Patients with Diverticular Disease Have Different Dietary Habits Compared to Control Subjects: Results from an Observational Italian Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
- (a)
- Controls (C): asymptomatic for upper and lower gastrointestinal symptoms, without endoscopic evidence of colonic diverticula or other organic disease (colonoscopy performed within two years);
- (b)
- Diverticulosis patients (D): with presence of colonic diverticula in the absence of abdominal symptoms;
- (c)
- SUDD patients: with recurrent abdominal pain, mainly in the lower abdominal quadrants, with a frequency of at least once weekly, present for at least six months, and/or changes in bowel habit, without a well-defined previous attack of acute diverticulitis;
- (d)
- Previous diverticulitis patients (PD): with at least one past episode of acute diverticulitis, complicated or not.
2.3. Statistical Analysis
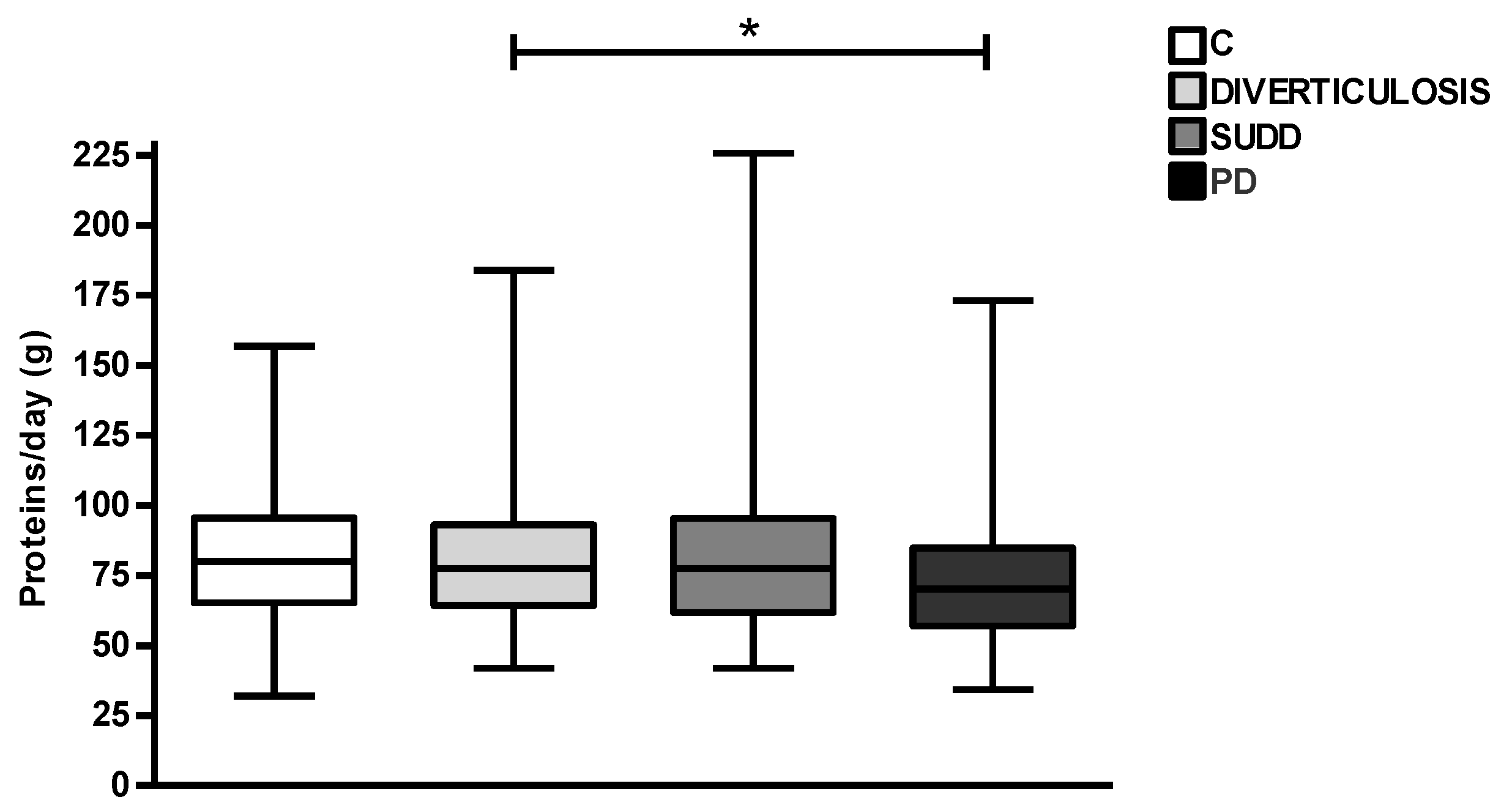
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peery, A.F.; Dellon, E.S.; Lund, J.; Crockett, S.D.; McGowan, C.E.; Bulsiewicz, W.J.; Gangarosa, L.M.; Thiny, M.T.; Stizenberg, K.; Morgan, D.R.; et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012, 143, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Stollman, N.; Raskin, J.B. Diverticular disease of the colon. Lancet 2004, 363, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Cuomo, R.; Barbara, G.; Pace, F.; Annese, V.; Bassotti, G.; Binda, G.A.; Casetti, T.; Colecchia, A.; Festi, D.; Fiocca, R.; et al. Italian consensus conference for colonic diverticulosis and diverticular disease. United Eur. Gastroenterol. J. 2014, 2, 413–442. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, A.E.; Parthasarathy, G.; Ditah, I.; Fletcher, J.G.; Ewelukwa, O.; Pendlimari, R.; Yawn, B.P.; Melton, L.J.; Schleck, C.; Zinsmeister, A.R. Temporal Trends in the Incidence and Natural History of Diverticulitis: A Population-Based Study. Am. J. Gastroenterol. 2015, 110, 1589–1596. [Google Scholar] [CrossRef] [PubMed]
- Paterson, H.M.; Arnott, I.D.; Nicholls, R.J.; Clark, D.; Bauer, J.; Bridger, P.C.; Crowe, A.M.; Knight, A.D.; Hodgkins, P.; Solomon, D.; et al. Diverticular disease in Scotland: 2000–2010. Color. Dis. 2015, 17, 329–334. [Google Scholar] [CrossRef]
- Wheat, C.L.; Strate, L.L. Trends in Hospitalization for Diverticulitis and Diverticular Bleeding in the United States From 2000 to 2010. Clin. Gastroenterol. Hepatol. 2016, 14, 96–103. [Google Scholar] [CrossRef]
- Binda, G.A.; Mataloni, F.; Bruzzone, M.; Carabotti, M.; Cirocchi, R.; Nascimbeni, R.; Gambassi, G.; Amato, A.; Vettoretto, N.; Pinnarelli, L.; et al. Trends in hospital admission for acute diverticulitis in Italy from 2008 to 2015. Tech. Coloproctol. 2018, 22, 597–604. [Google Scholar] [CrossRef]
- Ma, W.; Nguyen, L.H.; Song, M.; Jovani, M.; Liu, P.H.; Cao, Y.; Tam, I.; Wu, K.; Giovannucci, E.L.; Strate, L.L.; et al. Intake of Dietary Fiber, Fruits, and Vegetables and Risk of Diverticulitis. Am. J. Gastroenterol. 2019, 114, 1531–1538. [Google Scholar] [CrossRef]
- Crowe, F.L.; Balkwill, A.; Cairns, B.J.; Appleby, P.N.; Green, J.; Reeves, G.K.; Key, T.J.; Beral, V. Million Women Study Collaborators; Million Women Study Collaborators. Source of dietary fibre and diverticular disease incidence: A prospective study of UK women. Gut 2014, 63, 1450–1456. [Google Scholar] [CrossRef]
- Strate, L.L.; Keeley, B.R.; Cao, Y.; Wu, K.; Giovannucci, E.L.; Chan, A.T. Western Dietary Pattern Increases, and Prudent Dietary Pattern Decreases, Risk of Incident Diverticulitis in a Prospective Cohort Study. Gastroenterology 2017, 152, 1023–1030. [Google Scholar] [CrossRef]
- Cao, Y.; Strate, L.L.; Keeley, B.R.; Tam, I.; Wu, K.; Giovannucci, E.L.; Chan, A.T. Meat intake and risk of diverticulitis among men. Gut 2018, 67, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Maguire, L.H.; Song, M.; Strate, L.L.; Giovannucci, E.L.; Chan, A.T. Association of geographic and seasonal variation with diverticulitis admissions. JAMA Surg. 2015, 150, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Cuomo, R.; Barbara, G.; Pace, F.; Andreozzi, P.; Cremon, C.; Annibale, B. Demographic and clinical features distinguish subgroups of diverticular disease patients: Results from an Italian nationwide registry. United Eur. Gastroenterol. J. 2018, 6, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Cremon, C.; Carabotti, M.; Cuomo, R.; Pace, F.; Andreozzi, P.; Barbaro, M.R.; Annibale, B.; Barbara, G. Italian nationwide survey of pharmacologic treatments in diverticular disease: Results from the REMAD registry. United Eur. Gastroenterol. J. 2019, 7, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Carabotti, M.; Morselli Labate, A.M.; Cremon, C.; Cuomo, R.; Pace, F.; Andreozzi, P.; Falangone, F.; Barbara, G.; Annibale, B. Distinguishing features between patients with acute diverticulitis and diverticular bleeding: Results from the REMAD registry. Dig. Liver Dis. 2021, 53, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Rimm, E.; Smith-Warner, S.A.; Feskanich, D.; Stampfer, M.J.; Ascherio, A.; Sampson, L.; Willett, W.C. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am. J. Clin. Nutr. 1999, 69, 243–249. [Google Scholar] [CrossRef]
- Sette, S.; Le Donne, C.; Piccinelli, R.; Arcella, D.; Turrini, A.; Leclercq, C. INRAN-SCAI 2005-6 Study Group. The third Italian National Food Consumption Survey, INRAN-SCAI 2005-06-Part 1: Nutrient intakes in Italy. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 922–932. [Google Scholar] [CrossRef]
- Spiller, R. Is it diverticular disease or is it irritable bowel syndrome? Dig. Dis. 2012, 30, 64–69. [Google Scholar] [CrossRef]
- Strate, L.L.; Modi, R.; Cohen, E.; Spiegel, B.M. Diverticular disease as a chronic illness: Evolving epidemiologic and clinical insights. Am. J. Gastroenterol. 2012, 107, 1486–1493. [Google Scholar] [CrossRef]
- Violi, A.; Cambiè, G.; Miraglia, C.; Barchi, A.; Nouvenne, A.; Capasso, M.; Leandro, G.; Meschi, T.; De’ Angelis, G.L.; Di Mario, F. Epidemiology and risk factors for diverticular disease. Acta Biomed. 2018, 89, 107–112. [Google Scholar]
- Crowe, F.L.; Appleby, P.N.; Allen, N.E.; Key, T.J. Diet and risk of diverticular disease in Oxford cohort of European Prospective Investigation into Cancer and Nutrition (EPIC): Prospective study of British vegetarians and non-vegetarians. BMJ 2011, 343, d4131. [Google Scholar] [CrossRef] [PubMed]
- Aldoori, W.H.; Giovannucci, E.L.; Rockett, H.R.; Sampson, L.; Rimm, E.B.; Willett, W.C. A prospective study of dietary fiber types and symptomatic diverticular disease in men. J. Nutr. 1998, 128, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.W.; Abraham-Nordling, M.; Håkansson, N.; Wolk, A.; Hjern, F. High intake of dietary fibre from fruit and vegetables reduces the risk of hospitalisation for diverticular disease. Eur. J. Nutr. 2019, 58, 2393–2400. [Google Scholar] [CrossRef]
- Casanova, M.J.; Chaparro, M.; Molina, B.; Merino, O.; Batanero, R.; Dueñas-Sadornil, C.; Robledo, P.; Garcia-Albert, A.M.; Gómez-Sánchez, M.B.; Calvet, X.; et al. Prevalence of Malnutrition and Nutritional Characteristics of Patients with Inflammatory Bowel Disease. J. Crohns Colitis 2017, 11, 1430–1439. [Google Scholar] [CrossRef]
- Murray, H.B.; Bailey, A.P.; Keshishian, A.C.; Silvernale, C.J.; Staller, K.; Eddy, K.T.; Thomas, J.J.; Kuo, B. Prevalence and Characteristics of Avoidant/Restrictive Food Intake Disorder in Adult Neurogastroenterology Patients. Clin. Gastroenterol. Hepatol. 2020, 18, 1995–2002. [Google Scholar] [CrossRef] [PubMed]
- Cândido, F.G.; Valente, F.X.; Grześkowiak, Ł.M.; Moreira, A.P.B.; Rocha, D.M.U.P.; Alfenas, R.C.G. Impact of dietary fat on gut microbiota and low-grade systemic inflammation: Mechanisms and clinical implications on obesity. Int. J. Food Sci. Nutr. 2018, 69, 125–143. [Google Scholar] [CrossRef]
- Ley, S.H.; Sun, Q.; Willett, W.C.; Eliassen, A.H.; Wu, K.; Pan, A.; Grodstein, F.; Hu, F.B. Associations between red meat intake and biomarkers of inflammation and glucose metabolism in women. Am. J. Clin. Nutr. 2014, 99, 352–360. [Google Scholar] [CrossRef]
- Micha, R.; Michas, G.; Mozaffarian, D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes: An updated review of the evidence. Curr. Atheroscler. Rep. 2012, 14, 515–524. [Google Scholar] [CrossRef]
- Pan, A.; Sun, Q.; Bernstein, A.M.; Schulze, M.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Red meat consumption and risk of type 2 diabetes: 3 cohorts of US adults and an updated meta-analysis. Am. J. Clin. Nutr. 2011, 94, 1088–1096. [Google Scholar] [CrossRef]
- Kim, E.; Coelho, D.; Blachier, F. Review of the association between meat consumption and risk of colorectal cancer. Nutr. Res. 2013, 33, 983–994. [Google Scholar] [CrossRef]
- Lordan, C.; Thapa, D.; Ross, R.P.; Cotter, P.D. Potential for enriching next-generation health-promoting gut bacteria through prebiotics and other dietary components. Gut Microbes 2020, 11, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Strate, L.L.; Liu, Y.L.; Syngal, S.; Aldoori, W.H.; Giovannucci, E.L. Nut, corn, and popcorn consumption and the incidence of diverticular disease. JAMA 2008, 300, 907–914. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.H.; Hill, M.J.; Jenkins, D.J.; Pearson, J.R.; Wiggins, H.S. Changes in fecal composition and colonic function due to cereal fiber. Am. J. Clin. Nutr. 1976, 29, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Forsum, E.; Eriksson, C.; Göranzon, H.; Sohlström, A. Composition of faeces from human subjects consuming diets based on conventional foods containing different kinds and amounts of dietary fibre. Br. J. Nutr. 1990, 64, 171–186. [Google Scholar] [CrossRef]
- Lampe, J.W.; Slavin, J.L.; Melcher, E.A.; Potter, J.D. Effects of cereal and vegetable fiber feeding on potential risk factors for colon cancer. Cancer Epidemiol. Biomarkers Prev. 1992, 1, 207–211. [Google Scholar]
- Albenberg, L.G.; Wu, G.D. Diet and the intestinal microbiome: Associations, functions, and implications for health and disease. Gastroenterology 2014, 146, 1564–1572. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Barbara, G.; Scaioli, E.; Barbaro, M.R.; Biagi, E.; Laghi, L.; Cremon, C.; Marasco, G.; Colecchia, A.; Picone, G.; Salfi, N.; et al. Gut microbiota, metabolome and immune signatures in patients with uncomplicated diverticular disease. Gut 2017, 66, 1252–1261. [Google Scholar] [CrossRef]
- Pallotta, L.; Vona, R.; Maselli, M.A.; Cicenia, A.; Bella, A.; Ignazzi, A.; Carabotti, M.; Cappelletti, M.; Gioia, A.; Tarallo, M.; et al. Oxidative imbalance and muscular alterations in diverticular disease. Dig. Liver Dis. 2022, 54, 1186–1194. [Google Scholar] [CrossRef]
- Maguire, L.H.; Song, M.; Strate, L.E.; Giovannucci, E.L.; Chan, A.T. Higher serum levels of vitamin D are associated with a reduced risk of diverticulitis. Clin. Gastroenterol. Hepatol. 2013, 11, 1631–1635. [Google Scholar] [CrossRef]
- Wu, Z.; Broad, J.; Sluyter, J.; Waayer, D.; Camargo, C.A.; Scragg, R. Effect of monthly vitamin D on diverticular disease hospitalization: Post-hoc analysis of a randomized controlled trial. Clin. Nutr. 2021, 40, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Varghese, C.; Wu, Z.; Bissett, I.P.; Connolly, M.J.; Broad, J.B. Seasonal variations in acute diverticular disease hospitalisations in New Zealand. Int. J. Color. Dis. 2023, 38, 46. [Google Scholar] [CrossRef] [PubMed]






| C n = 119 | D n = 344 | SUDD n = 154 | PD n = 88 | p Value | |
|---|---|---|---|---|---|
| Female sex % (n) | 52.9 (63) | 52.6 (181) | 53.2 (82) | 52.3 (46) | 0.99 |
| Age, y | 64.6 ± 9.2 | 66.4 ± 8.8 | 66.2 ± 9.6 | 64.3 ± 11.8 | 0.11 |
| BMI, kg/m2 | 25.8 ± 3.8 | 26.6 ± 3.9 | 26.5 ± 3.8 | 25.7 ± 3.5 | 0.06 |
| C n = 119 | D n = 344 | SUDD n = 154 | PD n = 88 | p Value | ||
|---|---|---|---|---|---|---|
| Unprocessed Red Meat, (%) | Low | 15.3 | 11 | 5.8 | 18.2 | 0.02 |
| Normal | 50.8 | 44.5 | 47.4 | 36.4 | ||
| High | 33.9 | 44.5 | 46.8 | 45.5 | ||
| Processed Red Meat, (%) | Low | 6.8 | 7.6 | 7.8 | 6.8 | 0.44 |
| Normal | 33.9 | 24 | 24.8 | 31.8 | ||
| High | 59.3 | 68.4 | 67.3 | 61.4 | ||
| White Meat, (%) | Low | 32.2 | 8.5 | 26.6 | 15.9 | <0.01 |
| Normal | 61.9 | 88 | 69.5 | 80.7 | ||
| High | 5.9 | 3.5 | 3.9 | 3.4 | ||
| Fish, (%) | Low | 54.2 | 41.9 | 39 | 42 | 0.06 |
| Normal | 44.9 | 57.3 | 60.4 | 54.5 | ||
| High | 0.8 | 0.9 | 0.6 | 3.4 | ||
| Eggs, (%) | Low | 20.3 | 30.9 | 35.3 | 45.5 | 0.02 |
| Normal | 77.1 | 67.3 | 63.4 | 54.5 | ||
| High | 2.5 | 1.7 | 1.3 | 0 | ||
| Cheese, (%) | Low | 0.8 | 6.7 | 6.5 | 5.7 | 0.17 |
| Normal | 17.8 | 14 | 15.6 | 21.6 | ||
| High | 81.4 | 79.2 | 77.9 | 72.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polese, B.; Carabotti, M.; Rurgo, S.; Ritieni, C.; Sarnelli, G.; Barbara, G.; Pace, F.; Cuomo, R.; Annibale, B.; on behalf of REMAD Group. Patients with Diverticular Disease Have Different Dietary Habits Compared to Control Subjects: Results from an Observational Italian Study. Nutrients 2023, 15, 2119. https://doi.org/10.3390/nu15092119
Polese B, Carabotti M, Rurgo S, Ritieni C, Sarnelli G, Barbara G, Pace F, Cuomo R, Annibale B, on behalf of REMAD Group. Patients with Diverticular Disease Have Different Dietary Habits Compared to Control Subjects: Results from an Observational Italian Study. Nutrients. 2023; 15(9):2119. https://doi.org/10.3390/nu15092119
Chicago/Turabian StylePolese, Barbara, Marilia Carabotti, Sara Rurgo, Camilla Ritieni, Giovanni Sarnelli, Giovanni Barbara, Fabio Pace, Rosario Cuomo, Bruno Annibale, and on behalf of REMAD Group. 2023. "Patients with Diverticular Disease Have Different Dietary Habits Compared to Control Subjects: Results from an Observational Italian Study" Nutrients 15, no. 9: 2119. https://doi.org/10.3390/nu15092119
APA StylePolese, B., Carabotti, M., Rurgo, S., Ritieni, C., Sarnelli, G., Barbara, G., Pace, F., Cuomo, R., Annibale, B., & on behalf of REMAD Group. (2023). Patients with Diverticular Disease Have Different Dietary Habits Compared to Control Subjects: Results from an Observational Italian Study. Nutrients, 15(9), 2119. https://doi.org/10.3390/nu15092119







