The Antitumor Potential of λ-Carrageenan Oligosaccharides on Gastric Carcinoma by Immunomodulation
Abstract
1. Introduction
2. Materials and Methods
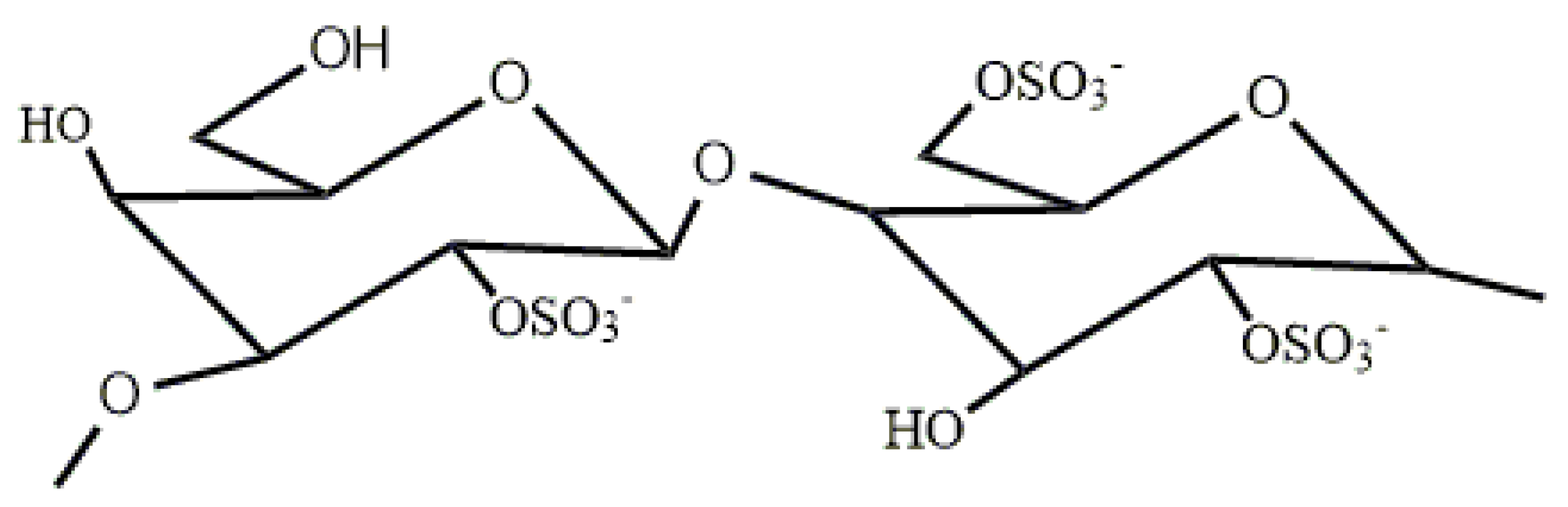
2.1. Preparation and Purification of λ-COS
2.2. Cell Lines and Reagents
2.3. Collection of Conditioned Medium
2.4. Cell Counting Kit-8 (CCK-8) Assay
2.5. DAPI Staining
2.6. Annexin-V/PI Apoptosis Detection
2.7. Measurement of Mitochondrial Transmembrane Potential
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Neutral Red Uptake Assay
2.10. Western Blotting Analysis
2.11. Xenografted Model and Treatment Procedures
2.12. Histopathological Assessment
2.13. In Vivo Phagocytosis Assay
2.14. Analysis of Cytokine Levels
2.15. Immunohistochemical Detection
2.16. Statistical Analysis
3. Results
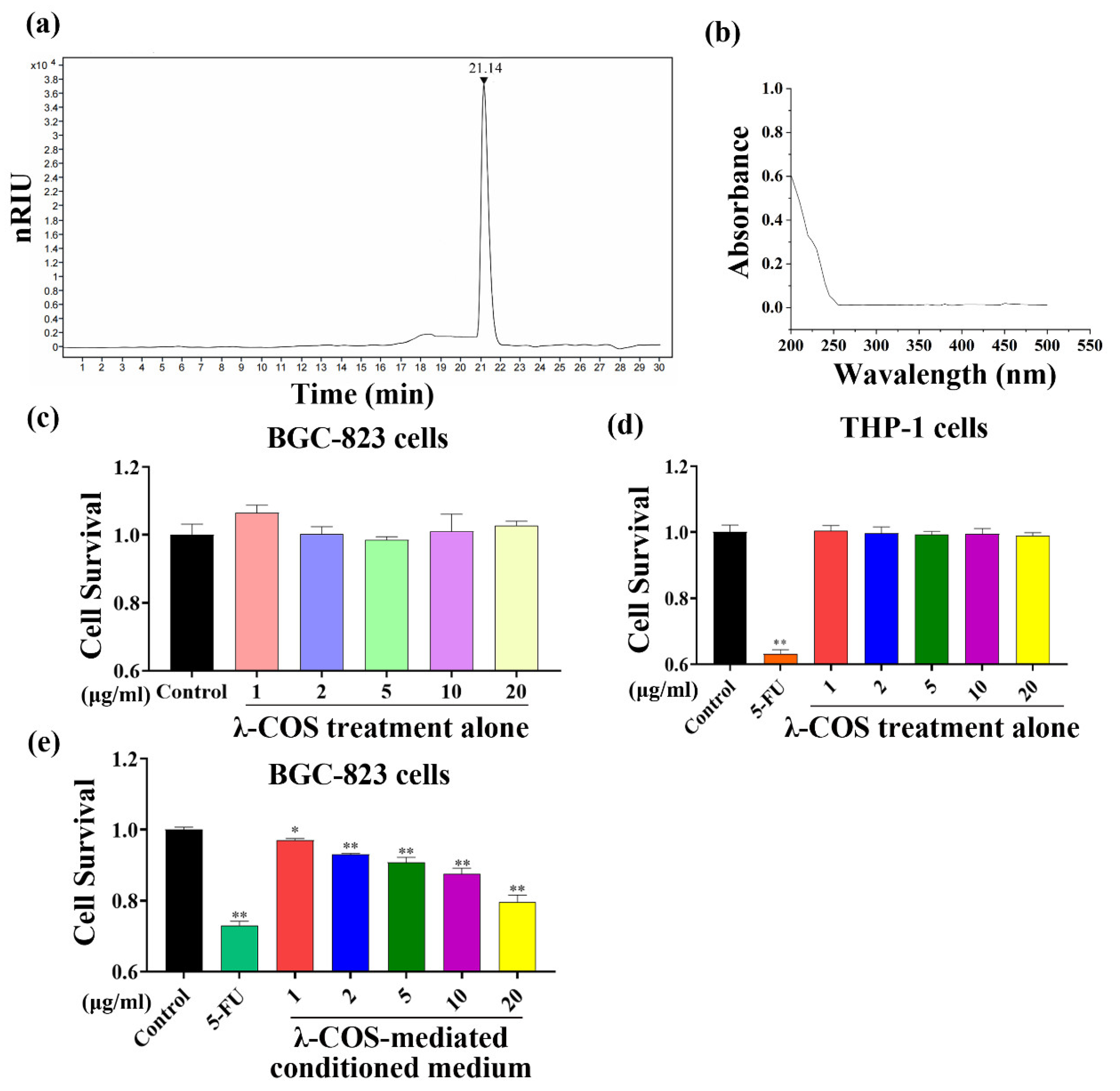
3.1. The Effects of λ-COS and Conditioned Medium on the Growth of BGC-823 Cells
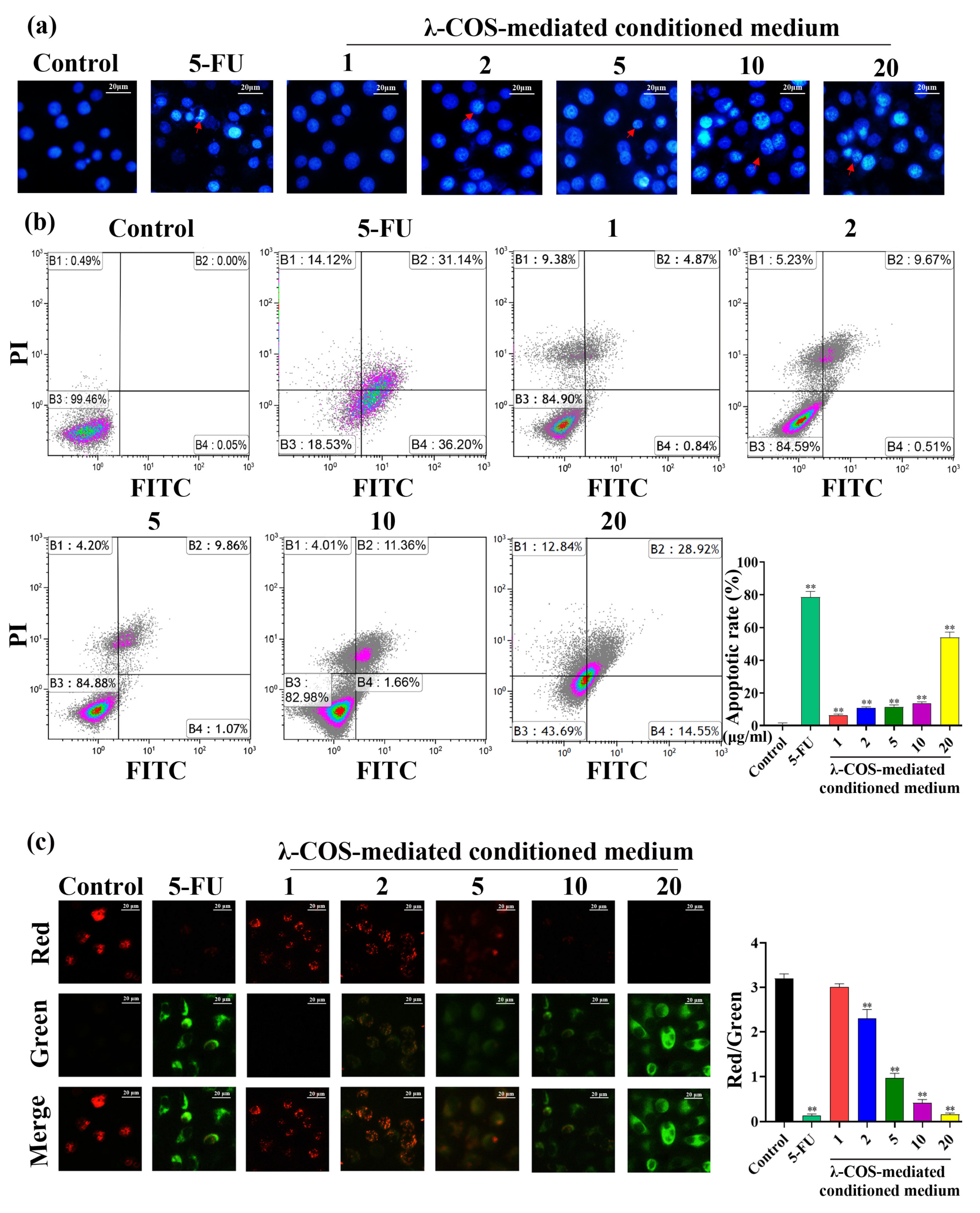
3.2. Conditioned Medium Promotes the Apoptosis of BGC-823 Cells
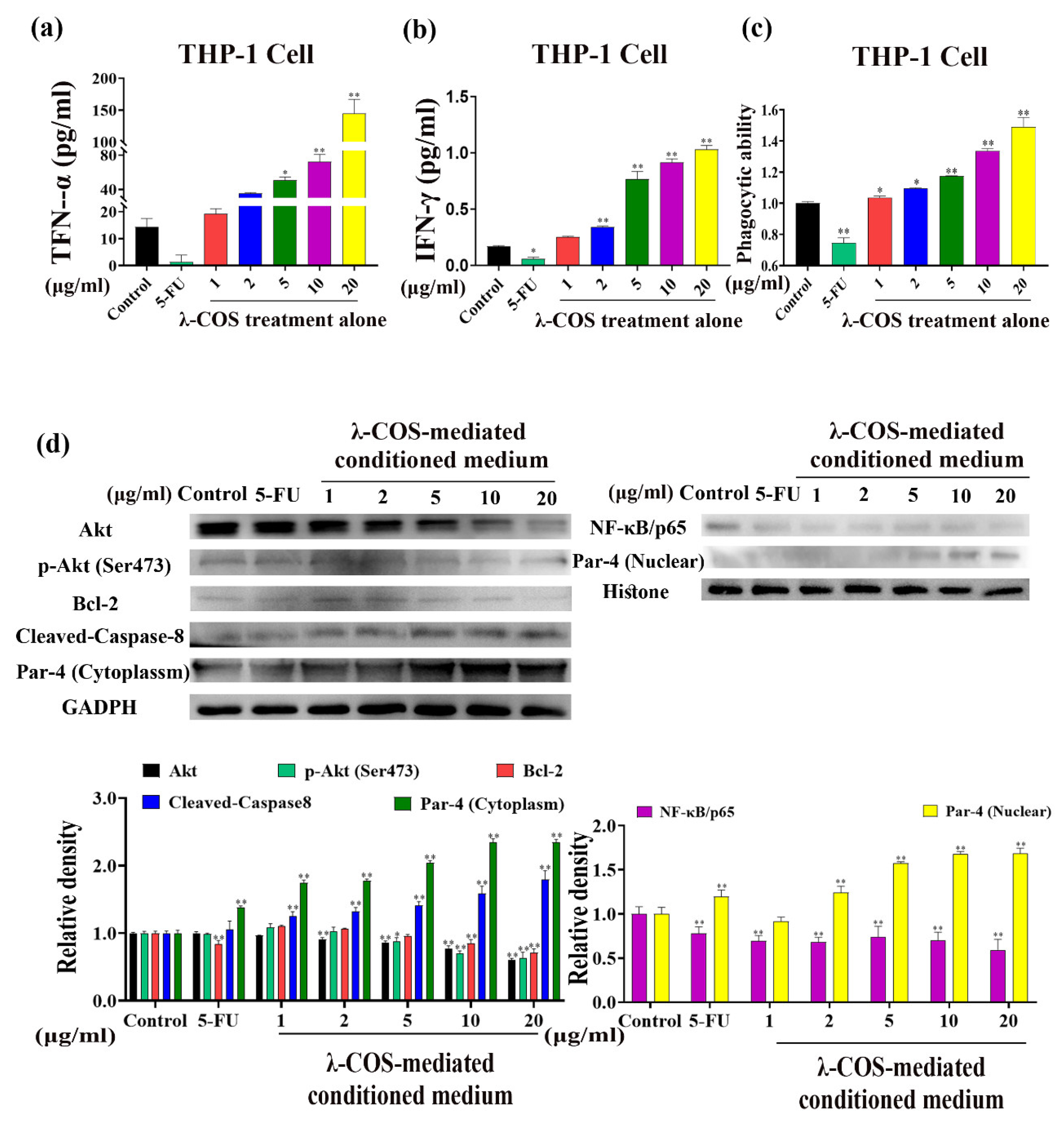
3.3. λ-COS Activate Macrophages and Mediate Apoptosis by the Par-4 Signaling Pathway
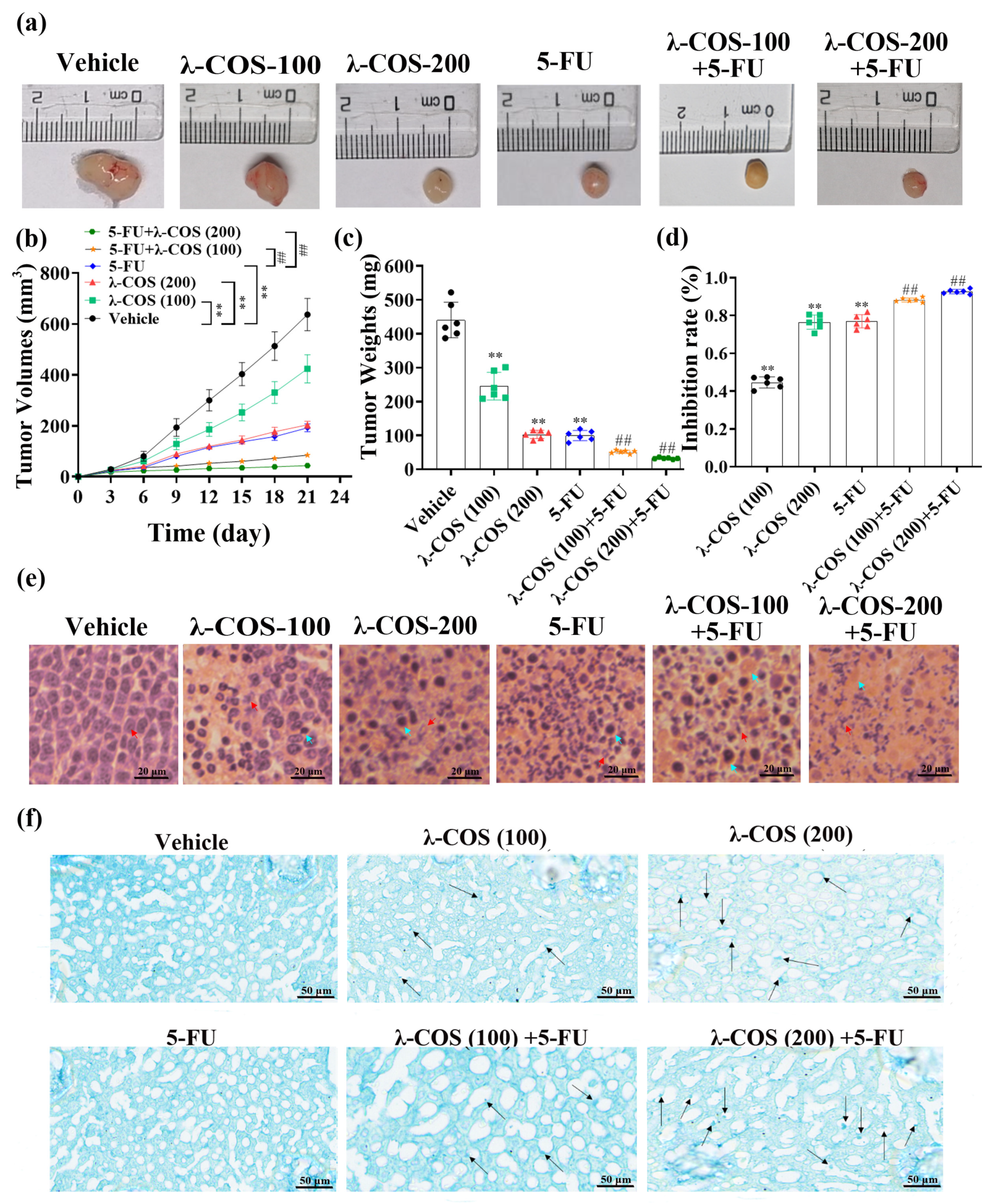
3.4. λ-COS Inhibit Tumor Growth in Mice
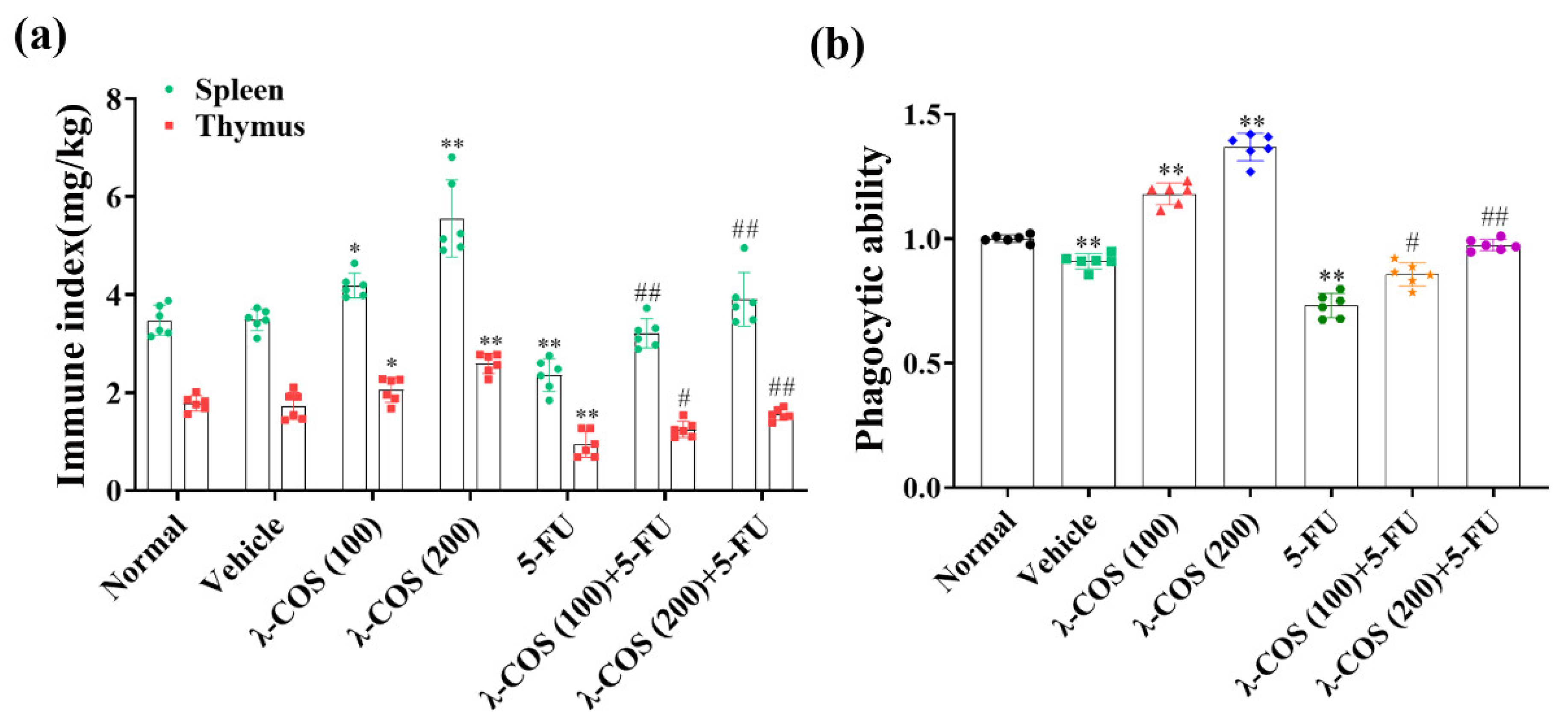
3.5. λ-COS Enhances Immune Function and Alleviates 5-FU-Induced Immunosuppression in Mice
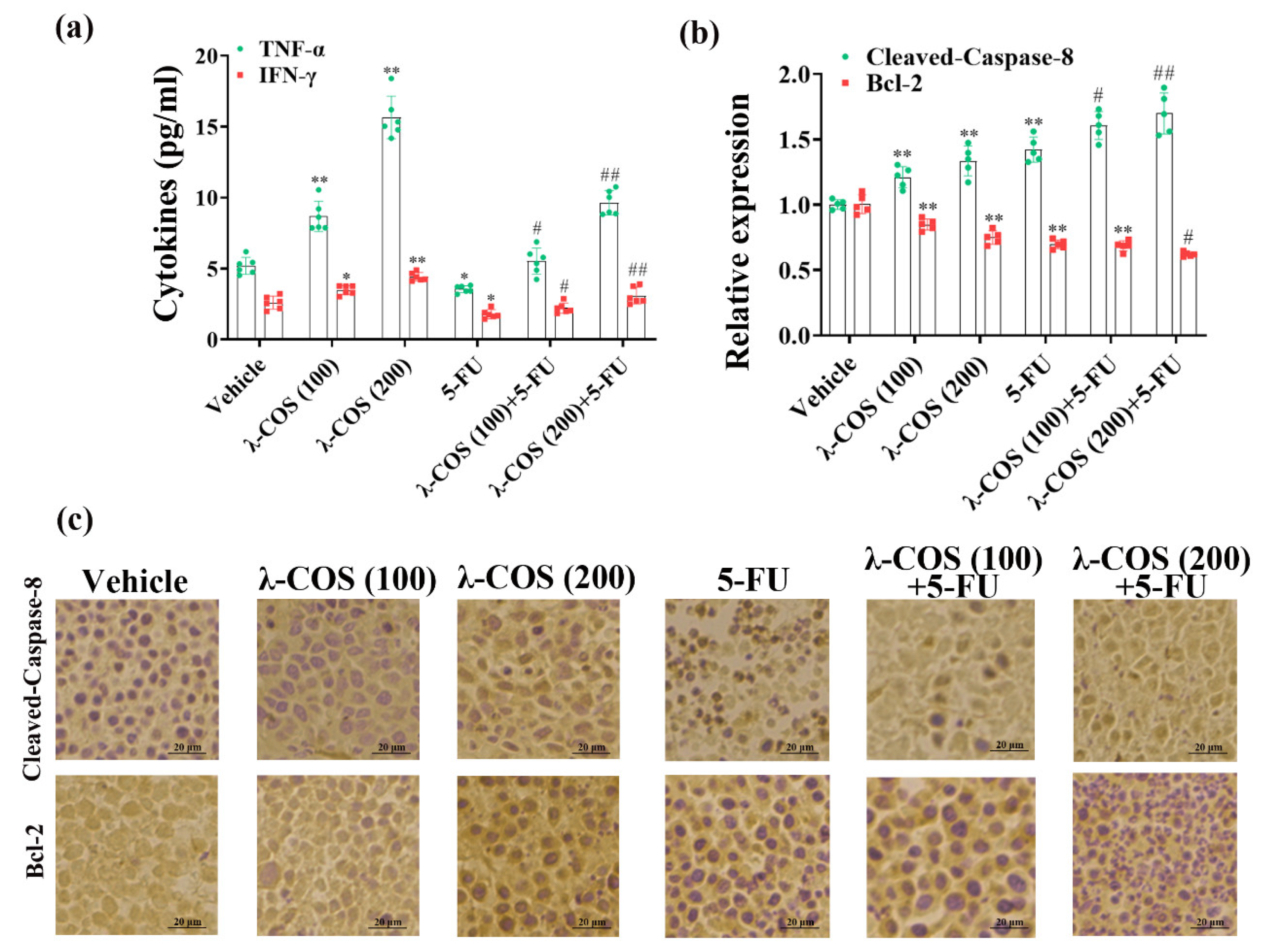
3.6. λ-COS Increase Cytokine Expression and Promote Pro-Apoptotic Proteins Levels in Tumor Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Gao, J.; Cui, Q.; Wang, Q. Strategies to Improve the Antitumor Effect of Immunotherapy for Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 783236. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Tang, J.N.; Xie, H.X.; Du, Y.A.; Huang, L.; Yu, P.F.; Cheng, X.D. 5-Fluorouracil chemotherapy of gastric cancer generates residual cells with properties of cancer stem cells. Int. J. Biol. Sci. 2015, 11, 284–294. [Google Scholar] [CrossRef] [PubMed]
- He, X.X.; Huang, C.K.; Xie, B.S. Autophagy inhibition enhanced 5-FU-induced cell death in human gastric carcinoma BGC-823 cells. Mol. Med. Rep. 2018, 17, 6768–6776. [Google Scholar] [CrossRef] [PubMed]
- Fata, F.; Ron, I.G.; Kemeny, N.; O’Reilly, E.; Klimstra, D.; Kelsen, D.P. 5-fluorouracil-induced small bowel toxicity in patients with colorectal carcinoma. Cancer 1999, 86, 1129–1134. [Google Scholar] [CrossRef]
- Wu, Y.; Deng, Z.; Wang, H.; Ma, W.; Zhou, C.; Zhang, S. Repeated cycles of 5-fluorouracil chemotherapy impaired anti-tumor functions of cytotoxic T cells in a CT26 tumor-bearing mouse model. BMC Immunol. 2016, 17, 29. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, D.; Xuan, R.; Zhou, J.; Liu, J.; Chen, J.; Han, H.; Niu, T.; Li, X.; Chen, H.; et al. λ-carrageenan exacerbates Citrobacter rodentium-induced infectious colitis in mice by targeting gut microbiota and intestinal barrier integrity. Pharmacol. Res. 2021, 174, 105940. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, Y.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef]
- Sun, Y.; Yang, B.; Wu, Y.; Liu, Y.; Gu, X.; Zhang, H.; Wang, C.; Cao, H.; Huang, L.; Wang, Z. Structural characterization and antioxidant activities of kappa-carrageenan oligosaccharides degraded by different methods. Food Chem. 2015, 178, 311–318. [Google Scholar] [CrossRef]
- Groult, H.; Cousin, R.; Chot-Plassot, C.; Maura, M.; Bridiau, N.; Piot, J.M.; Maugard, T.; Fruitier-Arnaudin, I. λ-Carrageenan Oligosaccharides of Distinct Anti-Heparanase and Anticoagulant Activities Inhibit MDA-MB-231 Breast Cancer Cell Migration. Mar. Drugs 2019, 17, 140. [Google Scholar] [CrossRef]
- Zeng, X.; Zhu, X.; Tian, Q.; Tan, X.; Sun, N.; Yan, M.; Zhao, J.; Wu, X.; Li, R.; Zhang, Z.; et al. Celastrol-conjugated chitosan oligosaccharide for the treatment of pancreatic cancer. Drug. Deliv. 2022, 29, 89–98. [Google Scholar] [CrossRef]
- Toole, B.P.; Ghatak, S.; Misra, S. Hyaluronan oligosaccharides as a potential anticancer therapeutic. Curr. Pharm. Biotechnol. 2008, 9, 249–252. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, L.; Yu, X.; Wang, S.; Xu, C.; Yin, H.; Wang, S. Alginate oligosaccharide attenuates alpha2,6-sialylation modification to inhibit prostate cancer cell growth via the Hippo/YAP pathway. Cell Death Dis. 2019, 10, 374. [Google Scholar] [CrossRef] [PubMed]
- Stephanie, B.; Eric, D.; Sophie, F.M.; Christian, B.; Yu, G. Carrageenan from Solieria chordalis (Gigartinales): Structural analysis and immunological activities of the low molecular weight fractions. Carbohydr. Polym. 2010, 81, 448–460. [Google Scholar] [CrossRef]
- Calvo, G.H.; Cosenza, V.A.; Saenz, D.A.; Navarro, D.A.; Stortz, C.A.; Cespedes, M.A.; Mamone, L.A.; Casas, A.G.; Di Venosa, G.M. Disaccharides obtained from carrageenans as potential antitumor agents. Sci. Rep. 2019, 9, 6654. [Google Scholar] [CrossRef] [PubMed]
- Tiasto, V.A.; Goncharov, N.V.; Romanishin, A.O.; Zhidkov, M.E.; Khotimchenko, Y.S. κ- and λ-Carrageenans from Marine Alga Chondrus armatus Exhibit Anticancer In Vitro Activity in Human Gastrointestinal Cancers Models. Mar. Drugs 2022, 20, 741. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Wang, F.; Chen, H.M.; Yan, X.J. κ-carrageenan induces the disruption of intestinal epithelial Caco-2 monolayers by promoting the interaction between intestinal epithelial cells and immune cells. Mol. Med. Rep. 2013, 8, 1635–1642. [Google Scholar] [CrossRef]
- Chen, H.; Wang, F.; Mao, H.; Yan, X. Degraded λ-carrageenan activates NF-κB and AP-1 pathways in macrophages and enhances LPS-induced TNF-α secretion through AP-1. Biochim. Biophys. Acta 2014, 1840, 2162–2170. [Google Scholar] [CrossRef]
- Wu, W.; Zhou, J.; Xuan, R.; Chen, J.; Han, H.; Liu, J.; Niu, T.; Chen, H.; Wang, F. Dietary κ-carrageenan facilitates gut microbiota-mediated intestinal inflammation. Carbohydr. Polym. 2022, 277, 118830. [Google Scholar] [CrossRef]
- Song, W.; Thakor, P.; Vesey, D.A.; Gobe, G.C.; Morais, C. Conditioned medium from stimulated macrophages inhibits growth but induces an inflammatory phenotype in breast cancer cells. Biomed. Pharmacother. 2018, 106, 247–254. [Google Scholar] [CrossRef]
- Gangolli, S.D.; Wright, M.G.; Grasso, P. Identification of carrageenan in mammalian tissues: An analytical and histochemical study. Histochem. J. 1973, 5, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Shelke, G.V.; Jagtap, J.C.; Kim, D.K.; Shah, R.D.; Das, G.; Shivayogi, M.; Pujari, R.; Shastry, P. TNF-α and IFN-γ Together Up-Regulates Par-4 Expression and Induce Apoptosis in Human Neuroblastomas. Biomedicines 2017, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Cao, Q.; Wang, Y.; Xiao, H.; Zhao, J.; Zhang, Q.; Ji, A.; Song, S. Advances in Research on the Bioactivity of Alginate Oligosaccharides. Mar. Drugs 2020, 18, 144. [Google Scholar] [CrossRef] [PubMed]
- McKim, J.M.; Willoughby, J.A., Sr.; Blakemore, W.R.; Weiner, M.L. Clarifying the confusion between poligeenan, degraded carrageenan, and carrageenan: A review of the chemistry, nomenclature, and in vivo toxicology by the oral route. Crit. Rev. Food Sci. Nutr. 2019, 59, 3054–3073. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, S.; Miller, K. Intestinal uptake and immunological effects of carrageenan—Current concepts. Food Addit. Contam. 1989, 6, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Maeß, M.B.; Wittig, B.; Lorkowski, S. Highly efficient transfection of human THP-1 macrophages by nucleofection. J. Vis. Exp. 2014, 91, e51960. [Google Scholar]
- Yan, D.; Qin, N.; Zhang, H.; Liu, T.; Yu, M.; Jiang, X.; Feng, W.; Wang, J.; Yin, B.; Zhang, T.; et al. Expression of TNF-α leader sequence renders MCF-7 tumor cells resistant to the cytotoxicity of soluble TNF-α. Breast Cancer Res. Treat. 2009, 116, 91–102. [Google Scholar] [CrossRef]
- Xia, L.; Mo, P.; Huang, W.; Zhang, L.; Wang, Y.; Zhu, H.; Tian, D.; Liu, J.; Chen, Z.; Zhang, Y.; et al. The TNF-α/ROS/HIF-1-induced upregulation of FoxMI expression promotes HCC proliferation and resistance to apoptosis. Carcinogenesis 2012, 33, 2250–2259. [Google Scholar] [CrossRef]
- Tu, S.P.; Quante, M.; Bhagat, G.; Takaishi, S.; Cui, G.; Yang, X.D.; Muthuplani, S.; Shibata, W.; Fox, J.G.; Pritchard, D.M.; et al. IFN-γ inhibits gastric carcinogenesis by inducing epithelial cell autophagy and T-cell apoptosis. Cancer Res. 2011, 71, 4247–4259. [Google Scholar] [CrossRef]
- Genin, M.; Clement, F.; Fattaccioli, A.; Raes, M.; Michiels, C. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015, 15, 577. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Rao, L.; Yao, H.; Wang, Z.; Ning, P.; Chen, X. Engineering Macrophages for Cancer Immunotherapy and Drug Delivery. Adv. Mater. 2020, 32, e2002054. [Google Scholar] [CrossRef] [PubMed]
- Ling, N.; Zhou, X.; Ji, Y.; Li, W.; Ji, C.; Qi, Z. Immuno-modulatory and cellular antioxidant activities of κ-selenocarrageenan in combination with Epirubicin in H22 hepatoma-bearing mice. Biomed. Pharmacother. 2017, 91, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Cheratta, A.R.; Thayyullathil, F.; Pallichankandy, S.; Subburayan, K.; Alakkal, A.; Galadari, S. Prostate apoptosis response-4 and tumor suppression: It’s not just about apoptosis anymore. Cell. Death Dis. 2021, 12, 47. [Google Scholar] [CrossRef]
- Irby, R.B.; Kline, C.L. Par-4 as a potential target for cancer therapy. Expert. Opin. Ther. Targets 2013, 17, 77–87. [Google Scholar] [CrossRef]
- Gurumurthy, S.; Goswami, A.; Vasudevan, K.M.; Rangnekar, V.M. Phosphorylation of Par-4 by protein kinase A is critical for apoptosis. Mol. Cell. Biol. 2005, 25, 1146–1161. [Google Scholar] [CrossRef] [PubMed]
- El-Guendy, N.; Zhao, Y.; Gurumurthy, S.; Burikhanov, R.; Rangnekar, V.M. Identification of a unique core domain of par-4 sufficient for selective apoptosis induction in cancer cells. Mol. Cell. Biol. 2003, 23, 5516–5525. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, G.; Jiang, P.; Xiang, Y.; Li, W.; Lee, W.; Zhang, Y. Decreased expression of protease-activated receptor 4 in human gastric cancer. Int. J. Biochem. Cell. Biol. 2011, 43, 1277–1283. [Google Scholar] [CrossRef]
- Kaboli, P.J.; Imani, S.; Jomhori, M.; Ling, K.H. Chemoresistance in breast cancer: PI3K/Akt pathway inhibitors vs the current chemotherapy. Am. J. Cancer Res. 2021, 11, 5155–5183. [Google Scholar]
- Goswami, A.; Burikhanov, R.; de Thonel, A.; Fujita, N.; Goswami, M.; Zhao, Y.; Eriksson, J.E.; Tsuruo, T.; Rangnekar, V.M. Binding and phosphorylation of par-4 by akt is essential for cancer cell survival. Mol. Cell. 2005, 20, 33–44. [Google Scholar] [CrossRef]
- Chen, X.; Sahasrabuddhe, A.A.; Szankasi, P.; Chung, F.; Basrur, V.; Rangnekar, V.M.; Pagano, M.; Lim, M.S.; Elenitoba-Johnson, K.S. Fbxo45-mediated degradation of the tumor-suppressor Par-4 regulates cancer cell survival. Cell. Death Differ. 2014, 21, 1535–1545. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.C.; Ho, Y.C.; Lai, C.Y.; Chou, D.; Chen, G.D.; Lin, T.B.; Peng, H.Y. Spinal TNF-α impedes Fbxo45-dependent Munc13-1 ubiquitination to mediate neuropathic allodynia in rats. Cell. Death Dis. 2018, 9, 811. [Google Scholar] [CrossRef]
- QiNan, W.; XiaGuang, G.; XiaoTian, L.; WuQuan, D.; Ling, Z.; Bing, C. Par-4/NF-κB Mediates the Apoptosis of Islet β Cells Induced by Glucolipotoxicity. J. Diabetes Res. 2016, 2016, 4692478. [Google Scholar] [CrossRef]
- Yao, Z.A.; Xu, L.; Jin, L.M.; Wang, B.X.; Fu, C.Z.; Bai, Y.; Wu, H.G. κ-Carrageenan Oligosaccharides Inhibit the Inflammation of Lipopolysaccharide-Activated Microglia Via TLR4/NF-κB and p38/JNK MAPKs Pathways. Neurochem. Res. 2022, 47, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Galante, J.M.; Mortenson, M.M.; Schlieman, M.G.; Virudachalam, S.; Bold, R.J. Targeting NF-kB/BCL-2 pathway increases apoptotic susceptibility to chemotherapy in pancreatic cancer. J. Surg. Res. 2004, 121, 306–307. [Google Scholar] [CrossRef]
- Chaudhry, S.; Joshi, V.; Bojjireddy, N.; Thoh, M.; Sandur, S.K.; Subrahmanyam, G. Silencing of type II phosphatidylinositol 4-kinase β stabilizes prostate apoptosis response-4 and induces apoptosis in cancer cells. Biochem. J. 2019, 476, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Treude, F.; Kappes, F.; Fahrenkamp, D.; Müller-Newen, G.; Dajas-Bailador, F.; Krämer, O.H.; Lüscher, B.; Hartkamp, J. Caspase-8-mediated PAR-4 cleavage is required for TNFα-induced apoptosis. Oncotarget 2014, 5, 2988–2998. [Google Scholar] [CrossRef]
- Chakraborty, M.; Qiu, S.G.; Vasudevan, K.M.; Rangnekar, V.M. Par-4 drives trafficking and activation of Fas and Fasl to induce prostate cancer cell apoptosis and tumor regression. Cancer Res. 2001, 61, 7255–7263. [Google Scholar]
- Yin, Y.; Li, M.; Gu, W.; Zeng, B.; Liu, W.; Zhu, L.; Pi, X.; Primerano, D.A.; Yu, H.D.; Wei, H.; et al. Carrageenan oligosaccharides and associated carrageenan-degrading bacteria induce intestinal inflammation in germ-free mice. J. Genet. Genom. 2021, 48, 815–824. [Google Scholar] [CrossRef]
- Ashraf, N.; Hoffe, S.; Kim, R. Adjuvant treatment for gastric cancer: Chemotherapy versus radiation. Oncologist 2013, 18, 1013–1021. [Google Scholar] [CrossRef]
- Guo, X.F.; Yang, Z.R.; Wang, J.; Lei, X.F.; Lv, X.G.; Dong, W.G. Synergistic antitumor effect of puerarin combined with 5-fluorouracil on gastric carcinoma. Mol. Med. Rep. 2015, 11, 2562–2568. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, M.; Zhai, L.; Chen, J.; Wang, F.; Chen, H.; Wu, W. The Antitumor Potential of λ-Carrageenan Oligosaccharides on Gastric Carcinoma by Immunomodulation. Nutrients 2023, 15, 2044. https://doi.org/10.3390/nu15092044
Tang M, Zhai L, Chen J, Wang F, Chen H, Wu W. The Antitumor Potential of λ-Carrageenan Oligosaccharides on Gastric Carcinoma by Immunomodulation. Nutrients. 2023; 15(9):2044. https://doi.org/10.3390/nu15092044
Chicago/Turabian StyleTang, Min, Leilei Zhai, Juanjuan Chen, Feng Wang, Haimin Chen, and Wei Wu. 2023. "The Antitumor Potential of λ-Carrageenan Oligosaccharides on Gastric Carcinoma by Immunomodulation" Nutrients 15, no. 9: 2044. https://doi.org/10.3390/nu15092044
APA StyleTang, M., Zhai, L., Chen, J., Wang, F., Chen, H., & Wu, W. (2023). The Antitumor Potential of λ-Carrageenan Oligosaccharides on Gastric Carcinoma by Immunomodulation. Nutrients, 15(9), 2044. https://doi.org/10.3390/nu15092044





