Diet Quality and Sleep Characteristics in Midlife: The Bogalusa Heart Study
Abstract
:1. Introduction
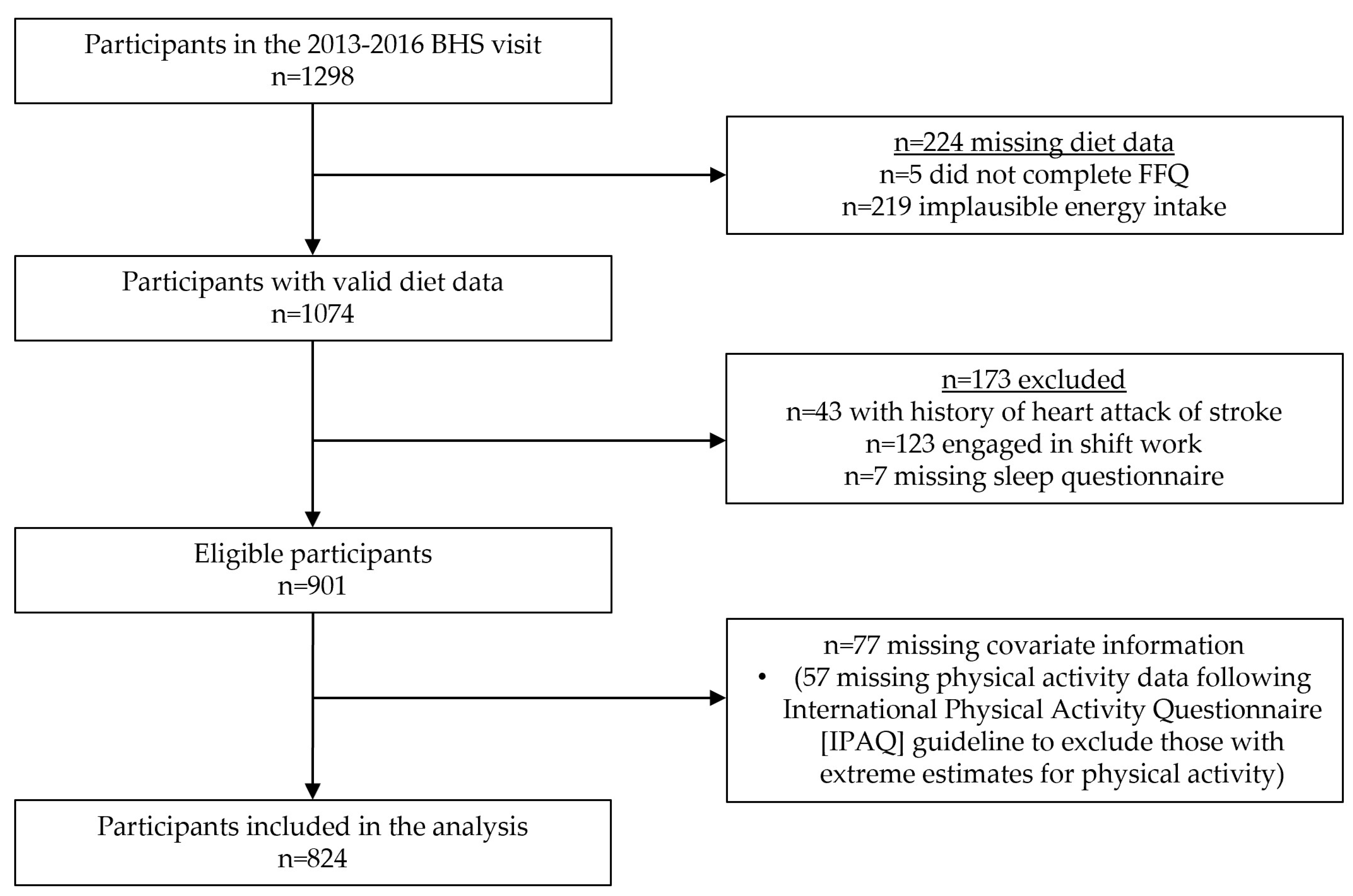
2. Materials and Methods
2.1. Study Design and Population
2.2. Measures
2.2.1. Dietary Assessment
2.2.2. Measurement of Sleep Outcomes
2.2.3. Measurement of Covariates
2.3. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murphy, S.L.; Kochanek, K.D.; Xu, J.; Arias, E. Mortality in the United States, 2020; NCHS Data Brief No. 427; National Center for Health Statistics: Hyattsville, MD, USA, 2021; 8p. [Google Scholar]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Rehm, C.D.; Peñalvo, J.L.; Afshin, A.; Mozaffarian, D. Dietary Intake Among US Adults, 1999–2012. JAMA 2016, 315, 2542–2553. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef]
- Shan, Z.; Li, Y.; Baden, M.Y.; Bhupathiraju, S.N.; Wang, D.D.; Sun, Q.; Rexrode, K.M.; Rimm, E.B.; Qi, L.; Willett, W.C.; et al. Association Between Healthy Eating Patterns and Risk of Cardiovascular Disease. JAMA Intern. Med. 2020, 180, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wheaton, A.G.; Chapman, D.P.; Cunningham, T.J.; Lu, H.; Croft, J.B. Prevalence of Healthy Sleep Duration among Adults—United States, 2014. MMWR. Morb. Mortal. Wkly. Rep. 2016, 65, 137–141. [Google Scholar] [CrossRef]
- Consensus Conference Panel, C.C.; Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. Sleep 2015, 38, 1161–1183. [Google Scholar] [CrossRef] [PubMed]
- Roth, T. Insomnia: Definition, Prevalence, Etiology, and Consequences. J. Clin. Sleep. Med. 2007, 3, S7–S10. [Google Scholar] [CrossRef]
- Reid, M.; Maras, J.E.; Shea, S.; Wood, A.C.; Castro-Diehl, C.; Johnson, D.A.; Huang, T.; Jacobs, D.R.; Crawford, A.; St-Onge, M.-P.; et al. Association between Diet Quality and Sleep Apnea in the Multi-Ethnic Study of Atherosclerosis. Sleep 2019, 42, zsy194. [Google Scholar] [CrossRef]
- Zuraikat, F.M.; Wood, R.A.; Barragán, R.; St-Onge, M.-P. Sleep and Diet: Mounting Evidence of a Cyclical Relationship. Annu. Rev. Nutr. 2021, 41, 309–332. [Google Scholar] [CrossRef]
- Gao, Q.; Kou, T.; Zhuang, B.; Ren, Y.; Dong, X.; Wang, Q. The Association between Vitamin D Deficiency and Sleep Disorders: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1395. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Annunziata, G.; Somma, C.D.; Laudisio, D.; Colao, A.; Savastano, S. Obesity and Sleep Disturbance: The Chicken or the Egg? Crit. Rev. Food Sci. Nutr. 2019, 59, 2158–2165. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Patel, N.P.; Perlis, M.L.; Gehrman, P.R.; Xie, D.; Sha, D.; Pigeon, W.R.; Teff, K.; Weaver, T.; Gooneratne, N.S. Obesity, Diabetes, and Exercise Associated with Sleep-Related Complaints in the American Population. Z. Gesundh. Wiss. 2011, 19, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Campanini, M.Z.; Guallar-Castillón, P.; Rodríguez-Artalejo, F.; Lopez-Garcia, E. Mediterranean Diet and Changes in Sleep Duration and Indicators of Sleep Quality in Older Adults. Sleep 2017, 40, zsw083. [Google Scholar] [CrossRef]
- Zuraikat, F.M.; Makarem, N.; St-Onge, M.-P.; Xi, H.; Akkapeddi, A.; Aggarwal, B. A Mediterranean Dietary Pattern Predicts Better Sleep Quality in US Women from the American Heart Association Go Red for Women Strategically Focused Research Network. Nutrients 2020, 12, 2830. [Google Scholar] [CrossRef]
- Stern, J.H.; Grant, A.S.; Thomson, C.A.; Tinker, L.; Hale, L.; Brennan, K.M.; Woods, N.F.; Chen, Z. Short Sleep Duration Is Associated with Decreased Serum Leptin, Increased Energy Intake and Decreased Diet Quality in Postmenopausal Women. Obesity 2014, 22, E55–E61. [Google Scholar] [CrossRef] [PubMed]
- Mossavar-Rahmani, Y.; Weng, J.; Wang, R.; Shaw, P.A.; Jung, M.; Sotres-Alvarez, D.; Castañeda, S.F.; Gallo, L.C.; Gellman, M.D.; Qi, Q.; et al. Actigraphic Sleep Measures and Diet Quality in the Hispanic Community Health Study/Study of Latinos Sueño Ancillary Study. J. Sleep Res. 2017, 26, 739–746. [Google Scholar] [CrossRef]
- Castro-Diehl, C.; Wood, A.C.; Redline, S.; Reid, M.; Johnson, D.A.; Maras, J.E.; Jacobs, D.R.; Shea, S.; Crawford, A.; St-Onge, M.-P. Mediterranean Diet Pattern and Sleep Duration and Insomnia Symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep 2018, 41, zsy158. [Google Scholar] [CrossRef]
- Jaussent, I.; Bouyer, J.; Ancelin, M.L.; Akbaraly, T.; Peres, K.; Ritchie, K.; Besset, A.; Dauvilliers, Y. Insomnia and Daytime Sleepiness Are Risk Factors for Depressive Symptoms in the Elderly. Sleep 2011, 34, 1103–1110. [Google Scholar] [CrossRef]
- Mamalaki, E.; Anastasiou, C.A.; Ntanasi, E.; Tsapanou, A.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.M.; Sakka, P.; Scarmeas, N.; Yannakoulia, M. Associations between the Mediterranean Diet and Sleep in Older Adults: Results from the Hellenic Longitudinal Investigation of Aging and Diet Study. Geriatr. Gerontol. Int. 2018, 18, 1543–1548. [Google Scholar] [CrossRef]
- Godos, J.; Ferri, R.; Caraci, F.; Cosentino, I.F.; Castellano, S.; Galvano, F.; Grosso, G. Adherence to the Mediterranean Diet Is Associated with Better Sleep Quality in Italian Adults. Nutrients 2019, 11, 976. [Google Scholar] [CrossRef]
- Mondin, T.C.; Stuart, A.L.; Williams, L.J.; Jacka, F.N.; Pasco, J.A.; Ruusunen, A. Diet Quality, Dietary Patterns and Short Sleep Duration: A Cross-Sectional Population-Based Study. Eur. J. Nutr. 2019, 58, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Grandner, M.A.; Jackson, N.J.; Izci-Balserak, B.; Gallagher, R.A.; Murray-Bachmann, R.; Williams, N.J.; Patel, N.P.; Jean-Louis, G. Social and Behavioral Determinants of Perceived Insufficient Sleep. Front. Neurol. 2015, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Duncan, D.T.; Kawachi, I.; Redline, S. The Social Epidemiology of Sleep; Oxford University Press: Oxford, UK, 2019; ISBN 978-0-19-093046-2. [Google Scholar]
- Shepherd, R. Social Determinants of Food Choice. Proc. Nutr. Soc. 1999, 58, 807–812. [Google Scholar] [CrossRef]
- Caspi, C.E.; Sorensen, G.; Subramanian, S.V.; Kawachi, I. The Local Food Environment and Diet: A Systematic Review. Health Place 2012, 18, 1172–1187. [Google Scholar] [CrossRef]
- Potts, K.; Bazzano, L. Diet Quality and Sleep Characteristics in Midlife: The Bogalusa Heart Study. Curr. Dev. Nutr. 2022, 6, 387. [Google Scholar] [CrossRef]
- Berenson, G.S. Bogalusa Heart Study: A Long-Term Community Study of a Rural Biracial (Black/White) Population. Am. J. Med. Sci. 2001, 322, 267–274. [Google Scholar] [CrossRef]
- Tucker, K.L.; Maras, J.; Champagne, C.; Connell, C.; Goolsby, S.; Weber, J.; Zaghloul, S.; Carithers, T.; Bogle, M.L. A Regional Food-Frequency Questionnaire for the US Mississippi Delta. Public. Health Nutr. 2005, 8, 87–96. [Google Scholar] [CrossRef]
- Carithers, T.C.; Talegawkar, S.A.; Rowser, M.L.; Henry, O.R.; Dubbert, P.M.; Montgomery, S.; Bogle, M.L.; Taylor, H.A.; Tucker, K.L. Validity and Calibration of Food Frequency Questionnaires Used with African American Adults in the Jackson Heart Study. J. Am. Diet. Assoc. 2009, 109, 1184–1193. [Google Scholar] [CrossRef]
- USDA. Agricultural Research Service Food Patterns Equivalent Database, Overview. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/food-surveys-research-group/docs/fped-overview/ (accessed on 3 February 2022).
- NCC: Nutrition Coordinating Center NDSR Software Food and Nutrient Database. Available online: http://www.ncc.umn.edu/ndsr-database-page/ (accessed on 28 July 2020).
- Reedy, J.; Lerman, J.L.; Krebs-Smith, S.M.; Kirkpatrick, S.I.; Pannucci, T.R.E.; Wilson, M.M.; Subar, A.F.; Kahle, L.L.; Tooze, J.A. Evaluation of the Healthy Eating Index-2015. J. Acad. Nutr. Diet. 2018, 118, 1622–1633. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Fung, T.T.; Rimm, E.B.; Hu, F.B.; McCullough, M.L.; Wang, M.; Stampfer, M.J.; Willett, W.C. Alternative Dietary Indices Both Strongly Predict Risk of Chronic Disease. J. Nutr. 2012, 142, 1009–1018. [Google Scholar] [CrossRef]
- Fung Teresa, T.; Rexrode Kathryn, M.; Mantzoros Christos, S.; Manson JoAnn, E.; Willett Walter, C.; Hu Frank, B. Mediterranean Diet and Incidence of and Mortality From Coronary Heart Disease and Stroke in Women. Circulation 2009, 119, 1093–1100. [Google Scholar] [CrossRef]
- Levine, D.W.; Lewis, M.A.; Bowen, D.J.; Kripke, D.F.; Kaplan, R.M.; Naughton, M.J.; Shumaker, S.A. Reliability and Validity of Women’s Health Initiative Insomnia Rating Scale. Psychol. Assess. 2003, 15, 137–148. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.W.; Dailey, M.E.; Rockhill, B.; Tipping, D.; Naughton, M.J.; Shumaker, S.A. Validation of the Women’s Health Initiative Insomnia Rating Scale in a Multicenter Controlled Clinical Trial. Psychosom. Med. 2005, 67, 98–104. [Google Scholar] [CrossRef]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire to Identify Patients at Risk for the Sleep Apnea Syndrome. Ann. Intern. Med. 1999, 131, 485. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Sun, D.; Zhou, T.; Heianza, Y.; Lv, J.; Li, L.; Qi, L. Sleep Patterns, Genetic Susceptibility, and Incident Cardiovascular Disease: A Prospective Study of 385 292 UK Biobank Participants. Eur. Heart J. 2020, 41, 1182–1189. [Google Scholar] [CrossRef]
- Adan, A.; Almirall, H. Horne & Östberg Morningness-Eveningness Questionnaire: A Reduced Scale. Personal. Individ. Differ. 1991, 12, 241–253. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sport. Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef]
- Andresen, E.M.; Malmgren, J.A.; Carter, W.B.; Patrick, D.L. Screening for Depression in Well Older Adults: Evaluation of a Short Form of the CES-D. Am. J. Prev. Med. 1994, 10, 77–84. [Google Scholar] [CrossRef]
- MMQGIS. Available online: https://michaelminn.com/linux/mmqgis/ (accessed on 3 February 2022).
- QGIS.org QGIS Geographic Information System. Available online: https://www.qgis.org (accessed on 3 February 2022).
- US Census Bureau American Community Survey 5-Year Data (2009–2019). Available online: https://www.census.gov/data/developers/data-sets/acs-5year.html (accessed on 3 February 2022).
- Feldman, J.M.; Waterman, P.D.; Coull, B.A.; Krieger, N. Spatial Social Polarisation: Using the Index of Concentration at the Extremes Jointly for Income and Race/Ethnicity to Analyse Risk of Hypertension. J. Epidemiol. Community Health 2015, 69, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- National Center for Chronic Disease Prevention and Health Promotion (U.S. Division of Nutrition, Physical Activity, and Obesity) Census Tract Level State Maps of the Modified Retail Food Environment Index (MRFEI). Available online: https://stacks.cdc.gov/view/cdc/61367 (accessed on 12 December 2021).
- Haghighatdoost, F.; Karimi, G.; Esmaillzadeh, A.; Azadbakht, L. Sleep Deprivation Is Associated with Lower Diet Quality Indices and Higher Rate of General and Central Obesity among Young Female Students in Iran. Nutrition 2012, 28, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Ferranti, R.; Marventano, S.; Castellano, S.; Giogianni, G.; Nolfo, F.; Rametta, S.; Matalone, M.; Mistretta, A. Sleep Quality and Duration Is Related with Diet and Obesity in Young Adolescent Living in Sicily, Southern Italy. Sleep Sci. 2016, 9, 117–122. [Google Scholar] [CrossRef]
- Cheng, F.W.; Li, Y.; Winkelman, J.W.; Hu, F.B.; Rimm, E.B.; Gao, X. Probable Insomnia Is Associated with Future Total Energy Intake and Diet Quality in Men. Am. J. Clin. Nutr. 2016, 104, 462–469. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Peppard, P.E.; Taheri, S. Excess Weight and Sleep-Disordered Breathing. J. Appl. Physiol. 2005, 99, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Yoshiike, T.; Nagao, K.; Utsumi, T.; Tsuru, A.; Otsuki, R.; Ayabe, N.; Hazumi, M.; Suzuki, M.; Saitoh, K.; et al. Association of Subjective Quality and Quantity of Sleep with Quality of Life among a General Population. Int. J. Environ. Res. Public. Health 2021, 18, 12835. [Google Scholar] [CrossRef] [PubMed]
| Total Sample a | Quintile of Alternate Healthy Eating Index-2010 b | p-Value c | |||
|---|---|---|---|---|---|
| Q1 | Q3 | Q5 | |||
| n = 824 | n = 163 | n = 166 | n = 169 | ||
| Demographic characteristics | |||||
| Age in years | 48.29 ± 5.18 | 47.44 ± 5.31 | 48.43 ± 5.21 | 49.23 ± 4.80 | 0.002 |
| Male (%) | 36.17 | 35.58 | 31.93 | 29.59 | 0.049 |
| Black persons (%) | 29.85 | 24.54 | 37.35 | 27.98 | 0.113 |
| Years of education (%) | |||||
| Less than high school | 10.56 | 14.72 | 11.45 | 5.92 | 0.004 |
| High school | 34.71 | 39.26 | 38.55 | 26.63 | |
| Some college or higher | 54.73 | 46.01 | 50.00 | 67.46 | |
| Employed (%) | 63.35 | 58.90 | 60.24 | 68.64 | 0.300 |
| Bed partner (%) | 62.26 | 62.58 | 55.42 | 63.91 | 0.220 |
| Household size, persons | 2.89 ± 1.45 | 2.86 ± 1.36 | 2.99 ± 1.44 | 2.80 ± 1.24 | 0.602 |
| Number of children in the house | 0.85 ± 1.08 | 0.86 ±1.02 | 0.95 ± 1.20 | 0.76 ± 0.99 | 0.174 |
| Neighborhood characteristics (census tract) | |||||
| ACS: % persons in poverty | 26.29 ± 10.96 | 27.11 ±9.70 | 27.08 ±10.99 | 22.92 ± 12.01 | 0.0002 |
| ACS: median income | 36,364.08 ± 17,408.19 | 34,595.61 ± 17,450.58 | 34,879.18 ± 16,114.36 | 42,479.30 ± 21,679.29 | <0.0001 |
| ACS: % households with no vehicle | 10.36 ± 6.69 | 10.93 ± 6.78 | 10.44 ± 6.24 | 8.90 ± 6.74 | 0.019 |
| ACS: Index of Concentration at the Extremes | −0.05 ± 0.20 | −0.05 ± 0.17 | −0.08 ± 0.20 | 0.00 ± 0.21 | 0.001 |
| ACS: total households | 1749.99 ± 775.36 | 1727.52 ± 1035.20 | 1740.71 ± 669.04 | 1834.49 ± 785.86 | 0.310 |
| Modified retail food environment index | 11.60 ± 9.16 | 11.32 ± 9.63 | 11.71 ± 9.20 | 11.36 ±8.99 | 0.953 |
| Health and lifestyle factors | |||||
| Smoking status (%) | |||||
| Never | 53.88 | 47.24 | 57.23 | 63.91 | <0.0001 |
| Former | 22.45 | 16.56 | 15.66 | 26.63 | |
| Current | 23.67 | 36.20 | 27.11 | 9.47 | |
| Current alcohol use (%) | 56.07 | 42.94 | 46.99 | 77.51 | <0.0001 |
| Total energy intake, kcal/d | 2319.30 ± 1019.58 | 2660.54 ± 901.85 | 2070.36 ± 998.06 | 2057.09 ± 934.93 | <0.0001 |
| Caffeine intake, mg/d | 257.84 ± 329.26 | 296.17 ± 346.17 | 220.87 ± 312.87 | 229.10 ± 260.86 | 0.173 |
| Physical activity, MET minutes per week | 4941.33 ± 5135.69 | 4285.45 ± 4755.41 | 4996.31 ± 5688.94 | 5827.31 ± 5348.46 | 0.079 |
| Illicit drug use (%) | 33.62 | 42.33 | 29.52 | 30.77 | 0.053 |
| Frequent sleeping pill use (%) | 17.84 | 19.02 | 22.89 | 14.20 | 0.217 |
| Depressive symptoms (%) | 28.28 | 36.20 | 32.53 | 19.53 | 0.004 |
| CESD-10 score | 7.44 ± 5.53 | 8.23 ± 6.03 | 7.94 ± 5.55 | 6.02 ± 4.45 | 0.0004 |
| Body mass index, kg/m2 | 31.37 ± 7.64 | 31.05 ± 6.73 | 32.27 ± 8.41 | 30.27 ± 7.62 | 0.159 |
| Obesity (%) | 52.18 | 57.06 | 53.01 | 44.38 | 0.198 |
| Waist circumference, cm | 96.02 ± 19.01 | 95.79 ± 16.64 | 97.14 ± 19.82 | 92.20 ± 20.84 | 0.047 |
| Sleep outcomes | |||||
| High risk for insomnia (%) | 44.05 | 51.53 | 44.58 | 36.09 | 0.029 |
| High risk for sleep apnea (%) | 44.05 | 52.76 | 50.00 | 30.77 | 0.0006 |
| Healthy sleep pattern (%) | 22.94 | 19.63 | 19.88 | 29.59 | 0.175 |
| Model | Q1 | Q2 a | Q3 a | Q4 a | Q5 a | p for Trend b | Per SD Increase a | |
|---|---|---|---|---|---|---|---|---|
| Alternate Healthy Eating Index 2010 | ||||||||
| Q n (median) | 163 (32.52) | 162 (39.41) | 166 (44.32) | 164 (50.36) | 169 (57.91) | SD = 10.01 | ||
| Insomnia risk | 1 | 1.00 | 0.95 (0.75, 1.20) | 0.87 (0.73, 1.03) | 0.77 (0.64, 0.93) ** | 0.70 (0.52, 0.94) * | 0.0004 | 0.88 (0.82, 0.95) *** |
| 2 | 1.00 | 1.03 (0.83, 1.27) | 0.91 (0.74, 1.10) | 0.83 (0.67, 1.04) | 0.75 (0.57, 0.99) * | 0.009 | 0.90 (0.83, 0.97) ** | |
| 3 | 1.00 | 1.06 (0.87, 1.29) | 0.90 (0.70, 1.15) | 0.95 (0.78, 1.15) | 0.86 (0.67, 1.11) | NS | 0.94 (0.87, 1.01) | |
| Sleep apnea risk | 1 | 1.00 | 0.83 (0.67, 1.02) | 0.95 (0.79, 1.13) | 0.82 (0.69, 0.98) * | 0.58 (0.45, 0.76) *** | <0.0001 | 0.86 (0.80, 0.93) *** |
| 2 | 1.00 | 0.81 (0.65, 1.01) | 1.00 (0.85, 1.17) | 0.84 (0.72, 0.97) * | 0.61 (0.47, 0.79) *** | <0.0001 | 0.88 (0.82, 0.94) *** | |
| 3 | 1.00 | 0.77 (0.61, 0.97) * | 0.91 (0.79, 1.05) | 0.80 (0.68, 0.95) * | 0.59 (0.44, 0.79) *** | <0.0001 | 0.88 (0.81, 0.95) ** | |
| Healthy sleep pattern | 1 | 1.00 | 1.10 (0.81, 1.49) | 1.01 (0.62, 1.66) | 1.21 (0.85, 1.72) | 1.51 (0.97, 2.34) | 0.048 | 1.15 (1.00, 1.33) |
| 2 | 1.00 | 1.10 (0.82, 1.49) | 0.98 (0.61, 1.57) | 1.15 (0.80, 1.67) | 1.39 (0.92, 2.10) | NS | 1.13 (0.98, 1.30) | |
| 3 | 1.00 | 1.14 (0.80, 1.64) | 0.97 (0.60, 1.55) | 1.05 (0.78, 1.41) | 1.19 (0.80, 1.77) | NS | 1.05 (0.93, 1.19) | |
| Healthy Eating Index 2015 | ||||||||
| Q n (median) | 160 (46.82) | 172 (54.39) | 165 (58.97) | 165 (63.93) | 162 (71.04) | SD = 9.17 | ||
| Insomnia risk | 1 | 1.00 | 1.03 (0.86, 1.23) | 0.88 (0.65, 1.19) | 0.94 (0.79, 1.13) | 0.72 (0.55, 0.94) * | 0.003 | 0.91 (0.85, 0.98) ** |
| 2 | 1.00 | 1.07 (0.88, 1.32) | 0.89 (0.67, 1.17) | 0.99 (0.80, 1.23) | 0.71 (0.55, 0.91) ** | 0.002 | 0.91 (0.85, 0.98) ** | |
| 3 | 1.00 | 1.05 (0.87, 1.26) | 0.89 (0.72, 1.10) | 1.01 (0.84, 1.23) | 0.85 (0.67, 1.10) | NS | 0.96 (0.89, 1.03) | |
| Sleep apnea risk | 1 | 1.00 | 0.91 (0.70, 1.17) | 1.03 (0.83, 1.29) | 0.88 (0.73, 1.07) | 0.76 (0.61, 0.94) * | 0.014 | 0.92 (0.85, 0.98) * |
| 2 | 1.00 | 0.94 (0.73, 1.21) | 1.06 (0.84, 1.33) | 0.93 (0.76, 1.14) | 0.77 (0.62, 0.95) * | 0.017 | 0.92 (0.86, 0.99) * | |
| 3 | 1.00 | 0.87 (0.70, 1.09) | 1.00 (0.81, 1.23) | 0.90 (0.72, 1.12) | 0.83 (0.66, 1.05) | NS | 0.95 (0.87, 1.01) | |
| Healthy sleep pattern | 1 | 1.00 | 1.15 (0.62, 2.12) | 0.97 (0.58, 1.63) | 0.97 (0.61, 1.55) | 1.31 (0.84, 2.03) | NS | 1.08 (0.97, 1.21) |
| 2 | 1.00 | 1.08 (0.62, 1.87) | 0.96 (0.60, 1.55) | 0.88 (0.58, 1.33) | 1.32 (0.86, 2.02) | NS | 1.08 (0.97, 1.21) | |
| 3 | 1.00 | 1.13 (0.66, 1.96) | 0.94 (0.58, 1.53) | 0.85 (0.57, 1.26) | 1.07 (0.67, 1.72) | NS | 1.02 (0.90, 1.15) | |
| Alternate Mediterranean Dietary Pattern | ||||||||
| Q n (median) | 201 (2.00) | 154 (3.00) | 164 (4.00) | 132 (5.00) | 173 (6.00) | SD = 1.78 | ||
| Insomnia risk | 1 | 1.00 | 0.83 (0.67, 1.02) | 0.85 (0.64, 1.14) | 1.05 (0.91, 1.20) | 0.75 (0.62, 0.90) ** | 0.031 | 0.94 (0.89, 1.00) * |
| 2 | 1.00 | 0.83 (0.68, 1.03) | 0.86 (0.63, 1.17) | 1.03 (0.84, 1.27) | 0.68 (0.55, 0.85) *** | 0.017 | 0.91 (0.85, 0.99) * | |
| 3 | 1.00 | 0.86 (0.73, 1.01) | 0.91 (0.69, 1.21) | 1.04 (0.81, 1.33) | 0.81 (0.66, 0.99) * | NS | 0.97 (0.89, 1.04) | |
| Sleep apnea risk | 1 | 1.00 | 0.99 (0.81, 1.22) | 1.08 (0.90, 1.29) | 1.20 (1.02, 1.41) * | 1.04 (0.84, 1.28) | NS | 1.02 (0.96, 1.09) |
| 2 | 1.00 | 0.98 (0.80, 1.19) | 1.02 (0.86, 1.21) | 1.11 (0.92, 1.33) | 0.89 (0.73, 1.08) | NS | 0.97 (0.91, 1.03) | |
| 3 | 1.00 | 0.95 (0.76, 1.20) | 0.95 (0.81, 1.12) | 1.02 (0.84, 1.24) | 0.94 (0.78, 1.14) | NS | 0.98 (0.91, 1.06) | |
| Healthy sleep pattern | 1 | 1.00 | 1.08 (0.83, 1.42) | 1.02 (0.78, 1.33) | 0.87 (0.60, 1.28) | 0.91 (0.70, 1.19) | NS | 0.95 (0.88, 1.03) |
| 2 | 1.00 | 1.09 (0.85, 1.40) | 1.02 (0.79, 1.31) | 0.87 (0.56, 1.36) | 0.99 (0.74, 1.31) | NS | 0.97 (0.87, 1.08) | |
| 3 | 1.00 | 1.04 (0.81, 1.36) | 0.98 (0.78, 1.24) | 0.84 (0.51, 1.39) | 0.77 (0.59, 1.01) | 0.049 | 0.90 (0.81, 0.99) * | |
| AHEI 2010 | Q1 | Q2 d | Q3 d | Q4 d | Q5 d | p for Trend e | Per One SD (10.01) Increase | p for Interaction f | |
|---|---|---|---|---|---|---|---|---|---|
| Insomnia risk a | Men | 1.00 | 1.12 (0.78, 1.62) | 0.81 (0.44, 1.48) | 0.97 (0.69, 1.36) | 0.91 (0.52, 1.57) | NS | 0.96 (0.84, 1.08) | NS |
| Women | 1.00 | 1.01 (0.76, 1.34) | 0.89 (0.73, 1.09) | 0.89 (0.66, 1.19) | 0.84 (0.66, 1.07) | NS | 0.92 (0.84, 1.01) | ||
| Sleep apnea risk a | Men | 1.00 | 0.76 (0.56, 1.03) | 1.11 (0.87, 1.41) | 0.81 (0.59, 1.13) | 0.78 (0.52, 1.18) | NS | 1.00 (0.88, 1.13) | 0.028 |
| Women | 1.00 | 0.76 (0.59, 0.98) * | 0.76 (0.60, 0.96) * | 0.79 (0.62, 1.01) | 0.48 (0.35, 0.66) *** | <0.0001 | 0.80 (0.74, 0.88) *** | ||
| Healthy sleep a | Men | 1.00 | 1.36 (0.73, 2.53) | 1.49 (0.46, 4.84) | 1.81 (1.05, 3.14) | 1.52 (0.61, 3.83) | NS | 1.14 (0.92, 1.42) | NS |
| Women | 1.00 | 1.14 (0.67, 1.91) | 0.83 (0.53, 1.30) | 0.77 (0.51, 1.15) | 1.01 (0.68, 1.49) | NS | 0.99 (0.87, 1.14) | ||
| Insomnia risk b | Black | 1.00 | 1.40 (1.01, 1.95) * | 0.98 (0.62, 1.55) | 1.08 (0.61, 1.89) | 1.30 (0.82, 2.04) | NS | 1.03 (0.92, 1.15) | NS |
| White | 1.00 | 1.00 (0.78, 1.27) | 0.89 (0.74, 1.08) | 0.96 (0.76, 1.21) | 0.77 (0.57, 1.05) | NS | 0.91 (0.83, 1.00) | ||
| Sleep apnea risk b | Black | 1.00 | 1.00 (0.68, 1.47) | 0.96 (0.60, 1.54) | 0.78 (0.47, 1.30) | 0.77 (0.37, 1.57) | NS | 0.90 (0.72, 1.12) | NS |
| White | 1.00 | 0.70 (0.51, 0.94) * | 0.92 (0.76, 1.11) | 0.86 (0.71, 1.04) | 0.54 (0.39, 0.74) *** | <0.0001 | 0.88 (0.81, 0.95) ** | ||
| Healthy sleep b | Black | 1.00 | 1.43 (1.04, 1.96) | 1.50 (0.74, 3.03) | 1.51 (0.52, 4.32) | 1.71 (0.93, 3.12) | NS | 1.11 (0.87, 1.40) | NS |
| White | 1.00 | 1.15 (0.72, 1.83) | 0.87 (0.51, 1.49) | 1.05 (0.73, 1.52) | 1.16 (0.72, 1.88) | NS | 1.08 (0.93, 1.25) | ||
| Insomnia risk c | Low ed. | 1.00 | 1.03 (0.83, 1.28) | 0.95 (0.71, 1.27) | 1.04 (0.83, 1.29) | 0.85 (0.59, 1.23) | NS | 0.95 (0.87, 1.04) | NS |
| High ed. | 1.00 | 1.06 (0.83, 1.35) | 0.81 (0.59, 1.13) | 0.84 (0.61, 1.16) | 0.90 (0.67, 1.22) | NS | 0.93 (0.84, 1.04) | ||
| Sleep apnea risk c | Low ed. | 1.00 | 0.73 (0.56, 0.94) * | 0.90 (0.71, 1.14) | 0.86 (0.66, 1.13) | 0.86 (0.61, 1.22) | NS | 0.97 (0.88, 1.08) | 0.009 |
| High ed. | 1.00 | 0.80 (0.58, 1.11) | 0.95 (0.72, 1.26) | 0.79 (0.59, 1.06) | 0.50 (0.34, 0.72) *** | 0.0001 | 0.83 (0.75, 0.92) *** | ||
| Healthy sleep c | Low ed. | 1.00 | 1.37 (0.92, 2.04) | 1.22 (0.77, 1.93) | 1.16 (0.62, 2.17) | 1.35 (0.74, 2.47) | NS | 1.01 (0.85, 1.20) | NS |
| High ed. | 1.00 | 0.95 (0.51, 1.77) | 0.79 (0.45, 1.39) | 0.95 (0.58, 1.54) | 1.02 (0.63, 1.64) | NS | 1.08 (0.93, 1.26) |
| Component of AHEI-2010 Dietary Pattern | Q1 | Q2 a | Q3 a | Q4 a | Q5 a | p for Trend | Per One Unit Increase a |
|---|---|---|---|---|---|---|---|
| 1. Fruits | 1.00 | 0.90 (0.78, 1.04) | 0.90 (0.74, 1.09) | 0.95 (0.79, 1.15) | 0.77 (0.60, 1.00) * | NS | 0.97 (0.95, 1.00) |
| 2. Vegetables (no potatoes) | 1.00 | 0.87 (0.72, 1.05) | 0.93 (0.74, 1.18) | 1.18 (1.00, 1.40) | 0.85 (0.68, 1.07) | NS | 1.00 (0.98, 1.02) |
| 3. Nuts and legumes | 1.00 | 1.28 (1.02, 1.61) * | 1.15 (0.91, 1.45) | 1.04 (0.85, 1.27) | 1.16 (0.94, 1.42) | NS | 1.00 (0.97, 1.04) |
| 4. Whole grains | 1.00 | 1.03 (0.83, 1.28) | 1.20 (0.87, 1.65) | 0.87 (0.65, 1.14) | 1.03 (0.77, 1.38) | NS | 0.99 (0.96, 1.03) |
| 5. Long-chain (n-3) fats | 1.00 | 0.75 (0.57, 0.98) * | 0.88 (0.74, 1.05) | 0.81 (0.68, 0.97) * | 0.70 (0.50, 0.98) * | NS | 0.97 (0.95, 1.00) * |
| 6. Polyunsaturated fats | 1.00 | 1.02 (0.74, 1.40) | 0.94 (0.73, 1.19) | 0.88 (0.62, 1.24) | 0.96 (0.68, 1.36) | NS | 0.99 (0.95, 1.03) |
| 7. Sugar-sweetened beverages + fruit juice b | 1.00 | 0.83 (0.69, 0.98) * | 0.87 (0.75, 1.00) | 0.75 (0.60, 0.93) * | NA | 0.010 | 0.98 (0.96, 0.99) ** |
| 8. Red and processed meats b | 1.00 | 0.89 (0.68, 1.17) | 0.87 (0.70, 1.07) | 0.73 (0.55, 0.96) * | NA | 0.017 | 0.97 (0.94, 1.00) * |
| 9. Trans fats | 1.00 | 0.98 (0.84, 1.16) | 0.97 (0.84, 1.11) | 0.91 (0.74, 1.11) | 0.98 (0.67, 1.45) | NS | 0.97 (0.93, 1.01) |
| 10. Sodium | 1.00 | 0.99 (0.84, 1.18) | 1.02 (0.82, 1.27) | 1.05 (0.84, 1.31) | 1.06 (0.72, 1.56) | NS | 1.00 (0.97, 1.04) |
| 11. Alcohol | 1.00 | 0.91 (0.68, 1.22) | 0.95 (0.73, 1.24) | 1.00 (0.79, 1.28) | 0.76 (0.64, 0.91) ** | 0.008 | 0.98 (0.95, 1.00) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potts, K.S.; Wallace, M.E.; Gustat, J.; Ley, S.H.; Qi, L.; Bazzano, L.A. Diet Quality and Sleep Characteristics in Midlife: The Bogalusa Heart Study. Nutrients 2023, 15, 2078. https://doi.org/10.3390/nu15092078
Potts KS, Wallace ME, Gustat J, Ley SH, Qi L, Bazzano LA. Diet Quality and Sleep Characteristics in Midlife: The Bogalusa Heart Study. Nutrients. 2023; 15(9):2078. https://doi.org/10.3390/nu15092078
Chicago/Turabian StylePotts, Kaitlin S., Maeve E. Wallace, Jeanette Gustat, Sylvia H. Ley, Lu Qi, and Lydia A. Bazzano. 2023. "Diet Quality and Sleep Characteristics in Midlife: The Bogalusa Heart Study" Nutrients 15, no. 9: 2078. https://doi.org/10.3390/nu15092078








