Heptadecanoic Acid Is Not a Key Mediator in the Prevention of Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Set Up
2.2. Metabolic Phenotyping
2.3. Tolerance Tests
2.4. Immunohistochemistry
2.5. Plasma and Tissue Measurements
2.6. Quantitative Real Time PCR (qRT PCR)
2.7. Western Blot
2.8. Long-Chain Fatty Acid (LCFA) Analysis
2.9. Primary Hepatocytes
2.10. Statistical Analysis
3. Results
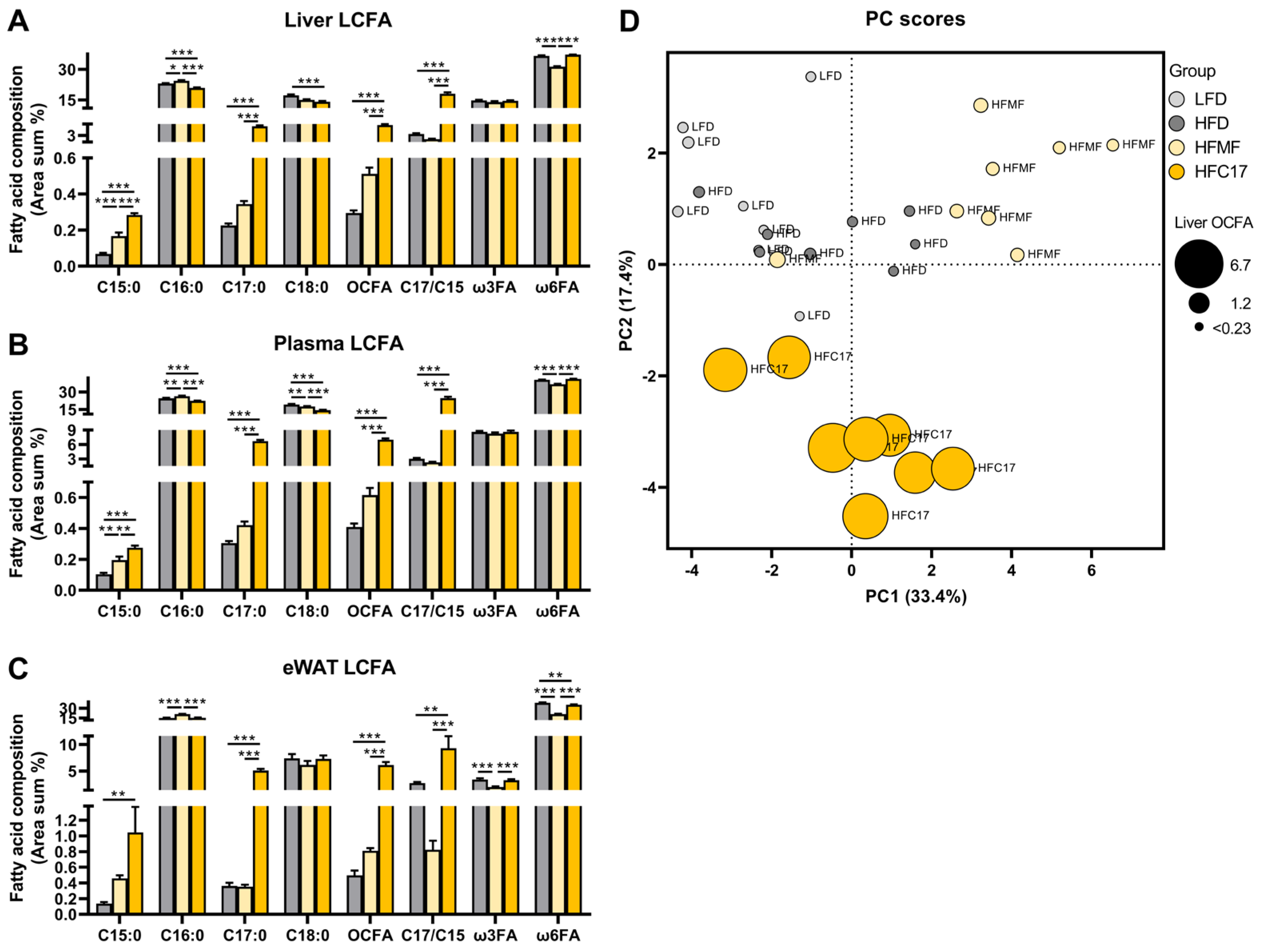
3.1. Long-Term Feeding of Milk Fat and C17:0 Elevates OCFA in Different Compartments
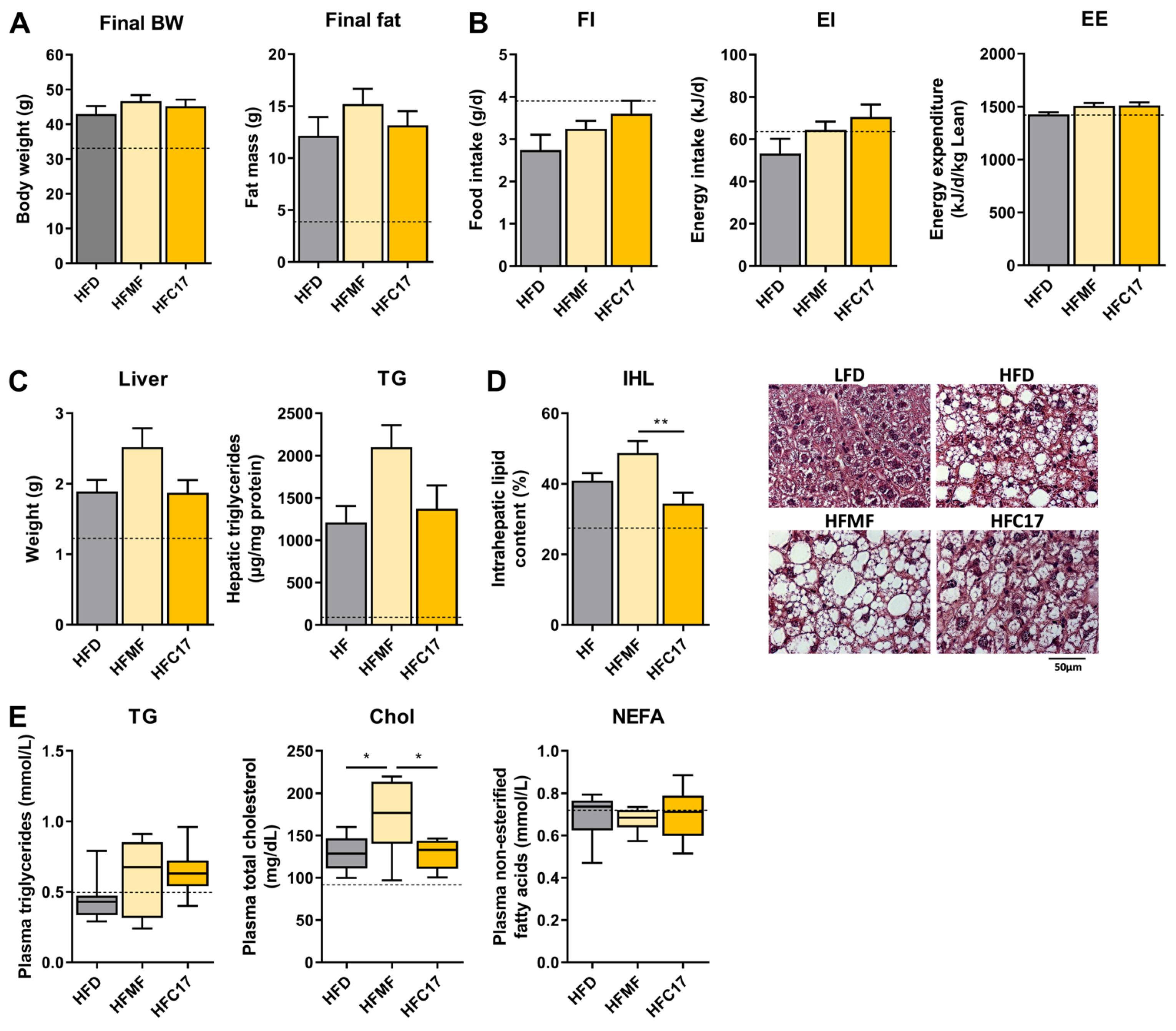
3.2. Long-Term Supplementation of C17:0 Does Not Improve Diet-Induced Hepatic Steatosis
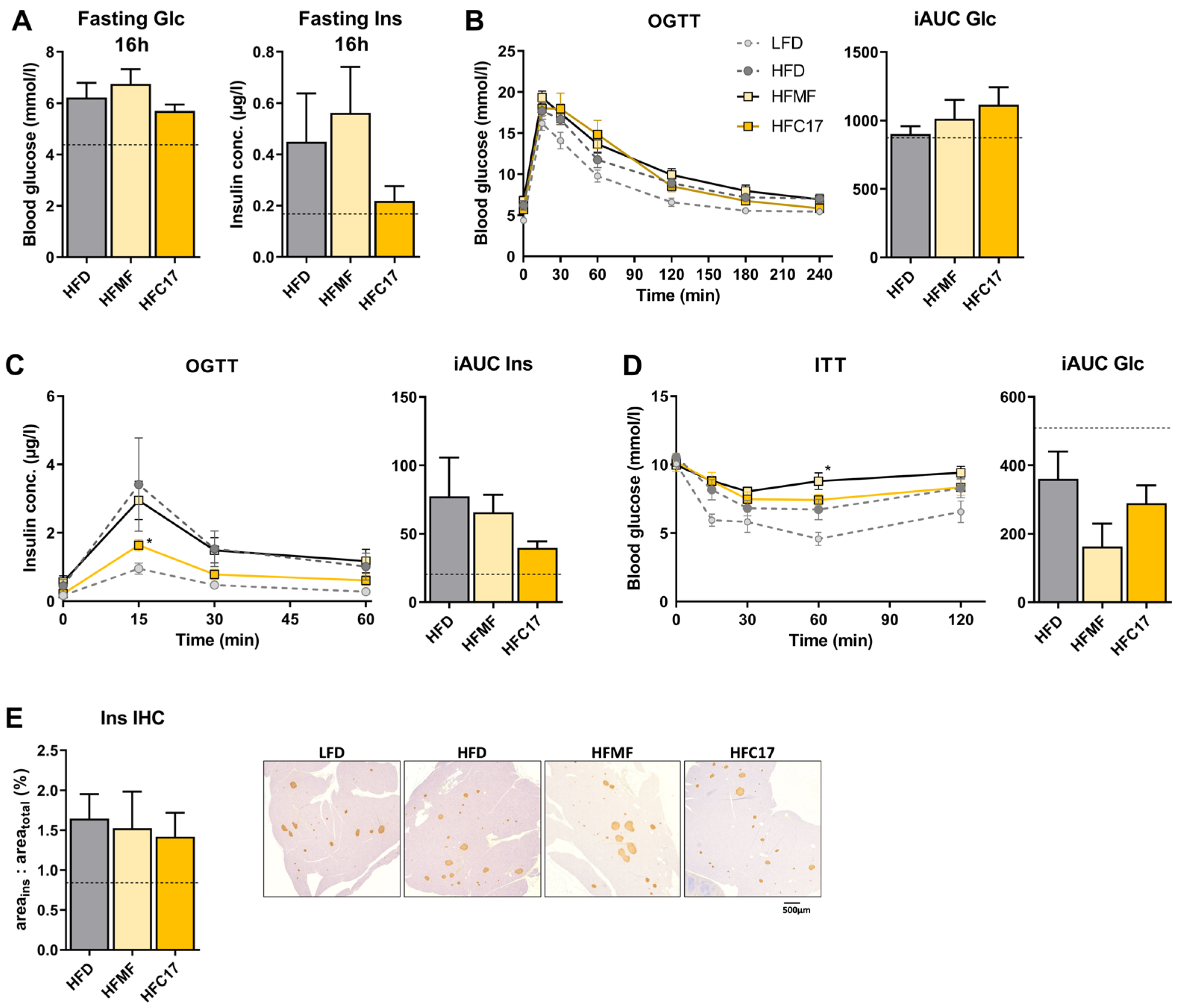
3.3. High-Fat C17:0 Supplementation Does Not Enhance Insulin Sensitivity
3.4. Supplementation of C17:0 Does Not Affect Hepatic Lipid Metabolism In Vivo
3.5. Hepatic Immune Signaling Is Diminished by OCFA Treatment In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoo, W.; Gjuka, D.; Stevenson, H.L.; Song, X.; Shen, H.; Yoo, S.Y.; Wang, J.; Fallon, M.; Ioannou, G.N.; Harrison, S.A.; et al. Fatty acids in non-alcoholic steatohepatitis: Focus on pentadecanoic acid. PLoS ONE 2017, 12, e0189965. [Google Scholar] [CrossRef] [PubMed]
- Sawh, M.C.; Wallace, M.; Shapiro, E.; Goyal, N.P.; Newton, K.P.; Yu, E.L.; Bross, C.; Durelle, J.; Knott, C.; Gangoiti, J.A.; et al. Dairy Fat Intake, Plasma Pentadecanoic Acid, and Plasma Iso-heptadecanoic Acid Are Inversely Associated With Liver Fat in Children. J. Pediatr. Gastroenterol. Nutr. 2021, 72, e90–e96. [Google Scholar] [CrossRef] [PubMed]
- Kroger, J.; Zietemann, V.; Enzenbach, C.; Weikert, C.; Jansen, E.H.; Doring, F.; Joost, H.G.; Boeing, H.; Schulze, M.B. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am. J. Clin. Nutr. 2011, 93, 127–142. [Google Scholar] [CrossRef]
- Forouhi, N.G.; Koulman, A.; Sharp, S.J.; Imamura, F.; Kroger, J.; Schulze, M.B.; Crowe, F.L.; Huerta, J.M.; Guevara, M.; Beulens, J.W.; et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: The EPIC-InterAct case-cohort study. Lancet. Diabetes Endocrinol. 2014, 2, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Iggman, D.; Arnlov, J.; Vessby, B.; Cederholm, T.; Sjogren, P.; Riserus, U. Adipose tissue fatty acids and insulin sensitivity in elderly men. Diabetologia 2010, 53, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Nestel, P.J.; Straznicky, N.; Mellett, N.A.; Wong, G.; De Souza, D.P.; Tull, D.L.; Barlow, C.K.; Grima, M.T.; Meikle, P.J. Specific plasma lipid classes and phospholipid fatty acids indicative of dairy food consumption associate with insulin sensitivity. Am. J. Clin. Nutr. 2014, 99, 46–53. [Google Scholar] [CrossRef]
- Prada, M.; Wittenbecher, C.; Eichelmann, F.; Wernitz, A.; Drouin-Chartier, J.P.; Schulze, M.B. Association of the odd-chain fatty acid content in lipid groups with type 2 diabetes risk: A targeted analysis of lipidomics data in the EPIC-Potsdam cohort. Clin. Nutr. 2021, 40, 4988–4999. [Google Scholar] [CrossRef]
- Warensjo, E.; Jansson, J.H.; Cederholm, T.; Boman, K.; Eliasson, M.; Hallmans, G.; Johansson, I.; Sjogren, P. Biomarkers of milk fat and the risk of myocardial infarction in men and women: A prospective, matched case-control study. Am. J. Clin. Nutr. 2010, 92, 194–202. [Google Scholar] [CrossRef]
- Weitkunat, K.; Schumann, S.; Nickel, D.; Hornemann, S.; Petzke, K.J.; Schulze, M.B.; Pfeiffer, A.F.; Klaus, S. Odd-chain fatty acids as a biomarker for dietary fiber intake: A novel pathway for endogenous production from propionate. Am. J. Clin. Nutr. 2017, 105, 1544–1551. [Google Scholar] [CrossRef]
- Pranger, I.G.; Muskiet, F.A.J.; Kema, I.P.; Singh-Povel, C.; Bakker, S.J.L. Potential Biomarkers for Fat from Dairy and Fish and Their Association with Cardiovascular Risk Factors: Cross-sectional Data from the LifeLines Biobank and Cohort Study. Nutrients 2019, 11, 1099. [Google Scholar] [CrossRef]
- Venalainen, T.; Schwab, U.; Agren, J.; de Mello, V.; Lindi, V.; Eloranta, A.M.; Kiiskinen, S.; Laaksonen, D.; Lakka, T.A. Cross-sectional associations of food consumption with plasma fatty acid composition and estimated desaturase activities in Finnish children. Lipids 2014, 49, 467–479. [Google Scholar] [CrossRef]
- Saadatian-Elahi, M.; Slimani, N.; Chajes, V.; Jenab, M.; Goudable, J.; Biessy, C.; Ferrari, P.; Byrnes, G.; Autier, P.; Peeters, P.H.; et al. Plasma phospholipid fatty acid profiles and their association with food intakes: Results from a cross-sectional study within the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2009, 89, 331–346. [Google Scholar] [CrossRef]
- Weitkunat, K.; Bishop, C.A.; Wittmuss, M.; Machate, T.; Schifelbein, T.; Schulze, M.B.; Klaus, S. Effect of Microbial Status on Hepatic Odd-Chain Fatty Acids Is Diet-Dependent. Nutrients 2021, 13, 1546. [Google Scholar] [CrossRef] [PubMed]
- Albani, V.; Celis-Morales, C.; O’Donovan, C.B.; Walsh, M.C.; Woolhead, C.; Forster, H.; Fallaize, R.; Macready, A.L.; Marsaux, C.F.M.; Navas-Carretero, S.; et al. Within-person reproducibility and sensitivity to dietary change of C15:0 and C17:0 levels in dried blood spots: Data from the European Food4Me Study. Mol. Nutr. Food Res. 2017, 61, 1700142. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.S.; Sharp, S.J.; Jansen, E.; Luben, R.N.; Khaw, K.T.; Wareham, N.J.; Forouhi, N.G. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: A pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am. J. Clin. Nutr. 2010, 92, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Imamura, F.; Fretts, A.; Marklund, M.; Ardisson Korat, A.V.; Yang, W.S.; Lankinen, M.; Qureshi, W.; Helmer, C.; Chen, T.A.; Wong, K.; et al. Fatty acid biomarkers of dairy fat consumption and incidence of type 2 diabetes: A pooled analysis of prospective cohort studies. PLoS Med. 2018, 15, e1002670. [Google Scholar] [CrossRef]
- To, N.B.; Truong, V.N.; Ediriweera, M.K.; Cho, S.K. Effects of Combined Pentadecanoic Acid and Tamoxifen Treatment on Tamoxifen Resistance in MCF-7/SC Breast Cancer Cells. Int. J. Mol. Sci. 2022, 23, 11340. [Google Scholar] [CrossRef]
- To, N.B.; Nguyen, Y.T.; Moon, J.Y.; Ediriweera, M.K.; Cho, S.K. Pentadecanoic Acid, an Odd-Chain Fatty Acid, Suppresses the Stemness of MCF-7/SC Human Breast Cancer Stem-Like Cells through JAK2/STAT3 Signaling. Nutrients 2020, 12, 1663. [Google Scholar] [CrossRef]
- Ediriweera, M.K.; To, N.B.; Lim, Y.; Cho, S.K. Odd-chain fatty acids as novel histone deacetylase 6 (HDAC6) inhibitors. Biochimie 2021, 186, 147–156. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Weitkunat, K.; Stuhlmann, C.; Postel, A.; Rumberger, S.; Fankhanel, M.; Woting, A.; Petzke, K.J.; Gohlke, S.; Schulz, T.J.; Blaut, M.; et al. Short-chain fatty acids and inulin, but not guar gum, prevent diet-induced obesity and insulin resistance through differential mechanisms in mice. Sci. Rep. 2017, 7, 6109. [Google Scholar] [CrossRef] [PubMed]
- Weitkunat, K.; Schumann, S.; Nickel, D.; Kappo, K.A.; Petzke, K.J.; Kipp, A.P.; Blaut, M.; Klaus, S. Importance of propionate for the repression of hepatic lipogenesis and improvement of insulin sensitivity in high-fat diet-induced obesity. Mol. Nutr. Food Res. 2016, 60, 2611–2621. [Google Scholar] [CrossRef]
- Xu, C.; Wu, P.; Gao, J.; Zhang, L.; Ma, T.; Ma, B.; Yang, S.; Shao, G.; Yu, Y.; Huang, X.; et al. Heptadecanoic acid inhibits cell proliferation in PC9 nonsmallcell lung cancer cells with acquired gefitinib resistance. Oncol. Rep. 2019, 41, 3499–3507. [Google Scholar] [CrossRef]
- Jakobsen, M.U.; Trolle, E.; Outzen, M.; Mejborn, H.; Gronberg, M.G.; Lyndgaard, C.B.; Stockmarr, A.; Veno, S.K.; Bysted, A. Intake of dairy products and associations with major atherosclerotic cardiovascular diseases: A systematic review and meta-analysis of cohort studies. Sci. Rep. 2021, 11, 1303. [Google Scholar] [CrossRef]
- Soedamah-Muthu, S.S.; Ding, E.L.; Al-Delaimy, W.K.; Hu, F.B.; Engberink, M.F.; Willett, W.C.; Geleijnse, J.M. Milk and dairy consumption and incidence of cardiovascular diseases and all-cause mortality: Dose-response meta-analysis of prospective cohort studies. Am. J. Clin. Nutr. 2011, 93, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Sbai, D.; Narcy, C.; Thompson, G.N.; Mariotti, A.; Poggi, F.; Saudubray, J.M.; Bresson, J.L. Contribution of odd-chain fatty acid oxidation to propionate production in disorders of propionate metabolism. Am. J. Clin. Nutr. 1994, 59, 1332–1337. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.A.; Machate, T.; Henning, T.; Henkel, J.; Puschel, G.; Weber, D.; Grune, T.; Klaus, S.; Weitkunat, K. Detrimental effects of branched-chain amino acids in glucose tolerance can be attributed to valine induced glucotoxicity in skeletal muscle. Nutr. Diabetes 2022, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Noatsch, A.; Petzke, K.J.; Millrose, M.K.; Klaus, S. Body weight and energy homeostasis was not affected in C57BL/6 mice fed high whey protein or leucine-supplemented low-fat diets. Eur. J. Nutr. 2011, 50, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Weitkunat, K.; Schumann, S.; Petzke, K.J.; Blaut, M.; Loh, G.; Klaus, S. Effects of dietary inulin on bacterial growth, short-chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice. J. Nutr. Biochem. 2015, 26, 929–937. [Google Scholar] [CrossRef]
- Vessby, B.; Gustafsson, I.B.; Tengblad, S.; Berglund, L. Indices of fatty acid desaturase activity in healthy human subjects: Effects of different types of dietary fat. Br. J. Nutr. 2013, 110, 871–879. [Google Scholar] [CrossRef]
- Bishop, C.A.; Schulze, M.B.; Klaus, S.; Weitkunat, K. The branched-chain amino acids valine and leucine have differential effects on hepatic lipid metabolism. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2020, 34, 9727–9739. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, I.F.; Migliaccio, V.; Lepretti, M.; Paolella, G.; Di Gregorio, I.; Caputo, I.; Ribeiro, E.B.; Lionetti, L. Dose- and Time-Dependent Effects of Oleate on Mitochondrial Fusion/Fission Proteins and Cell Viability in HepG2 Cells: Comparison with Palmitate Effects. Int. J. Mol. Sci. 2021, 22, 9812. [Google Scholar] [CrossRef] [PubMed]
- Eynaudi, A.; Diaz-Castro, F.; Borquez, J.C.; Bravo-Sagua, R.; Parra, V.; Troncoso, R. Differential Effects of Oleic and Palmitic Acids on Lipid Droplet-Mitochondria Interaction in the Hepatic Cell Line HepG2. Front. Nutr. 2021, 8, 775382. [Google Scholar] [CrossRef] [PubMed]
- Kratz, M.; Marcovina, S.; Nelson, J.E.; Yeh, M.M.; Kowdley, K.V.; Callahan, H.S.; Song, X.; Di, C.; Utzschneider, K.M. Dairy fat intake is associated with glucose tolerance, hepatic and systemic insulin sensitivity, and liver fat but not beta-cell function in humans. Am. J. Clin. Nutr. 2014, 99, 1385–1396. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, S.; Thelin, A. High dairy fat intake related to less central obesity: A male cohort study with 12 years’ follow-up. Scand. J. Prim. Health Care 2013, 31, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Milard, M.; Laugerette, F.; Durand, A.; Buisson, C.; Meugnier, E.; Loizon, E.; Louche-Pelissier, C.; Sauvinet, V.; Garnier, L.; Viel, S.; et al. Milk Polar Lipids in a High-Fat Diet Can Prevent Body Weight Gain: Modulated Abundance of Gut Bacteria in Relation with Fecal Loss of Specific Fatty Acids. Mol. Nutr. Food Res. 2019, 63, e1801078. [Google Scholar] [CrossRef]
- Nestel, P.J.; Chronopulos, A.; Cehun, M. Dairy fat in cheese raises LDL cholesterol less than that in butter in mildly hypercholesterolaemic subjects. Eur. J. Clin. Nutr. 2005, 59, 1059–1063. [Google Scholar] [CrossRef]
- American Heart Association Nutrition, C.; Lichtenstein, A.H.; Appel, L.J.; Brands, M.; Carnethon, M.; Daniels, S.; Franch, H.A.; Franklin, B.; Kris-Etherton, P.; Harris, W.S.; et al. Diet and lifestyle recommendations revision 2006: A scientific statement from the American Heart Association Nutrition Committee. Circulation 2006, 114, 82–96. [Google Scholar] [CrossRef]
- Fuchs, M.; Sanyal, A.J. Lipotoxicity in NASH. J. Hepatol. 2012, 56, 291–293. [Google Scholar] [CrossRef]
- Rada, P.; Gonzalez-Rodriguez, A.; Garcia-Monzon, C.; Valverde, A.M. Understanding lipotoxicity in NAFLD pathogenesis: Is CD36 a key driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef]
- Tu, Q.Q.; Zheng, R.Y.; Li, J.; Hu, L.; Chang, Y.X.; Li, L.; Li, M.H.; Wang, R.Y.; Huang, D.D.; Wu, M.C.; et al. Palmitic acid induces autophagy in hepatocytes via JNK2 activation. Acta Pharm. Sin. 2014, 35, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Malhi, H.; Bronk, S.F.; Werneburg, N.W.; Gores, G.J. Free fatty acids induce JNK-dependent hepatocyte lipoapoptosis. J. Biol. Chem. 2006, 281, 12093–12101. [Google Scholar] [CrossRef] [PubMed]
- Gaundal, L.; Myhrstad, M.C.W.; Leder, L.; Byfuglien, M.G.; Gjovaag, T.; Rud, I.; Retterstol, K.; Holven, K.B.; Ulven, S.M.; Telle-Hansen, V.H. Beneficial effect on serum cholesterol levels, but not glycaemic regulation, after replacing SFA with PUFA for 3 d: A randomised crossover trial. Br. J. Nutr. 2021, 125, 915–925. [Google Scholar] [CrossRef]
- Sun, Y.; Neelakantan, N.; Wu, Y.; Lote-Oke, R.; Pan, A.; van Dam, R.M. Palm Oil Consumption Increases LDL Cholesterol Compared with Vegetable Oils Low in Saturated Fat in a Meta-Analysis of Clinical Trials. J. Nutr. 2015, 145, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy Fats and Cardiovascular Disease: Do We Really Need to be Concerned? Foods 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Serviddio, G.; Bellanti, F.; Villani, R.; Tamborra, R.; Zerbinati, C.; Blonda, M.; Ciacciarelli, M.; Poli, G.; Vendemiale, G.; Iuliano, L. Effects of dietary fatty acids and cholesterol excess on liver injury: A lipidomic approach. Redox Biol. 2016, 9, 296–305. [Google Scholar] [CrossRef] [PubMed]
- Krachler, B.; Norberg, M.; Eriksson, J.W.; Hallmans, G.; Johansson, I.; Vessby, B.; Weinehall, L.; Lindahl, B. Fatty acid profile of the erythrocyte membrane preceding development of Type 2 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. NMCD 2008, 18, 503–510. [Google Scholar] [CrossRef]
- Morales-Ruiz, M.; Santel, A.; Ribera, J.; Jimenez, W. The Role of Akt in Chronic Liver Disease and Liver Regeneration. Semin. Liver Dis. 2017, 37, 11–16. [Google Scholar] [CrossRef]
- Fu, W.C.; Li, H.Y.; Li, T.T.; Yang, K.; Chen, J.X.; Wang, S.J.; Liu, C.H.; Zhang, W. Pentadecanoic acid promotes basal and insulin-stimulated glucose uptake in C2C12 myotubes. Food Nutr. Res. 2021, 65, 4527. [Google Scholar] [CrossRef]
- Matejcic, M.; Lesueur, F.; Biessy, C.; Renault, A.L.; Mebirouk, N.; Yammine, S.; Keski-Rahkonen, P.; Li, K.; Hemon, B.; Weiderpass, E.; et al. Circulating plasma phospholipid fatty acids and risk of pancreatic cancer in a large European cohort. Int. J. Cancer 2018, 143, 2437–2448. [Google Scholar] [CrossRef]
- Venn-Watson, S.K.; Butterworth, C.N. Broader and safer clinically-relevant activities of pentadecanoic acid compared to omega-3: Evaluation of an emerging essential fatty acid across twelve primary human cell-based disease systems. PLoS ONE 2022, 17, e0268778. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H. Cytokines and liver diseases. Can. J. Gastroenterol. 2001, 15, 661–668. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bishop, C.A.; Machate, T.; Henkel, J.; Schulze, M.B.; Klaus, S.; Piepelow, K. Heptadecanoic Acid Is Not a Key Mediator in the Prevention of Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice. Nutrients 2023, 15, 2052. https://doi.org/10.3390/nu15092052
Bishop CA, Machate T, Henkel J, Schulze MB, Klaus S, Piepelow K. Heptadecanoic Acid Is Not a Key Mediator in the Prevention of Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice. Nutrients. 2023; 15(9):2052. https://doi.org/10.3390/nu15092052
Chicago/Turabian StyleBishop, Christopher A., Tina Machate, Janin Henkel, Matthias B. Schulze, Susanne Klaus, and Karolin Piepelow. 2023. "Heptadecanoic Acid Is Not a Key Mediator in the Prevention of Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice" Nutrients 15, no. 9: 2052. https://doi.org/10.3390/nu15092052
APA StyleBishop, C. A., Machate, T., Henkel, J., Schulze, M. B., Klaus, S., & Piepelow, K. (2023). Heptadecanoic Acid Is Not a Key Mediator in the Prevention of Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice. Nutrients, 15(9), 2052. https://doi.org/10.3390/nu15092052






