Association of Serum Albumin Levels and Long-Term Prognosis in Patients with Biopsy-Confirmed Nonalcoholic Fatty Liver Disease
(This article belongs to the Section Nutrition and Metabolism)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Sources
2.3. Study Cohort
2.4. Clinical Assessment
2.5. Liver Histology
2.6. Follow-Up Evaluation
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics
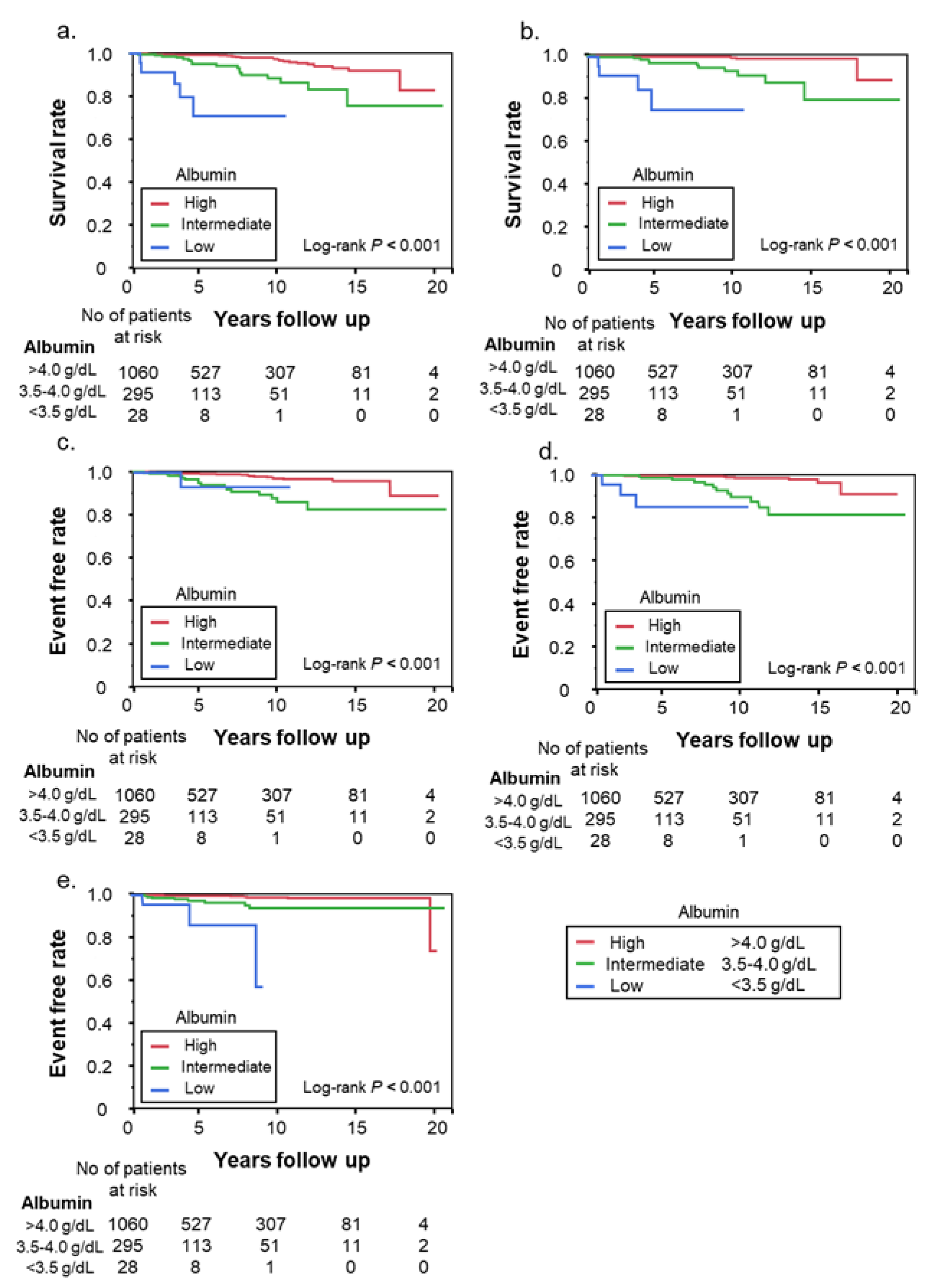
3.2. Clinically Important Outcomes According to Albumin Level
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Wong, V.W.; Chan, W.K.; Chitturi, S.; Chawla, Y.; Dan, Y.Y.; Duseja, A.; Fan, J.; Goh, K.L.; Hamaguchi, M.; Hashimoto, E.; et al. Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease guidelines 2017—Part 1: Definition, risk factors and assessment. J. Gastroenterol. Hepatol. 2018, 33, 70–85. [Google Scholar] [CrossRef]
- Jagdish, R.K.; Maras, J.S.; Sarin, S.K. Albumin in Advanced Liver Diseases: The Good and Bad of a Drug! Hepatology 2021, 74, 2848–2862. [Google Scholar] [CrossRef]
- Bernardi, M.; Angeli, P.; Claria, J.; Moreau, R.; Gines, P.; Jalan, R.; Caraceni, P.; Fernandez, J.; Gerbes, A.L.; O’Brien, A.J.; et al. Albumin in decompensated cirrhosis: New concepts and perspectives. Gut 2020, 69, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, G.; Garcia-Tsao, G.; Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J. Hepatol. 2006, 44, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.R.; Verdelho Machado, M. New Insights About Albumin and Liver Disease. Ann. Hepatol. 2018, 17, 547–560. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Izumi, N.; Charlton, M.R.; Sata, M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology 2011, 54, 1063–1070. [Google Scholar] [CrossRef]
- Kim, S.; McClave, S.A.; Martindale, R.G.; Miller, K.R.; Hurt, R.T. Hypoalbuminemia and Clinical Outcomes: What is the Mechanism behind the Relationship? Am. Surg. 2017, 83, 1220–1227. [Google Scholar] [CrossRef]
- Yoshiji, H.; Nagoshi, S.; Akahane, T.; Asaoka, Y.; Ueno, Y.; Ogawa, K.; Kawaguchi, T.; Kurosaki, M.; Sakaida, I.; Shimizu, M.; et al. Evidence-based clinical practice guidelines for Liver Cirrhosis 2020. J. Gastroenterol. 2021, 56, 593–619. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Bugianesi, E.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Barrera, F.; Haflidadottir, S.; Day, C.P.; George, J. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2013, 145, 782–789.E4. [Google Scholar] [CrossRef]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD fibrosis score: A noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Fujii, H.; Iwaki, M.; Hayashi, H.; Toyoda, H.; Oeda, S.; Hyogo, H.; Kawanaka, M.; Morishita, A.; Munekage, K.; Kawata, K.; et al. Clinical Outcomes in Biopsy-Proven Nonalcoholic Fatty Liver Disease Patients: A Multicenter Registry-based Cohort Study. Clin. Gastroenterol. Hepatol. 2023, 21, 370–379. [Google Scholar] [CrossRef]
- American Diabetes, A. Diagnosis and classification of diabetes mellitus. Diabetes Care 2010, 33 (Suppl. 1), S62–S69. [Google Scholar] [CrossRef]
- Umemura, S.; Arima, H.; Arima, S.; Asayama, K.; Dohi, Y.; Hirooka, Y.; Horio, T.; Hoshide, S.; Ikeda, S.; Ishimitsu, T.; et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2019). Hypertens. Res. 2019, 42, 1235–1481. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, T.; Sasaki, J.; Ueshima, H.; Egusa, G.; Kinoshita, M.; Shimamoto, K.; Daida, H.; Biro, S.; Hirobe, K.; Funahashi, T.; et al. Diagnostic criteria for dyslipidemia. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerotic cardiovascular diseases for Japanese. J. Atheroscler. Thromb. 2007, 14, 155–158. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef]
- Bedossa, P.; Consortium, F.P. Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease. Hepatology 2014, 60, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Kawanaka, M.; Nishino, K.; Ishii, K.; Tanikawa, T.; Urata, N.; Suehiro, M.; Sasai, T.; Haruma, K.; Kawamoto, H. Combination of type IV collagen 7S, albumin concentrations, and platelet count predicts prognosis of non-alcoholic fatty liver disease. World J. Hepatol. 2021, 13, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Vilar-Gomez, E.; Calzadilla-Bertot, L.; Wai-Sun Wong, V.; Castellanos, M.; Aller-de la Fuente, R.; Metwally, M.; Eslam, M.; Gonzalez-Fabian, L.; Alvarez-Quinones Sanz, M.; Conde-Martin, A.F.; et al. Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study. Gastroenterology 2018, 155, 443–457.E17. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.R.; Mannalithara, A.; Heimbach, J.K.; Kamath, P.S.; Asrani, S.K.; Biggins, S.W.; Wood, N.L.; Gentry, S.E.; Kwong, A.J. MELD 3.0: The Model for End-Stage Liver Disease Updated for the Modern Era. Gastroenterology 2021, 161, 1887–1895.E4. [Google Scholar] [CrossRef] [PubMed]
- Muto, Y.; Sato, S.; Watanabe, A.; Moriwaki, H.; Suzuki, K.; Kato, A.; Kato, M.; Nakamura, T.; Higuchi, K.; Nishiguchi, S.; et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin. Gastroenterol. Hepatol. 2005, 3, 705–713. [Google Scholar] [CrossRef]
- Plauth, M.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schutz, T.; Bischoff, S.C. ESPEN guideline on clinical nutrition in liver disease. Clin. Nutr. 2019, 38, 485–521. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Sakai, Y.; Terashima, T.; Shimode, T.; Seki, A.; Orita, N.; Takeshita, Y.; Shimakami, T.; Takatori, H.; Arai, K.; et al. Decline in serum albumin concentration is a predictor of serious events in nonalcoholic fatty liver disease. Medicine 2021, 100, e26835. [Google Scholar] [CrossRef]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schutz, T.; Plauth, M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin. Nutr. 2020, 39, 3533–3562. [Google Scholar] [CrossRef]
- Yoneshiro, T.; Wang, Q.; Tajima, K.; Matsushita, M.; Maki, H.; Igarashi, K.; Dai, Z.; White, P.J.; McGarrah, R.W.; Ilkayeva, O.R.; et al. BCAA catabolism in brown fat controls energy homeostasis through SLC25A44. Nature 2019, 572, 614–619. [Google Scholar] [CrossRef]
- De Bandt, J.P.; Coumoul, X.; Barouki, R. Branched-Chain Amino Acids and Insulin Resistance, from Protein Supply to Diet-Induced Obesity. Nutrients 2023, 15, 68. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.P.; Brandon, A.E.; et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019, 1, 532–545. [Google Scholar] [CrossRef]
- Carpentier, A.C.; Blondin, D.P.; Haman, F.; Richard, D. Brown Adipose Tissue-A Translational Perspective. Endocr. Rev. 2023, 44, 143–192. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Kleiner, D.E.; Dam-Larsen, S.; Adams, L.A.; Bjornsson, E.S.; Charatcharoenwitthaya, P.; Mills, P.R.; Keach, J.C.; Lafferty, H.D.; Stahler, A.; et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015, 149, 389–397.E10. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.G.; Roelstraete, B.; Khalili, H.; Hagstrom, H.; Ludvigsson, J.F. Mortality in biopsy-confirmed nonalcoholic fatty liver disease: Results from a nationwide cohort. Gut 2021, 70, 1375–1382. [Google Scholar] [CrossRef]
- Hagstrom, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stal, P.; Hultcrantz, R.; Kechagias, S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017, 67, 1265–1273. [Google Scholar] [CrossRef]
- Sanyal, A.J.; Van Natta, M.L.; Clark, J.; Neuschwander-Tetri, B.A.; Diehl, A.; Dasarathy, S.; Loomba, R.; Chalasani, N.; Kowdley, K.; Hameed, B.; et al. Prospective Study of Outcomes in Adults with Nonalcoholic Fatty Liver Disease. N. Engl. J. Med. 2021, 385, 1559–1569. [Google Scholar] [CrossRef] [PubMed]


| Albumin (g/dL) | ||||
|---|---|---|---|---|
| Variables | Total N = 1383 | High (>4.0) n = 1060 | Intermediate (3.5–4.0) n = 295 | Low (<3.5) n = 28 |
| Sex, No. (%) | ||||
| Female | 790 (57.1) | 566 (53.4) | 203 (68.8) *** | 21 (75.0) * |
| Male | 593 (42.9) | 494 (46.6) | 92 (31.2) | 7 (25.0) |
| Age, mean (SD), y | 54.6 (14.3) | 53.0 (14.4) | 59.8 (12.4) *** | 60.5 (12.4) ** |
| Age ≥ 65 y, No. (%) | 388 (28.1) | 257 (24.2) | 120 (40.7) *** | 11 (39.3) |
| BMI, mean (SD), kg/m2 | 27.9 (4.7) | 27.8 (4.5) | 28.2 (5.2) | 29.8 (4.8) * |
| Medical history, No. (%) | ||||
| Hypertension | 581 (42.0) | 427 (40.3) | 140 (47.5) * | 14 (50.0) |
| DM | 498 (36.0) | 339 (32.0) | 145 (49.2) *** | 14 (50.0) |
| Dyslipidemia | 793 (57.3) | 617 (58.2) | 163 (55.3) | 13 (46.4) |
| Laboratory data, mean (SD) | ||||
| Albumin, g/dL | 4.3 (0.4) | 4.5 (0.3) | 3.8 (0.2) *** | 3.1 (0.6) *** |
| AST, U/L | 61.2 (39.7) | 58.9 (36.9) | 67.6 (43.7) ** | 81.7 (73.6) ** |
| ALT, U/L | 88.3 (61.1) | 90.5 (61.5) | 81.8 (59.5) * | 71.2 (56.2) |
| GGT, U/L | 88.8 (96.3) | 87.1 (88.2) | 91.6 (114) | 127 (164) * |
| Platelet count,×109/L | 220 (70.3) | 226 (67.3) | 202 (73.2) ** | 156 (86.7) ** |
| FIB-4 index | 2.01 (1.61) | 1.74 (1.27) | 2.71 (1.94) *** | 4.97 (3.30) *** |
| NFS | –1.57 (1.72) | –1.93 (1.56) | –0.51 (1.59) *** | 1.14 (2.00) *** |
| Liver histology, No. (%) | ||||
| NASH (yes) | 925 (66.9) | 693 (65.4) | 216 (73.2) * | 18 (64.3) |
| Fibrosis stage | ||||
| 1/2 | 1160 (83.9) | 926 (87.4) | 221 (74.9) *** | 13 (46.4) *** |
| 3/4 | 223 (16.1) | 134 (12.6) | 74 (25.1) | 15 (53.6) |
| Albumin (g/dL) | ||||
|---|---|---|---|---|
| Total N = 1383 | High (>4.0) N = 1060 | Intermediate (3.5–4.0) n = 295 | Low (<3.5) N = 28 | |
| Death or OLT | 46 | 24 | 17 | 5 |
| Liver-related death | 20 | 2 | 11 | 4 |
| Liver-related events | ||||
| Hepatocellular carcinoma | 36 | 18 | 17 | 1 |
| Decompensated cirrhosis | 23 | 8 | 12 | 3 |
| Gastroesophageal varices | 17 | 6 | 8 | 3 |
| Death or OLT | Liver-Related Death | ||||
|---|---|---|---|---|---|
| Albumin | HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Model 1 | High | 1 (reference) | 1 (reference) | ||
| Intermediate | 3.51 (1.85–6.63) | <0.001 | 11.4 (1.85–6.63) | <0.001 | |
| Low | 23.3 (8.42–64.6) | <0.001 | 99.8 (24.1–412) | <0.001 | |
| Model 2 | High | 1 (reference) | |||
| Intermediate | 3.26 (1.72–6.20) | <0.001 | |||
| Low | 23.6 (8.51–65.6) | <0.001 | |||
| Model 3 | High | 1 (reference) | |||
| Intermediate | 3.33 (1.74–6.38) | <0.001 | |||
| Low | 23.0 (8.21–64.3) | <0.001 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, H.; Kawanaka, M.; Fujii, H.; Iwaki, M.; Hayashi, H.; Toyoda, H.; Oeda, S.; Hyogo, H.; Morishita, A.; Munekage, K.; et al. Association of Serum Albumin Levels and Long-Term Prognosis in Patients with Biopsy-Confirmed Nonalcoholic Fatty Liver Disease. Nutrients 2023, 15, 2014. https://doi.org/10.3390/nu15092014
Takahashi H, Kawanaka M, Fujii H, Iwaki M, Hayashi H, Toyoda H, Oeda S, Hyogo H, Morishita A, Munekage K, et al. Association of Serum Albumin Levels and Long-Term Prognosis in Patients with Biopsy-Confirmed Nonalcoholic Fatty Liver Disease. Nutrients. 2023; 15(9):2014. https://doi.org/10.3390/nu15092014
Chicago/Turabian StyleTakahashi, Hirokazu, Miwa Kawanaka, Hideki Fujii, Michihiro Iwaki, Hideki Hayashi, Hidenori Toyoda, Satoshi Oeda, Hideyuki Hyogo, Asahiro Morishita, Kensuke Munekage, and et al. 2023. "Association of Serum Albumin Levels and Long-Term Prognosis in Patients with Biopsy-Confirmed Nonalcoholic Fatty Liver Disease" Nutrients 15, no. 9: 2014. https://doi.org/10.3390/nu15092014
APA StyleTakahashi, H., Kawanaka, M., Fujii, H., Iwaki, M., Hayashi, H., Toyoda, H., Oeda, S., Hyogo, H., Morishita, A., Munekage, K., Kawata, K., Tsutsumi, T., Sawada, K., Maeshiro, T., Tobita, H., Yoshida, Y., Naito, M., Araki, A., Arakaki, S., ... Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD). (2023). Association of Serum Albumin Levels and Long-Term Prognosis in Patients with Biopsy-Confirmed Nonalcoholic Fatty Liver Disease. Nutrients, 15(9), 2014. https://doi.org/10.3390/nu15092014











