Prenatal Vitamin D Levels Influence Growth and Body Composition until 11 Years in Boys
Abstract
1. Introduction
2. Materials and Methods
2.1. Population of Study
2.2. Vitamin D
2.3. Outcome Assessment
2.4. BMI Polygenic Risk Scores
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Characteristics of the Population
3.2. Vitamin D Associations with Body Composition Outcome and zBMI Trajectories
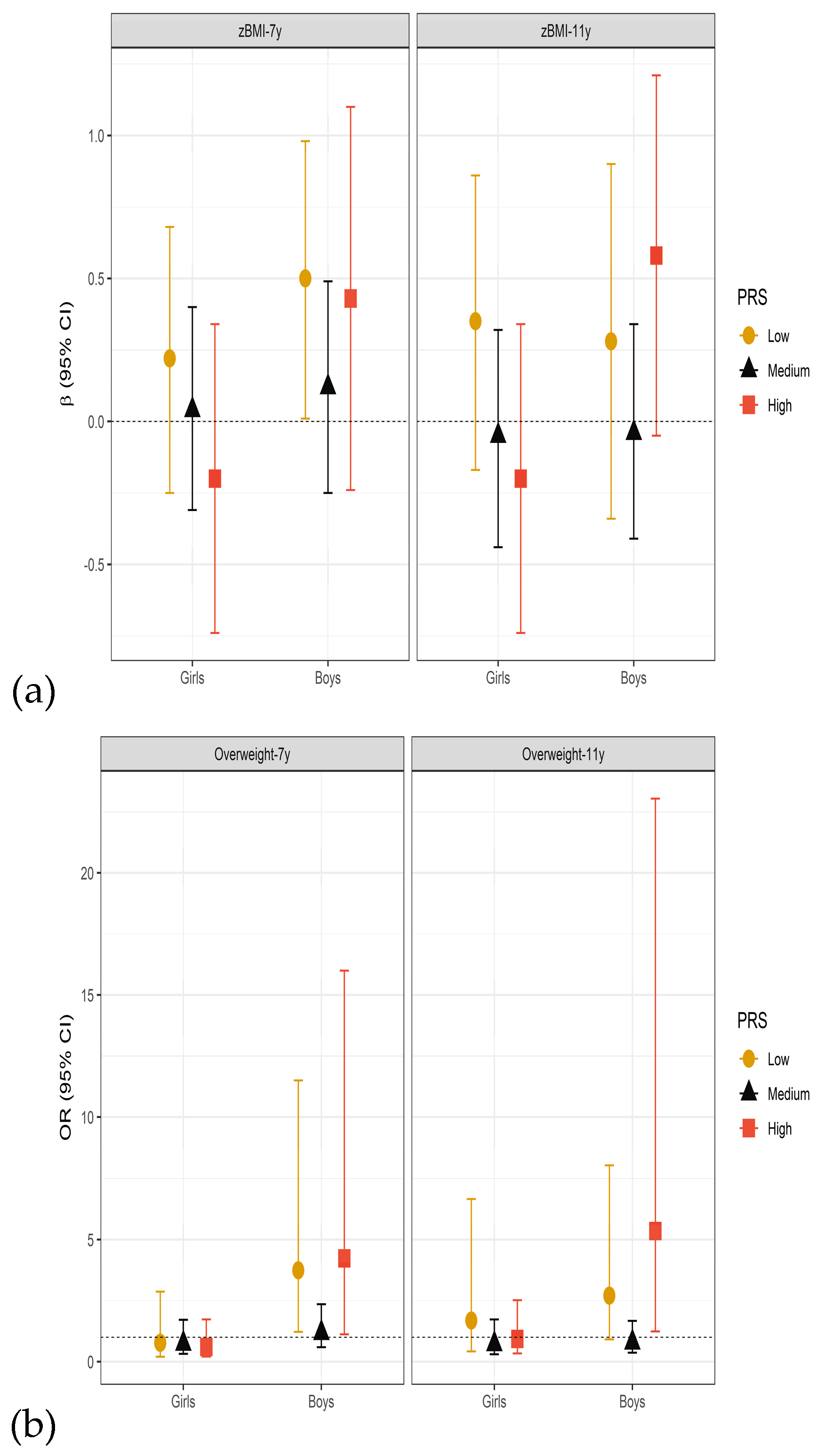
3.3. Sensitivity and Effect Modification Analyses
4. Discussion
4.1. Association between Early Pregnancy Maternal Vitamin D Levels and Child Body Composition
4.2. Sex-Specific Associations
4.3. Other Potential Effect Modifiers
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saraf, R.; Morton, S.M.B.; Camargo, C.A.; Grant, C.C. Global Summary of Maternal and Newborn Vitamin D Status–A Systematic Review. Matern. Child. Nutr. 2016, 12, 647–668. [Google Scholar] [CrossRef] [PubMed]
- Brannon, P.M.; Picciano, M.F. Vitamin D in Pregnancy and Lactation in Humans. Annu. Rev. Nutr. 2011, 31, 89–115. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Crozier, S.R.; Dennison, E.M.; Davies, J.H.; Robinson, S.M.; Inskip, H.M.; Godfrey, K.M.; Cooper, C.; Harvey, N.C. Tracking of 25-HydroxyVitamin D Status during Pregnancy: The Importance of Vitamin D Supplementation. Am. J. Clin. Nutr. 2015, 102, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.P.; Osmond, C.; Forsén, T.J.; Kajantie, E.; Eriksson, J.G. Trajectories of Growth among Children Who Have Coronary Events as Adults. N. Engl. J. Med. 2005, 353, 1802–1809. [Google Scholar] [CrossRef]
- Kumar, S.; Kelly, A.S. Review of Childhood Obesity: From Epidemiology, Etiology and Comorbidities to Clinical Assessment and Treatment. Mayo Clin. Proc. 2017, 92, 251–265. [Google Scholar] [CrossRef]
- Miliku, K.; Vinkhuyzen, A.; Blanken, L.M.E.; McGrath, J.J.; Eyles, D.W.; Burne, T.H.; Hofman, A.; Tiemeier, H.; Steegers, E.A.P.; Gaillard, R.; et al. Maternal Vitamin D Concentrations during Pregnancy, Fetal Growth Patterns, and Risks of Adverse Birth Outcomes. Am. J. Clin. Nutr. 2016, 103, 1514–1522. [Google Scholar] [CrossRef]
- Reichetzeder, C.; Dwi Putra, S.E.; Li, J.; Hocher, B. Developmental Origins of Disease-Crisis Precipitates Change. Cell. Physiol. Biochem. 2016, 39, 919–938. [Google Scholar] [CrossRef]
- Weaver, C.M. Vitamin D, Calcium Homeostasis, and Skeleton Accretion in Children. J. Bone Miner. Res. 2007, 22, V45–V49. [Google Scholar] [CrossRef]
- Dix, C.F.; Barcley, J.L.; Wright, O.R.L. The Role of Vitamin D in Adipogenesis. Nutr. Rev. 2018, 76, 47–59. [Google Scholar] [CrossRef]
- Wood, R.J. Vitamin D and Adipogenesis: New Molecular Insights. Nutr. Rev. 2008, 66, 40–46. [Google Scholar] [CrossRef]
- Mutt, S.J.; Hyppönen, E.; Saarnio, J.; Järvelin, M.R.; Herzig, H.K. Vitamin D and Adipose Tissue-More than Storage. Front. Physiol. 2014, 24, 228. [Google Scholar] [CrossRef]
- Ochs-Balcom, H.M.; Chennamaneni, R.; Millen, A.E.; Shields, P.G.; Marian, C.; Trevisan, M.; Freudenheim, J.L. Vitamin D Receptor Gene Polymorphisms Are Associated with Adiposity Phenotypes. Am. J. Clin. Nutr. 2011, 93, 5–10. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Anguita-Ruiz, A.; Leis, R.; Aguilera, C.M. Genetic Factors and Molecular Mechanisms of Vitamin D and Obesity Relationship. Ann. Nutr. Metab. 2018, 73, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Kim, Y.J.; Lee, H.; Gwak, H.S.; Park, E.A.; Cho, S.J.; Oh, S.Y.; Ha, E.H.; Kim, H.S.; Park, H. Association of Vitamin D Concentrations with Adiposity Indices among Preadolescent Children in Korea. J. Pediatr. Endocrinol. Metab. 2013, 26, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.M.S.; Novaes, J.F.; Azeredo, L.M.; Cândido, A.P.C.; Leite, I.C.G. Association of Vitamin D Insufficiency with Adiposity and Metabolic Disorders in Brazilian Adolescents. Public Health Nutr. 2014, 17, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rodríguez, E.; Navia-Lombán, B.; López-Sobaler, A.M.; Ortega, R.M. Associations between Abdominal Fat and Body Mass Index on Vitamin D Status in a Group of Spanish Schoolchildren. Eur. J. Clin. Nutr. 2010, 64, 461–467. [Google Scholar] [CrossRef]
- Rytter, D.; Bech, B.H.; Halldorsson, T.I.; Henriksen, T.B.; Grandström, C.; Cohen, A.; Olsen, S.F. Maternal Vitamin D Status at Week 30 of Gestation and Offspring Cardio-Metabolic Health at 20 Years: A Prospective Cohort Study over Two Decades. PLoS ONE 2016, 11, e0164758. [Google Scholar] [CrossRef]
- Larsen, S.D.; Christensen, M.E.; Dalgård, C.; Lykkedegn, S.; Andersen, L.B.; Andersen, M.S.; Glintborg, D.; Christesen, H.T. Pregnancy or Cord 25-Hydroxyvitamin D Is Not Associated with Measures of Body Fat or Adiposity in Children from Three Months to Three Years of Age. An Odense Child Cohort Study. Clin. Nutr. 2020, 39, 1832–1839. [Google Scholar] [CrossRef]
- Krishnaveni, G.V.; Veena, S.R.; Winder, N.R.; Hill, J.C.; Noonan, K.; Boucher, B.J.; Karat, S.C.; Fall, C.H.D. Maternal Vitamin D Status during Pregnancy and Body Composition and Cardiovascular Risk Markers in Indian Children: The Mysore Parthenon Study. Am. J. Clin. Nutr. 2011, 93, 628–635. [Google Scholar] [CrossRef]
- Gale, C.R.; Robinson, S.M.; Harvey, N.C.; Javaid, M.K.; Jiang, B.; Martyn, C.N.; Godfrey, K.M.; Cooper, C. Maternal Vitamin D Status during Pregnancy and Child Outcomes. Eur. J. Clin. Nutr. 2008, 62, 68–77. [Google Scholar] [CrossRef]
- Morales, E.; Rodriguez, A.; Valvi, D.; Iñiguez, C.; Esplugues, A.; Vioque, J.; Marina, L.S.; Jiménez, A.; Espada, M.; Dehli, C.R.; et al. Deficit of Vitamin D in Pregnancy and Growth and Overweight in the Offspring. Int. J. Obes. 2015, 39, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Daraki, V.; Roumeliotaki, T.; Chalkiadaki, G.; Katrinaki, M.; Karachaliou, M.; Leventakou, V.; Vafeiadi, M.; Sarri, K.; Vassilaki, M.; Papavasiliou, S.; et al. Low Maternal Vitamin D Status in Pregnancy Increases the Risk of Childhood Obesity. Pediatr. Obes. 2018, 13, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Hrudey, E.J.; Reynolds, R.M.; Oostvogels, A.J.J.M.; Brouwer, I.A.; Vrijkotte, T.G.M. The Association between Maternal 25-Hydroxyvitamin D Concentration during Gestation and Early Childhood Cardiometabolic Outcomes: Is There Interaction with Pre-Pregnancy BMI? PLoS ONE 2015, 10, e0133313. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wei, S.Q.; Bi, W.G.; Weiler, H.A.; Wen, S.W. Effect of Vitamin d Supplementation in Early Life on Children’s Growth and Body Composition: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 524. [Google Scholar] [CrossRef]
- Amberntsson, A.; Papadopoulou, E.; Winkvist, A.; Lissner, L.; Meltzer, H.M.; Brantsaeter, A.L.; Augustin, H. Maternal Vitamin D Intake and BMI during Pregnancy in Relation to Child’s Growth and Weight Status from Birth to 8 Years: A Large National Cohort Study. BMJ Open 2021, 11, 101–113. [Google Scholar] [CrossRef]
- Hyde, N.K.; Brennan-Olsen, S.L.; Wark, J.D.; Hosking, S.M.; Holloway-Kew, K.L.; Pasco, J.A. Vitamin D during Pregnancy and Offspring Body Composition: A Prospective Cohort Study. Pediatr. Obes. 2018, 13, 514–521. [Google Scholar] [CrossRef]
- Montazeri, P.; Vrijheid, M.; Martinez, D.; Basterrechea, M.; Fernandez-Somoano, A.; Guxens, M.; Iñiguez, C.; Lertxundi, A.; Murcia, M.; Tardon, A.; et al. Maternal Metabolic Health Parameters During Pregnancy in Relation to Early Childhood BMI Trajectories. Obesity 2018, 26, 588–596. [Google Scholar] [CrossRef]
- Guxens, M.; Ballester, F.; Espada, M.; Fernández, M.F.; Grimalt, J.O.; Ibarluzea, J.; Olea, N.; Rebagliato, M.; Tardon, A.; Torrent, M.; et al. Cohort Profile: The INMA—INfancia y Medio Ambiente—(Environment and Childhood) Project. Int. J. Epidemiol. 2012, 41, 930–940. [Google Scholar] [CrossRef]
- GmbH Laboratories Instruction Manual BIO-RAD. 25(OH)-Vitamin D3 by HPLC; GmbH Laboratories Instruction Manual BIO-RAD: Muchen, Germany, 2003. [Google Scholar]
- Morales, E.; Guxens, M.; Llop, S.; Rodríguez-Bernal, C.L.; Tardón, A.; Riaño, I.; Ibarluzea, J.; Lertxundi, N.; Espada, M.; Rodriguez, A.; et al. Circulating 25-Hydroxyvitamin D3 in Pregnancy and Infant Neuropsychological Development. Pediatrics 2012, 130, e913–e920. [Google Scholar] [CrossRef]
- De Onis, M. 4.1 The WHO Child Growth Standards. World Rev. Nutr. Diet. 2015, 113, 278–294. [Google Scholar] [CrossRef]
- Multicentre Growth Reference Study Group. WHO Child Growth Standards Based on Length/Height, Weight and Age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar] [CrossRef]
- Horlick, M.; Arpadi, S.M.; Bethel, J.; Wang, J.; Moye, J.; Cuff, P.; Pierson, R.N.; Kotler, D. Bioelectrical Impedance Analysis Models for Prediction of Total Body Water and Fat-Free Mass in Healthy and HIV-Infected Children and Adolescents. Am. J. Clin. Nutr. 2002, 76, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Montazeri, P.; Fossati, S.; Clemente, D.B.P.; Cirugeda, L.; Elosua, R.; Fernández-barrés, S.; Fochs, S.; Garcia-esteban, R.; Marquez, S.; Pey, N.; et al. Early-Childhood BMI Trajectories in Relation to Preclinical Cardiovascular Measurements in Adolescence. J. Dev. Orig. Heal. Dis. 2022, 13, 322–329. [Google Scholar] [CrossRef]
- Choi, S.W.; O’Reilly, P.F. PRSice-2: Polygenic Risk Score Software for Biobank-Scale Data. Gigascience 2019, 8, 1–6. [Google Scholar] [CrossRef]
- Wegienka, G.; Havstad, S.; Zoratti, E.M.; Kim, H.; Ownby, D.R.; Johnson, C.C. Association between Vitamin D Levels and Allergy-Related Outcomes Vary by Race and Other Factors. J. Allergy Clin. Immunol. 2015, 136, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Berents, T.L.; Lødrup Carlsen, K.C.; Mowinckel, P.; Sandvik, L.; Skjerven, H.O.; Rolfsjord, L.B.; Kvenshagen, B.; Hunderi, J.O.G.; Bradley, M.; Lieden, A.; et al. Vitamin D Levels and Atopic Eczema in Infancy and Early Childhood in Norway: A Cohort Study. Br. J. Dermatol. 2016, 175, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Van Buuren, S.; Boshuizen, H.C.; Knook, D.L. Multiple Imputation of Missing Blood Pressure Covariates in Survival Analysis. Stat. Med. 1999, 18, 681–694. [Google Scholar] [CrossRef]
- Miliku, K.; Felix, J.F.; Voortman, T.; Tiemeier, H.; Eyles, D.W.; Burne, T.H.; McGrath, J.J.; Jaddoe, V.W.V. Associations of Maternal and Fetal Vitamin D Status with Childhood Body Composition and Cardiovascular Risk Factors. Matern. Child Nutr. 2019, 15, e12672. [Google Scholar] [CrossRef]
- Boyle, V.T.; Thorstensen, E.B.; Thompson, J.M.D.; McCowan, L.M.E.; Mitchell, E.A.; Godfrey, K.M.; Poston, L.; Wall, C.R.; Murphy, R.; Cutfield, W.; et al. The Relationship between Maternal 25-Hydroxyvitamin D Status in Pregnancy and Childhood Adiposity and Allergy: An Observational Study. Int. J. Obes. 2017, 41, 1755–1760. [Google Scholar] [CrossRef]
- Crozier, S.R.; Harvey, N.C.; Inskip, H.M.; Godfrey, K.M. Maternal Vitamin D Status in Pregnancy Is Associated with Adiposity in the Offspring: Prospective Observational Study. Am. J. Clin. Nutr. 2012, 96, 57–63. [Google Scholar] [CrossRef]
- Poissonnet, C.M.; Burdi, A.R.; Garn, S.M. The Chronology of Adipose Tissue Appearance and Distribution in the Human Fetus. Early Hum. Dev. 1984, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Belenchia, A.M.; Jones, K.L.; Will, M.; Beversdorf, D.Q.; Vieira-Potter, V.; Rosenfeld, C.S.; Peterson, C.A. Maternal Vitamin D Deficiency during Pregnancy Affects Expression of Adipogenic-Regulating Genes Peroxisome Proliferator-Activated Receptor Gamma (PPARγ) and Vitamin D Receptor (VDR) in Lean Male Mice Offspring. Eur. J. Nutr. 2018, 57, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Hong, Q.; Wang, X.; Zhu, L.; Wu, T.; Xu, P.; Fu, Z.; You, L.; Ji, C.; Guo, X. The Effect of Maternal Vitamin D Deficiency during Pregnancy on Body Fat and Adipogenesis in Rat Offspring. Sci. Rep. 2018, 8, 365. [Google Scholar] [CrossRef]
- Bennour, I.; Haroun, N.; Sicard, F.; Mounien, L.; Landrier, J.F. Recent Insights into Vitamin D, Adipocyte, and Adipose Tissue Biology. Obes. Rev. 2022, 23, e13453. [Google Scholar] [CrossRef] [PubMed]
- Olmos-Ortiz, A.; García-Quiroz, J.; López-Marure, R.; González-Curiel, I.; Rivas-Santiago, B.; Olivares, A.; Avila, E.; Barrera, D.; Halhali, A.; Caldiño, F.; et al. Evidence of Sexual Dimorphism in Placental Vitamin D Metabolism: Testosterone Inhibits Calcitriol-Dependent Cathelicidin Expression. J. Steroid Biochem. Mol. Biol. 2016, 163, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Seipelt, E.M.; Tourniaire, F.; Couturier, C.; Astier, J.; Loriod, B.; Vachon, H.; Pucéat, M.; Mounien, L.; Landrier, J.F. Prenatal Maternal Vitamin D Deficiency Sex-Dependently Programs Adipose Tissue Metabolism and Energy Homeostasis in Offspring. FASEB J. 2020, 34, 14905–14919. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, C.; Bi, W.G.; Leduc, L.; Tabatabaei, N.; Jantchou, P.; Luo, Z.; Audibert, F.; Nuyt, A.M.; Wei, S.Q. Prenatal Vitamin D Status and Offspring ’ s Growth, Adiposity and Metabolic Health: A Systematic Review and Meta-Analysis. Br. J. Nutr. 2018, 25, 310–319. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased Bioavailability of Vitamin D in Obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Dirks, N.F.; Ackermans, M.T.; Lips, P.; De Jongh, R.T.; Vervloet, M.G.; de Jonge, R.; Heijboer, A.C. The When, What & How of Measuring Vitamin D Metabolism in Clinical Medicine. Nutrients 2018, 10, 482. [Google Scholar] [CrossRef]
- Lensmeyer, G.L.; Wiebe, D.A.; Binkley, N.; Drezner, M.K. HPLC Method for 25-Hydroxyvitamin D Measurement: Comparison with Contemporary Assays. Clin. Chem. 2006, 52, 1120–1126. [Google Scholar] [CrossRef]
- Roth, H.J.; Schmidt-Gayk, H.; Weber, H.; Niederau, C. Accuracy and Clinical Implications of Seven 25-Hydroxyvitamin D Methods Compared with Liquid Chromatography-Tandem Mass Spectrometry as a Reference. Ann. Clin. Biochem. 2008, 45, 153–159. [Google Scholar] [CrossRef]
- Sangüesa, J.; Sunyer, J.; Garcia-Esteban, R.; Abellan, A.; Esplugues, A.; Garcia-Aymerich, J.; Guxens, M.; Irizar, A.; Júlvez, J.; Luque-García, L.; et al. Prenatal and Child Vitamin D Levels and Allergy and Asthma in Childhood. Pediatr. Res. 2022, 1–7. [Google Scholar] [CrossRef]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.A.M.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Anderson, C.A.; Pettersson, F.H.; Clarke, G.M.; Cardon, L.R.; Morris, A.P.; Zondervan, K.T. Data quality control in genetic case-control association studies. Nat. Protoc. 2010, 5, 1564–1573. [Google Scholar] [CrossRef]
- Das, S.; Forer, L.; Schönherr, S.; Sidore, C.; Locke, A.E.; Kwong, A.; Vrieze, S.I.; Chew, E.Y.; Levy, S.; McGue, M.; et al. Next-generation genotype imputation service and methods. Nat. Genet. 2016, 48, 1284–1287. [Google Scholar] [CrossRef]
- McCarthy, S.; Das, S.; Kretzschmar, W.; Delaneau, O.; Wood, A.R.; Teumer, A.; Kang, H.M.; Fuchsberger, C.; Danecek, P.; Sharp, K.; et al. A reference panel of 64,976 haplotypes for genotype imputation. Nat. Genet. 2016, 48, 1279–1283. [Google Scholar] [PubMed]
- Loh, P.-R.; Danecek, P.; Palamara, P.F.; Fuchsberger, C.; Reshef, Y.A.; Finucane, H.K.; Schoenherr, S.; Forer, L.; McCarthy, S.; Abecasis, C.F.G.R.; et al. Reference-based phasing using the Haplotype Reference Consortium panel. Nat. Genet. 2016, 48, 1443–1448. [Google Scholar] [CrossRef] [PubMed]
- Fuchsberger, C.; Abecasis, G.R.; Hinds, D.A. Minimac2: Faster genotype imputation. Bioinformatics 2015, 31, 782–784. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.S.; de Andrade, M.; Tromp, G.; Kuivaniemi, H.; Epugh, E.; Namjou-Khales, B.; Mukherjee, S.; Jarvik, G.P.; Kottyan, L.; Eburt, A.; et al. Imputation and quality control steps for combining multiple genome-wide datasets. Front. Genet. 2014, 5, 370. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.S.; Quinlan, A.R. Who’s Who? Detecting and Resolving Sample Anomalies in Human DNA Sequencing Studies with Peddy. Am. J. Hum. Genet. 2017, 100, 406–413. [Google Scholar] [CrossRef] [PubMed]
| Study Population | |||||
|---|---|---|---|---|---|
| Total | Deficient | Adequate | |||
| % Missing | n = 2027 | n = 355 | N = 1672 | p-Value | |
| Maternal characteristics | |||||
| Region of residence; % | 0 | ||||
| Gipuzkoa | 26.7 | 26.2 | 26.8 | <0.001 | |
| Sabadell | 27.4 | 37.5 | 25.5 | ||
| Valencia | 32.4 | 21.9 | 34.4 | ||
| Menorca | 13.5 | 14.4 | 13.3 | ||
| Maternal country of birth; % Spain | 0.3 | 92.2 | 88.7 | 92.9 | 0.015 |
| Maternal age; years (SD) | 0 | 30.3 (4.2) | 29.9 (4.4) | 30.4 (4.2) | 0.043 |
| Maternal pregnancy BMI; kg/m2 (SD) | 0.3 | 23.4 (4.2) | 23.5 (4.3) | 23.4 (4.2) | 0.762 |
| Maternal pregnancy weight status; % | |||||
| Underweight | 4.4 | 6.6 | 4.0 | 0.093 | |
| Normal | 70.8 | 66.1 | 71.7 | ||
| Overweight | 17.5 | 19.7 | 17.0 | ||
| Obese | 7.3 | 7.5 | 7.3 | ||
| Maternal education; % | 0.5 | ||||
| Primary or less | 29.4 | 34.8 | 28.3 | 0.036 | |
| Secondary | 38.8 | 38.2 | 39.0 | ||
| High | 31.8 | 27.0 | 32.7 | ||
| Maternal social class; % | 3.4 | ||||
| Low | 21.2 | 17.1 | 22.0 | 0.020 | |
| Medium | 32.0 | 29.0 | 32.5 | ||
| High | 46.8 | 53.9 | 45.5 | ||
| Maternal smoking pregnancy; % | 1.9 | ||||
| Never | 67.7 | 65.1 | 68.2 | 0.033 | |
| Only early in pregnancy | 15.8 | 13.7 | 16.3 | ||
| During whole pregnancy | 16.5 | 21.3 | 15.5 | ||
| Parity; % | 2.3 | ||||
| No previous pregnancies | 54.8 | 56.8 | 54.4 | 0.461 | |
| One or more | 45.2 | 43.2 | 45.6 | ||
| Physical activity | 14.6 | 0.449 | |||
| Sedentary; % | 5.4 | 6.6 | 5.2 | ||
| Slightly active; % | 24.5 | 26.9 | 24.0 | ||
| Moderately active; % | 42.6 | 41.7 | 42.7 | ||
| Quite active/very active; % | 27.5 | 24.7 | 28.1 | ||
| Adherence Mediterranean diet | 14.2 | 0.509 | |||
| Low; % | 42.3 | 41.0 | 42.5 | ||
| Medium; % | 28.6 | 31.5 | 28.1 | ||
| High; % | 29.1 | 27.5 | 29.4 | ||
| Gestational diabetes mellitus; % yes | 15.7 | 4.6 | 5.2 | 4.4 | 0.686 |
| Child characteristics | |||||
| Sex; % male | 0 | 50.8 | 55.0 | 50.0 | 0.116 |
| Birth weight; grams (SD) | 0.4 | 3248 (475) | 3261 (461) | 3245 (478) | 0.578 |
| Gestational age; weeks (SD) | 0.1 | 39.6 (1.6) | 39.5 (1.6) | 39.6 (1.6) | 0.495 |
| Preterm (<37 weeks); % | 0.1 | 4.2 | 4.1 | 4.2 | 1 |
| Breastfeeding duration; weeks (SD) | 2.1 | 23.1 (20.1) | 23.0 (20.2) | 23.2 (20.0) | 0.886 |
| Total Study Population | Girls | Boys | |
|---|---|---|---|
| N = 2027 | N = 997 | N = 1030 | |
| Child outcome characteristics | |||
| Age—7 years (years) | 7.3 (0.6) | 7.3 (0.6) | 7.3 (0.6) |
| zBMI—7 years | 0.74 (1.2) | 0.68 (1.1) | 0.80 (1.3) |
| Overweight—7 years: % | 38.9 | 37.2 | 40.5 |
| Age—11 years (years) | 11.1 (0.5) | 11.1 (0.5) | 11.1 (0.5) |
| zBMI—11 years | 0.65 (1.2) | 0.57 (1.1) | 0.74 (1.3) |
| Overweight—11 years: % | 40.6 | 37.4 | 43.9 |
| Fat mass—11 years (%) | 25.5 (8.2) | 26.6 (7.9) | 24.3 (8.4) |
| BMI trajectories from birth to 11 years | |||
| Class 1: higher birth size/accelerated gain | 11.3 | 9.7 | 12.8 |
| Class 2: higher birth size—slower gain | 24.1 | 24.3 | 23.8 |
| Class 3: lower birth size—accelerated gain | 17.5 | 16.6 | 18.3 |
| Class 4: average birth size—slower gain (ref.) | 31.9 | 34.5 | 29.4 |
| Class 5: lower birth size—slower gain | 15.2 | 14.8 | 15.6 |
| Total Study Population | Girls | Boys | |||||||
| 5 ng/mL Decrease | Deficient | 5 ng/mL Decrease | Deficient | 5 ng/mL Decrease | Deficient | ||||
| N | β (95% CI) | β (95% CI) | N | β (95% CI) | β (95% CI) | N | β (95% CI) | β (95% CI) | |
| BMI z-score | |||||||||
| 7 years | 1536 | 0.01 (−0.02–0.04) | 0.16 (0.01–0.32) | 769 | −0.02 (−0.05–0.02) | 0.06 (−0.15–0.27) | 767 | 0.04 (0.00–0.09) | 0.29 (0.03–0.55) |
| 11 years | 1390 | 0.01 (−0.02–0.04) | 0.15 (−0.01–0.32) | 716 | −0.02 (−0.06–0.02) | 0.01 (−0.21–0.23) | 674 | 0.04 (−0.01–0.09) | 0.28 (0.03–0.52) |
| Body composition 11 years | |||||||||
| Body fat (%) | 1375 | 0.07 (−0.13–0.26) | 0.81 (−0.28–1.91) | 709 | −0.13 (−0.39–0.12) | 0.01 (−1.50–1.52) | 666 | 0.33 (0.04–0.63) | 1.78 (0.24–3.32) |
| 5 ng/mL decrease | Deficient | 5 ng/mL decrease | Deficient | 5 ng/mL decrease | Deficient | ||||
| N | OR (95% CI) | OR (95% CI) | N | OR (95% CI) | OR (95% CI) | N | OR (95% CI) | OR (95% CI) | |
| Overweight | |||||||||
| 7 years | 1536 | 1.00 (0.95–1.05) | 1.25 (0.93–1.68) | 769 | 0.95 (0.88–1.02) | 0.83 (0.53–1.30) | 767 | 1.07 (0.99–1.15) | 1.76 (1.18–2.64) |
| 11 years | 1390 | 1.02 (0.96–1.08) | 1.30 (0.96–1.76) | 716 | 0.97 (0.90–1.05) | 0.97 (0.61–1.53) | 674 | 1.08 (0.99–1.17) | 1.68 (1.10–2.56) |
| Total Study Population | Girls | Boys | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 5 ng/mL Decrease | Deficient | 5 ng/mL Decrease | Deficient | 5 ng/mL Decrease | Deficient | ||||
| N | RRR (95% CI) | RRR (95% CI) | N | RRR (95% CI) | β (95% CI) | N | RRR (95% CI) | β (95% CI) | |
| zBMI trajectories | 1.738 | 843 | 895 | ||||||
| 1: higher birth size & accelerated BMI gain | 197 | 1.01 (0.94–1.09) | 1.15 (0.73–1.82) | 82 | 1.02 (0.91–1.15) | 0.89 (0.42–1.86) | 115 | 1.00 (0.90–1.11) | 1.49 (0.81–2.73) |
| 2: higher birth size & slower BMI gain | 418 | 1.05 (0.99–1.12) | 1.23 (0.87–1.75) | 205 | 1.07 (0.98–1.17) | 1.08 (0.66–1.77) | 213 | 1.04 (0.95–1.13) | 1.41 (0.85–2.37) |
| 3: lower birth size & accelerated BMI gain | 304 | 1.02 (0.95–1.09) | 1.11 (0.74–1.66) | 140 | 0.95 (0.87–1.05) | 0.62 (0.32–1.20) | 164 | 1.08 (0.98–1.19) | 1.76 (1.02–3.02) |
| 5: lower birth size & average BMI gain | 265 | 0.99 (0.93–1.06) | 0.83 (0.53–1.30) | 125 | 1.00 (0.91–1.11) | 0.70 (0.37–1.33) | 140 | 0.98 (0.89–1.08) | 1.01 (0.54–1.91) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanguesa, J.; Marquez, S.; Bustamante, M.; Sunyer, J.; Iniguez, C.; Vioque, J.; Rodriguez, L.S.-M.; Jimeno-Romero, A.; Torrent, M.; Casas, M.; et al. Prenatal Vitamin D Levels Influence Growth and Body Composition until 11 Years in Boys. Nutrients 2023, 15, 2033. https://doi.org/10.3390/nu15092033
Sanguesa J, Marquez S, Bustamante M, Sunyer J, Iniguez C, Vioque J, Rodriguez LS-M, Jimeno-Romero A, Torrent M, Casas M, et al. Prenatal Vitamin D Levels Influence Growth and Body Composition until 11 Years in Boys. Nutrients. 2023; 15(9):2033. https://doi.org/10.3390/nu15092033
Chicago/Turabian StyleSanguesa, Julia, Sandra Marquez, Mariona Bustamante, Jordi Sunyer, Carmen Iniguez, Jesus Vioque, Loreto Santa-Marina Rodriguez, Alba Jimeno-Romero, Matias Torrent, Maribel Casas, and et al. 2023. "Prenatal Vitamin D Levels Influence Growth and Body Composition until 11 Years in Boys" Nutrients 15, no. 9: 2033. https://doi.org/10.3390/nu15092033
APA StyleSanguesa, J., Marquez, S., Bustamante, M., Sunyer, J., Iniguez, C., Vioque, J., Rodriguez, L. S.-M., Jimeno-Romero, A., Torrent, M., Casas, M., & Vrijheid, M. (2023). Prenatal Vitamin D Levels Influence Growth and Body Composition until 11 Years in Boys. Nutrients, 15(9), 2033. https://doi.org/10.3390/nu15092033









