsuji, K.; Mitsuoka, T.; Ueda, K. Genetic control in the susceptibility of germfree inbred mice to infection by Escherichia coli O115a,c:K(B). Infect. Immun. 1988, 56, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Petty, N.K.; Feltwell, T.; Pickard, D.; Clare, S.; Toribio, A.L.; Fookes, M.; Roberts, K.; Monson, R.; Nair, S.; Kingsley, R.A.; et al. Citrobacter rodentium is an Unstable Pathogen Showing Evidence of Significant Genomic Flux. PLoS Pathog. 2011, 7, e1002018. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Dai, C.; Brown, K.; Rajendiran, E.; Makarenko, S.; Baker, J.; Ma, C.; Halder, S.; Montero, M.; Ionescu, V.A.; et al. Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status, and ion transporter gene expression. Am. J. Physiol. Gastrointest Liver Physiol. 2011, 301, G39–G49. [Google Scholar] [CrossRef] [PubMed]
- Omidkhoda, S.F.; Razavi, B.M.; Hosseinzadeh, H. Protective effects of Ginkgo biloba L. against natural toxins, chemical toxicities, and radiation: A comprehensive review. Phytother. Res. PTR 2019, 33, 2821–2840. [Google Scholar] [CrossRef]
- Mohanta, T.K.; Tamboli, Y.; Zubaidha, P.K. Phytochemical and medicinal importance of Ginkgo biloba L. Nat. Prod. Res. 2014, 28, 746–752. [Google Scholar] [CrossRef]
- Klomsakul, P.; Aiumsubtub, A.; Chalopagorn, P. Evaluation of Antioxidant Activities and Tyrosinase Inhibitory Effects of Ginkgo biloba Tea Extract. Sci. World J. 2022, 2022, 4806889. [Google Scholar] [CrossRef]
- Guo, N.; Jiang, Y.W.; Song, X.R.; Li, Y.Y.; Liu, Z.M.; Fu, Y.J. Effect of Bacillus natto solid-state fermentation on the functional constituents and properties of Ginkgo seeds. J. Food Biochem. 2019, 43, e12820. [Google Scholar] [CrossRef]
- El-Ghazaly, M.A.; Sadik, N.A.; Rashed, E.R.; Abd-El-Fattah, A.A. Neuroprotective effect of GBE761(R) and low-dose whole-body gamma-irradiation in a rat model of Parkinson’s disease. Toxicol. Ind. Health 2015, 31, 1128–1143. [Google Scholar] [CrossRef]
- Dodge, H.H.; Zitzelberger, T.; Oken, B.S.; Howieson, D.; Kaye, J. A randomized placebo-controlled trial of Ginkgo biloba for the prevention of cognitive decline. Neurology 2008, 70, 1809–1817. [Google Scholar] [CrossRef]
- Zhou, Y.H.; Yu, J.P.; Liu, Y.F.; Teng, X.J.; Ming, M.; Lv, P.; An, P.; Liu, S.Q.; Yu, H.G. Effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-alpha, NF-kappaBp65, IL-6) in TNBS-induced colitis in rats. Mediat. Inflamm. 2006, 2006, 92642. [Google Scholar] [CrossRef]
- Kotakadi, V.S.; Jin, Y.; Hofseth, A.B.; Ying, L.; Cui, X.L.; Volate, S.; Chumanevich, A.; Wood, P.A.; Price, R.L.; McNeal, A.; et al. Ginkgo biloba extract GBE 761 has anti-inflammatory properties and ameliorates colitis in mice by driving effector T cell apoptosis. Carcinogenesis 2008, 29, 1799–1806. [Google Scholar] [CrossRef]
- Mustafa, A.; El-Medany, A.; Hagar, H.H.; El-Medany, G. Ginkgo biloba attenuates mucosal damage in a rat model of ulcerative colitis. Pharmacol. Res. 2006, 53, 324–330. [Google Scholar] [CrossRef]
- Kang, S.; Chen, T.; Hao, Z.; Yang, X.; Wang, M.; Zhang, Z.; Hao, S.; Lang, F.; Hao, H. Oxymatrine Alleviates Gentamicin-Induced Renal Injury in Rats. Molecules 2022, 27, 6209. [Google Scholar] [CrossRef]
- Kong, C.; Liang, L.; Liu, G.; Du, L.T.; Yang, Y.Z.; Liu, J.Q.; Shi, D.B.; Li, X.X.; Ma, Y.L. Integrated metagenomic and metabolomic analysis reveals distinct gut-microbiome-derived phenotypes in early-onset colorectal cancer. Gut 2022. ahead of print. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Jiang, D.; Jin, Y.H.; Jia, H.; Yang, Y.; Kim, I.H.; Dai, Z.L.; Zhang, J.H.; Ren, F.Z.; Wu, Z.L. Glycine Attenuates Citrobacter rodentium-Induced Colitis by Regulating ATF6-Mediated Endoplasmic Reticulum Stress in Mice. Mol. Nutr. Food Res. 2021, 65, e2001065. [Google Scholar] [CrossRef]
- Goncalves, P.; Araujo, J.R.; Di Santo, J.P. A Cross-Talk Between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 558–572. [Google Scholar] [CrossRef]
- Friedrich, M.; Pohin, M.; Powrie, F. Cytokine Networks in the Pathophysiology of Inflammatory Bowel Disease. Immunity 2019, 50, 992–1006. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Powell, D.N.; Kalman, D. Layered defense: How mucus and tight junctions seal the intestinal barrier. J. Mol. Med. 2017, 95, 927–934. [Google Scholar] [CrossRef]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Feng, Y.; Li, D.T.; Ma, C.; Tian, M.L.; Hu, X.S.; Chen, F. Barley Leaf Ameliorates Citrobacter rodentium-Induced Colitis through Preventive Effects. Nutrients 2022, 14, 3833. [Google Scholar] [CrossRef]
- Xu, H.M.; Huang, H.L.; Liu, Y.D.; Zhu, J.Q.; Zhou, Y.L.; Chen, H.T.; Xu, J.; Zhao, H.L.; Guo, X.; Shi, W.; et al. Selection strategy of dextran sulfate sodium-induced acute or chronic colitis mouse models based on gut microbial profile. BMC Microbiol. 2021, 21, 279. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Y.; Zou, G.L.; Li, B.; Du, X.F.; Sun, Z.; Sun, Y.; Jiang, X.F. Fecal Microbiota Transplantation (FMT) Alleviates Experimental Colitis in Mice by Gut Microbiota Regulation. J. Microbiol. Biotechn 2020, 30, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Raju, P.; Shashikanth, N.; Tsai, P.Y.; Pongkorpsakol, P.; Chanez-Paredes, S.; Steinhagen, P.R.; Kuo, W.T.; Singh, G.; Tsukita, S.; Turner, J.R. Inactivation of paracellular cation-selective claudin-2 channels attenuates immune-mediated experimental colitis in mice. J. Clin. Invest. 2020, 130, 5197–5208. [Google Scholar] [CrossRef] [PubMed]
- Parker, K.D.; Maurya, A.K.; Ibrahim, H.; Rao, S.; Hove, P.R.; Kumar, D.; Kant, R.; Raina, B.; Agarwal, R.; Kuhn, K.A.; et al. Dietary Rice Bran-Modified Human Gut Microbial Consortia Confers Protection against Colon Carcinogenesis Following Fecal Transfaunation. Biomedicines 2021, 9, 144. [Google Scholar] [CrossRef] [PubMed]
- Mikami, A.; Ogita, T.; Namai, F.; Shigemori, S.; Sato, T.; Shimosato, T. Oral Administration of Flavonifractor plautii, a Bacteria Increased with Green Tea Consumption, Promotes Recovery from Acute Colitis in Mice via Suppression of IL-17. Front. Nutr. 2021, 7, 610946. [Google Scholar] [CrossRef]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef]
- Kim, Y.J.; Jeon, H.; Na, S.H.; Kwon, H.I.; Selasi, G.N.; Nicholas, A.; Park, T.I.; Lee, S.H.; Lee, J.C. Stenotrophomonas maltophilia outer membrane vesicles elicit a potent inflammatory response in vitro and in vivo. Pathog. Dis. 2016, 74, ftw104. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshida, S.; Hara, H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Brit J. Nutr. 2008, 100, 297–305. [Google Scholar] [CrossRef]
- Peng, Y.J.; Yan, Y.M.; Wan, P.; Chen, D.; Ding, Y.; Ran, L.W.; Mi, J.; Lu, L.; Zhang, Z.J.; Li, X.Y.; et al. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radic. Bio. Med. 2019, 136, 96–108. [Google Scholar] [CrossRef]
- Guo, W.L.; Mao, B.Y.; Cui, S.M.; Tang, X.; Zhang, Q.X.; Zhao, J.X.; Zhang, H. Protective Effects of a Novel Probiotic Bifidobacterium pseudolongum on the Intestinal Barrier of Colitis Mice via Modulating the Ppar gamma/STAT3 Pathway and Intestinal Microbiota. Foods 2022, 11, 1551. [Google Scholar] [CrossRef]
- Wang, M.X.; Lin, L.; Chen, Y.D.; Zhong, Y.P.; Lin, Y.X.; Li, P.; Tian, X.; Han, B.; Xie, Z.Y.; Liao, Q.F. Evodiamine has therapeutic efficacy in ulcerative colitis by increasing Lactobacillus acidophilus levels and acetate production. Pharmacol. Res. 2020, 159, 104978. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, F.; Zhang, J.; Zhang, X.; Guo, Y.; Yao, Q. A Holistic View of Berberine Inhibiting Intestinal Carcinogenesis in Conventional Mice Based on Microbiome-Metabolomics Analysis. Front. Immunol. 2020, 11, 588079. [Google Scholar] [CrossRef]
- Lin, M.Y.; de Zoete, M.R.; van Putten, J.P.M.; Strijbis, K. Redirection of epithelial immune responses by short-chain fatty acids through inhibition of histone deacetylases. Front. Immunol. 2015, 6, 554. [Google Scholar] [CrossRef]
- Ma, X.; Fan, P.X.; Li, L.S.; Qiao, S.Y.; Zhang, G.L.; Li, D.F. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012, 90, 266–268. [Google Scholar] [CrossRef]
- Pirozzi, C.; Francisco, V.; Guida, F.D.; Gomez, R.; Lago, F.; Pino, J.; Meli, R.; Gualillo, O. Butyrate Modulates Inflammation in Chondrocytes via GPR43 Receptor. Cell Physiol. Biochem. 2018, 51, 228–243. [Google Scholar] [CrossRef]
- Zheng, S.; Zhuang, T.C.; Tang, Y.J.; Wu, R.H.; Xu, T.; Leng, T.; Wang, Y.; Lin, Z.; Ji, M.H. Leonurine protects against ulcerative colitis by alleviating inflammation and modulating intestinal microflora in mouse models. Exp. Ther. Med. 2021, 22, 1199. [Google Scholar] [CrossRef]
- Yang, W.J.; Yu, T.M.; Huang, X.S.; Bilotta, A.J.; Xu, L.Q.; Lu, Y.; Sun, J.R.; Pan, F.; Zhou, J.; Zhang, W.B.; et al. Intestinal microbiota-derived short-chain fatty acids regulation of immune cell IL-22 production and gut immunity. Nat. Commun. 2020, 11, 4457. [Google Scholar] [CrossRef]
- Xia, Z.B.; Han, Y.J.; Wang, K.; Guo, S.K.; Wu, D.Z.; Huang, X.Z.; Li, Z.R.; Zhu, L.B. Oral administration of propionic acid during lactation enhances the colonic barrier function. Lipids Health Dis. 2017, 16, 62. [Google Scholar] [CrossRef]
- Marino, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 2017, 18, 1271. [Google Scholar] [CrossRef]
- Neurath, M.F. Cytokines in inflammatory bowel disease. Nat. Rev. Immunol. 2014, 14, 329–342. [Google Scholar] [CrossRef]
- Li, X.; Huang, Q.; Ong, C.N.; Yang, X.F.; Shen, H.M. Chrysin sensitizes tumor necrosis factor-alpha-induced apoptosis in human tumor cells via suppression of nuclear factor-kappaB. Cancer Lett. 2010, 293, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Marafini, I.; Sedda, S.; Dinallo, V.; Monteleone, G. Inflammatory cytokines: From discoveries to therapies in IBD. Expert. Opin. Biol. Ther. 2019, 19, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.; Zhang, H.; Ke, Y. Phosphatase Shp2 exacerbates intestinal inflammation by disrupting macrophage responsiveness to interleukin-10. Eur. J. Immunol. 2019, 49, 1393. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Zhou, D.S.; Xuan, R.R.; Zhou, J.W.; Liu, J.W.W.; Chen, J.J.; Han, H.; Niu, T.T.; Li, X.X.; Chen, H.M.; et al. Lambda-carrageenan exacerbates Citrobacter rodentium-induced infectious colitis in mice by targeting gut microbiota and intestinal barrier integrity. Pharmacol. Res. 2021, 174, 105940. [Google Scholar] [CrossRef]
- Monteleone, I.; Pallone, F.; Monteleone, G. Th17-related cytokines: New players in the control of chronic intestinal inflammation. BMC Med. 2011, 9, 122. [Google Scholar] [CrossRef]
- Lin, R.; Piao, M.Y.; Song, Y. Dietary Quercetin Increases Colonic Microbial Diversity and Attenuates Colitis Severity in Citrobacter rodentium-Infected Mice. Fron Microbiol. 2019, 10, 1092. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Lu, Y.M.; Ou, Y.X.; Zhang, H.Z.; Chen, W.X. Dynamic progress of 2,4,6-trinitrobenzene sulfonic acid induced chronic colitis and fibrosis in rat model. J. Dig. Dis. 2012, 13, 421–429. [Google Scholar] [CrossRef]
- Dann, S.M.; Le, C.H.Y.; Hanson, E.M.; Ross, M.C.; Eckmann, L. Giardia Infection of the Small Intestine Induces Chronic Colitis in Genetically Susceptible Hosts. J. Immunol. 2018, 201, 548–559. [Google Scholar] [CrossRef]
- Jia, D.J.C.; Wang, Q.W.; He, J.M. Lactobacillus Johnsonii Alleviates Colitis by Tlr1/2-Stat3 Mediated M2-Like Macrophages Polarization. Gastroenterology 2022, 162, S985. [Google Scholar] [CrossRef]
- Qu, Y.F.; Li, X.Y.; Xu, F.Y.; Zhao, S.M.; Wu, X.M.; Wang, Y.Z.; Xie, J.M. Kaempferol Alleviates Murine Experimental Colitis by Restoring Gut Microbiota and Inhibiting the LPS-TLR4-NF-kappa B Axis. Front. Immunol. 2021, 12, 679897. [Google Scholar] [CrossRef]
- Zhang, H.L.; Wang, Y.; Su, Y.C.; Fang, X.D.; Guo, W.J. The alleviating effect and mechanism of Bilobalide on ulcerative colitis. Food Funct. 2021, 12, 6226–6239. [Google Scholar] [CrossRef]
- Van der Sluis, M.; De Koning, B.A.E.; De Bruijn, A.C.J.M.; Velcich, A.; Meijerink, J.P.P.; Van Goudoever, J.B.; Buller, H.A.; Dekker, J.; Van Seuningen, I.; Renes, I.B.; et al. Muc2-deficient mice spontaneously develop colitis, indicating that Muc2 is critical for colonic protection. Gastroenterology 2006, 131, 117–129. [Google Scholar] [CrossRef]
- Velcich, A.; Yang, W.C.; Heyer, J.; Fragale, A.; Nicholas, C.; Viani, S.; Kucherlapati, R.; Lipkin, M.; Yang, K.; Augenlicht, L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 2002, 295, 1726–1729. [Google Scholar] [CrossRef]
- Cai, J.; Chen, H.; Weng, M.; Jiang, S.; Gao, J. Diagnostic and Clinical Significance of Serum Levels of D-Lactate and Diamine Oxidase in Patients with Crohn’s Disease. Gastroenterol. Res. Pract. 2019, 2019, 8536952. [Google Scholar] [CrossRef]
- Xue, M.; Ji, X.; Liang, H.; Liu, Y.; Wang, B.; Sun, L.; Li, W. The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer. Food Funct. 2018, 9, 1214–1223. [Google Scholar] [CrossRef]
- Tsai, P.Y.; Zhang, B.K.; He, W.Q.; Zha, J.M.; Odenwald, M.A.; Singh, G.; Tamura, A.; Shen, L.; Sailer, A.; Yeruva, S.; et al. IL-22 Upregulates Epithelial Claudin-2 to Drive Diarrhea and Enteric Pathogen Clearance. Cell Host Microbe 2017, 21, 671–681.e4. [Google Scholar] [CrossRef]
- Dou, W.; Zhang, J.J.; Li, H.; Kortagere, S.; Sun, K.; Ding, L.L.; Ren, G.Y.; Wang, Z.T.; Mani, S. Plant flavonol isorhamnetin attenuates chemically induced inflammatory bowel disease via a PXR-dependent pathway. J. Nutr. Biochem. 2014, 25, 923–933. [Google Scholar] [CrossRef]




| Score | Weight Loss % | Stool Consistency | Health Status |
|---|---|---|---|
| 0 | none | normal | normal |
| 1 | 0–5 | ||
| 2 | 6–10 | loose stool | poor |
| 3 | 11–15 | ||
| 4 | >16 | diarrhea | terrible |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Chen, Y.; Li, K.; Chen, Z.; Zhao, Q.; Fan, Y.; Liu, Y.; Zhang, S.; Hao, Z. Ginkgo biloba Extract Preventively Intervenes in Citrobacter Rodentium-Induced Colitis in Mice. Nutrients 2023, 15, 2008. https://doi.org/10.3390/nu15082008
Chen T, Chen Y, Li K, Chen Z, Zhao Q, Fan Y, Liu Y, Zhang S, Hao Z. Ginkgo biloba Extract Preventively Intervenes in Citrobacter Rodentium-Induced Colitis in Mice. Nutrients. 2023; 15(8):2008. https://doi.org/10.3390/nu15082008
Chicago/Turabian StyleChen, Tingting, Yiqiang Chen, Kaiyuan Li, Zhuo Chen, Qingyu Zhao, Yimeng Fan, Ying Liu, Suxia Zhang, and Zhihui Hao. 2023. "Ginkgo biloba Extract Preventively Intervenes in Citrobacter Rodentium-Induced Colitis in Mice" Nutrients 15, no. 8: 2008. https://doi.org/10.3390/nu15082008
APA StyleChen, T., Chen, Y., Li, K., Chen, Z., Zhao, Q., Fan, Y., Liu, Y., Zhang, S., & Hao, Z. (2023). Ginkgo biloba Extract Preventively Intervenes in Citrobacter Rodentium-Induced Colitis in Mice. Nutrients, 15(8), 2008. https://doi.org/10.3390/nu15082008