Comparison of Clinical, Biochemical and Histological Features between Adult Celiac Patients with High and Low Anti-Transglutaminase IgA Titer at Diagnosis and Follow-Up
Abstract
1. Introduction
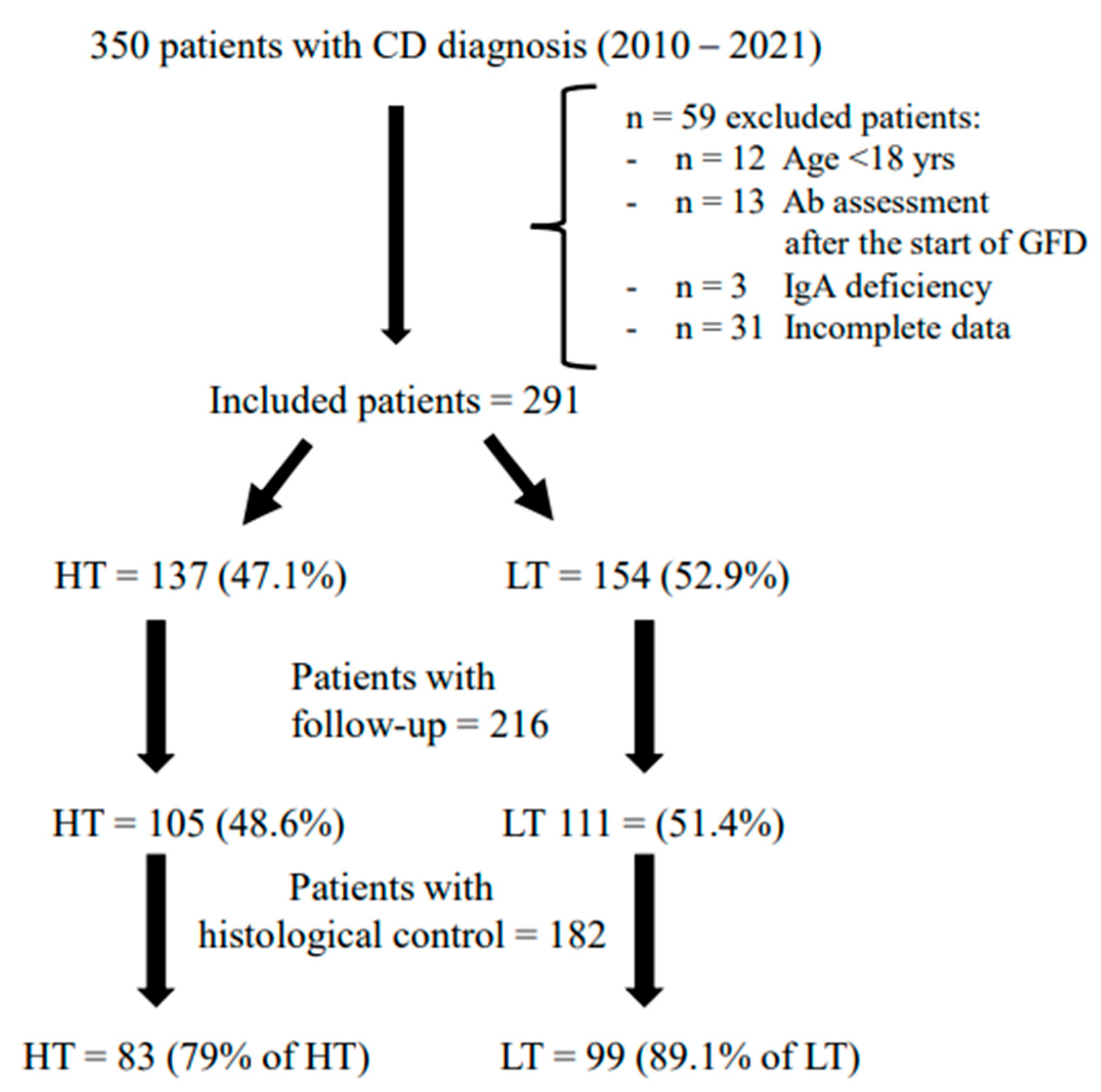
2. Materials and Methods
2.1. Endoscopic Procedures and Histological Classification
2.2. Serological Assays and DEXA Examination
2.3. Clinical Assessment and GFD Evaluation
2.4. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fasano, A.; Catassi, C. Clinical Practice. Celiac Disease. N. Engl. J. Med. 2012, 367, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol J. 2019, 7, 583–613. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; Giersiepen, K.; Branski, D.; Catassi, C.; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Working Group Appointed by the Finnish Medical Society Duodecim, the Finnish Society of Gastroenterology. Coeliac Disease. Current Care Summary. Available online: https://www.kaypahoito.fi/en/ccs00086 (accessed on 25 March 2023).
- Rubio-Tapia, A.; Hill, I.D.; Semrad, C.; Kelly, C.P.; Lebwohl, B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2023, 118, 59–76. [Google Scholar] [CrossRef]
- Tortora, R.; Imperatore, N.; Capone, P.; De Palma, G.D.; De Stefano, G.; Gerbino, N.; Caporaso, N.; Rispo, A. The presence of anti-endomysial antibodies and the level of anti-tissue transglutaminases can be used to diagnose adult coeliac disease without duodenal biopsy. Aliment. Pharm. 2014, 40, 1223–1229. [Google Scholar] [CrossRef]
- Zanini, B.M.; Magni, A.; Caselani, F.; Lanzarotto, F.; Carabellese, N.; Villanacci, V.; Ricci, C.; Lanzini, A. Hightissue-transglutaminase antibody level predicts small intestinal villous atrophy in adult patients at high risk of celiac disease. Dig. Liver Dis. 2012, 44, 280–285. [Google Scholar] [CrossRef]
- Hill, P.G.; Holmes, G.K. Coeliac disease: A biopsy is not always necessary for diagnosis. Aliment. Pharmacol. Ther. 2008, 27, 572–577. [Google Scholar] [CrossRef]
- Penny, H.A.; Raju, S.A.; Lau, M.S.; Marks, L.J.; Baggus, E.M.; Bai, J.C.; Bassotti, G.; Bontkes, H.J.; Carroccio, A.; Danciu, M.; et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut 2021, 70, 876–883. [Google Scholar] [CrossRef]
- Pallav, K.; Leffler, D.A.; Tariq, S.; Kabbani, T.; Hansen, J.; Peer, A.; Kelly, C.P. Noncoeliac enteropathy: The differential diagnosis of villous atrophy in contemporary clinical practice. Aliment Pharmacol Ther. 2012, 35, 380–390. [Google Scholar] [CrossRef]
- Green, P.H.; Fleischauer, A.T.; Bhagat, G.; Goyal, R.; Jabri, B.; Neugut, A.I. Risk of malignancy in patients with celiac disease. Am. J. Med. 2003, 115, 191–195. [Google Scholar] [CrossRef]
- Bardella, M.T.; Velio, P.; Cesana, B.M.; Prampolini, L.; Casella, G.; Di Bella, C.; Lanzini, A.; Gambarotti, M.; Bassotti, G.; Villanacci, V. Coeliac disease: A histological follow-up study. Histopathology 2007, 50, 465–471. [Google Scholar] [CrossRef]
- Wahab, P.J.; Meijer, J.W.; Mulder, C.J. Histologic follow-up of people with celiac disease on a gluten-free diet: Slow and incomplete recovery. Am. J. Clin. Pathol. 2002, 118, 459–463. [Google Scholar] [CrossRef]
- Losurdo, G.; Di Leo, M.; Santamato, E.; Arena, M.; Rendina, M.; Luigiano, C.; Ierardi, E.; Di Leo, A. Serologic diagnosis of celiac disease: May it be suitable for adults? World J. Gastroenterol. 2021, 27, 7233–7239. [Google Scholar] [CrossRef]
- Taavela, J.; Kurppa, K.; Collin, P.; Lähdeaho, M.L.; Salmi, T.; Saavalainen, P.; Haimila, K.; Huhtala, H.; Laurila, K.; Sievänen, H.; et al. Degree of damage to the small bowel and serum antibody titers correlate with clinical presentation of patients with celiac disease. Clin. Gastroenterol Hepatol. 2013, 11, 166–171.e1. [Google Scholar] [CrossRef]
- Di Tola, M.; Marino, M.; Goetze, S.; Casale, R.; Di Nardi, S.; Borghini, R.; Donato, G.; Tiberti, A.; Picarelli, A. Identification of a serum transglutaminase threshold value for the noninvasive diagnosis of symptomatic adult celiac disease patients: A retrospective study. J. Gastroenterol. 2016, 51, 1031–1039. [Google Scholar] [CrossRef]
- Singh, P.; Kurray, L.; Agnihotri, A.; Das, P.; Verma, A.K.; Sreenivas, V.; Dattagupta, S.; Makharia, G.K. Titers of anti-tissue transglutaminase antibody correlate well with severity of villous abnormalities in celiac disease. J. Clin. Gastroenterol. 2015, 49, 212–217. [Google Scholar] [CrossRef]
- Käräjämäki, A.J.; Taavela, J.; Nielsen, C.; Lönnqvist, M.; Svartbäck, M.; Kaukinen, K.; Tertti, R. Celiac disease antibody levels reflect duodenal mucosal damage but not clinical symptoms. Scand J. Gastroenterol. 2021, 56, 514–519. [Google Scholar] [CrossRef]
- Biagi, F.; Bianchi, P.I.; Marchese, A.; Trotta, L.; Vattiato, C.; Balduzzi, D.; Brusco, G.; Andrealli, A.; Cisarò, F.; Astegiano, M.; et al. A score that verifies adherence to a gluten-free diet: A cross-sectional, multicentre validation in real clinical life. Br. J. Nutr. 2012, 108, 1884–1888. [Google Scholar] [CrossRef]
- Marsh, M.N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic ap-proach to the spectrum of gluten sensitivity (’celiac sprue’). Gastroenterology 1992, 102, 330–354. [Google Scholar] [CrossRef]
- Oberhuber, G.; Granditsch, G.; Vogelsang, H. The Histopathology of Coeliac Disease: Time for a Standardized Report Scheme for Pathologists. Eur. J. Gastroenterol. Hepatol. 1999, 11, 1185–1194. [Google Scholar] [CrossRef]
- Carabotti, M.; Lahner, E.; Esposito, G.; Sacchi, M.C.; Severi, C.; Annibale, B. Upper Gastrointestinal Symptoms in Autoimmune Gastritis: A Cross-Sectional Study. Medicine 2017, 96, e5784. [Google Scholar] [CrossRef] [PubMed]
- Galli, G.; Carabotti, M.; Pilozzi, E.; Lahner, E.; Annibale, B.; Conti, L. Relationship between Persistent Gastrointestinal Symptoms and Duodenal Histological Findings after Adequate Gluten-Free Diet: A Gray Area of Celiac Disease Management in Adult Patients. Nutrients 2021, 13, 600. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.J.; Heaton, K.W. Stool form scale as a useful guide to intestinal transit time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef] [PubMed]
- Vakil, N.; van Zanten, S.V.; Kahrilas, P.; Dent, J.; Jones, R.; Global Consensus Group. The Montreal definition and classification of gastroesophageal reflux disease: A global evidence-based consensus. Am. J. Gastroenterol. 2006, 101, 1900–1920, quiz 1943. [Google Scholar] [CrossRef]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407. [Google Scholar] [CrossRef]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- Sugai, E.; Selvaggio, G.; Vazquez, H.; Viola, M.; Mazure, R.; Pizarro, B.; Smecuol, E.; Flores, D.; Pedreira, S.; Mauriño, E.; et al. Tissue transglutaminase antibodies in celiac disease: Assessment of a commercial kit. Am. J. Gastroenterol. 2000, 95, 2318–2322. [Google Scholar] [CrossRef]
- Sblattero, D.; Berti, I.; Trevisiol, C.; Marzari, R.; Tommasini, A.; Bradbury, A.; Fasano, A.; Ventura, A.; Not, T. Human recombinant tissue transglutaminase ELISA: An innovative diagnostic assay for celiac disease. Am. J. Gastroenterol. 2000, 95, 1253–1257. [Google Scholar] [CrossRef]
- Ylönen, V.; Lindfors, K.; Repo, M.; Huhtala, H.; Fuchs, V.; Saavalainen, P.; Musikka, A.; Laurila, K.; Kaukinen, K.; Kurppa, K. Non-Biopsy Serology-Based Diagnosis of Celiac Disease in Adults Is Accurate with Different Commercial Kits and Pre-Test Probabilities. Nutrients 2020, 12, 2736. [Google Scholar] [CrossRef]
- Donaldson, M.R.; Book, L.S.; Leiferman, K.M.; Zone, J.J.; Neuhausen, S.L. Strongly positive tissue transglutaminase antibodies are associated with Marsh 3 histopathology in adult and pediatric celiac disease. J. Clin. Gastroenterol. 2008, 42, 256–260. [Google Scholar] [CrossRef]
- Mubarak, A.; Wolters, V.M.; Gmelig-Meyling, F.H.; Ten Kate, F.J.; Houwen, R.H. Tissue transglutaminase levels above 100 U/mL and celiac disease: A prospective study. World J. Gastroenterol. 2012, 18, 4399–4403. [Google Scholar] [CrossRef]
- Talarico, V.; Giancotti, L.; Mazza, G.A.; Miniero, R.; Bertini, M. Iron Deficiency Anemia in Celiac Disease. Nutrients 2021, 13, 1695. [Google Scholar] [CrossRef]
- Di Stefano, M.; Mengoli, C.; Bergonzi, M.; Corazza, G.R. Bone mass and mineral metabolism alterations in adult celiac disease: Pathophysiology and clinical approach. Nutrients 2013, 5, 4786–4799. [Google Scholar] [CrossRef]
- Brar, P.; Kwon, G.Y.; Egbuna, I.I.; Holleran, S.; Ramakrishnan, R.; Bhagat, G.; Green, P.H. Lack of correlation of degree of villous atrophy with severity of clinical presentation of coeliac disease. Dig. Liver Dis. 2007, 39, 26–29, discussion 30-2. [Google Scholar] [CrossRef]
- Thomas, H.J.; Ahmad, T.; Rajaguru, C.; Barnardo, M.; Warren, B.F.; Jewell, D.P. Contribution of histological, serological, and genetic factors to the clinical heterogeneity of adult-onset coeliac disease. Scand. J. Gastroenterol. 2009, 44, 1076–1083. [Google Scholar] [CrossRef]
- Abu Daya, H.; Lebwohl, B.; Lewis, S.K.; Green, P.H. Celiac disease patients presenting with anemia have more severe disease than those presenting with diarrhea. Clin. Gastroenterol. Hepatol. 2013, 11, 1472–1477. [Google Scholar] [CrossRef]
- Galli, G.; Esposito, G.; Lahner, E.; Pilozzi, E.; Corleto, V.D.; Di Giulio, E.; Aloe Spiriti, M.A.; Annibale, B. Histological recovery and gluten-free diet adherence: A prospective 1-year follow-up study of adult patients with coeliac disease. Aliment Pharmacol. Ther. 2014, 40, 639–647. [Google Scholar] [CrossRef]
- Pekki, H.; Kurppa, K.; Mäki, M.; Huhtala, H.; Sievänen, H.; Laurila, K.; Collin, P.; Kaukinen, K. Predictors and Significance of Incomplete Mucosal Recovery in Celiac Disease After 1 Year on a Gluten-Free Diet. Am. J. Gastroenterol. 2015, 110, 1078–1085. [Google Scholar] [CrossRef]
- Galli, G.; Amici, G.; Conti, L.; Lahner, E.; Annibale, B.; Carabotti, M. Sex-Gender Differences in Adult Coeliac Disease at Diagnosis and Gluten-Free-Diet Follow-Up. Nutrients 2022, 14, 3192. [Google Scholar] [CrossRef]
| HT Patients with Ab > 10 ULN n = 137 (47.1%) | LT Patients with Ab < 10 ULN n = 154 (52.9%) | p Value | |
|---|---|---|---|
| Females | 75.2% | 67.5% | 0.156 |
| Median age, years (range) | 38 (18–72) | 38 (18–76) | 0.857 |
| Median BMI kg/m2 (range) | 21.8 (15.9–33.8) | 22.5 (16.4–38.2) | 0.218 |
| Underweight (BMI < 18 kg/m2) | 12.8% | 6.9% | 0.728 |
| Autoimmune comorbidities | 30.6% | 31.8% | 0.899 |
| Family history of CD | 17.5% | 18.5% | 0.878 |
| >3 years duration of symptoms/signs before the CD diagnosis | 42.2% | 34.1% | 0.194 |
| Marsh IIIC | 67.1% | 47.4% | 0.005 |
| Clinical presentation Classical presentation | 23.5% | 35.1% | 0.039 |
| GI symptoms Total pts with GI symptoms Nausea/Vomit Heartburn Regurgitation Dysphagia Postprandial fullness/early satiety Abdominal pain Abdominal bloating Constipation Diarrhoea | 73.1% 19.4% 26.1% 13.4% 8.2% 37.3% 43.6% 51.5% 14.2% 22.4% | 82.5% 24.7% 31.2% 25.3% 11% 43% 50% 58.4% 15.6% 27.9% | 0.063 0.321 0.363 0.012 0.434 0.151 0.288 0.285 0.743 0.341 |
| Signs of malabsorption Total pts with signs of malabsorption Anaemia Hypoferritinaemia Hypocholesterolaemia Hypotriglyceridaemia | 68.6% 45.9% 66.6% 7.7% 6.1% | 67.7% 35.3% 45.2% 13% 8% | 0.899 0.071 0.006 0.220 0.629 |
| Osteopenia/Osteoporosis | 63.5% | 50% | 0.039 |
| HT Patients with Ab > 10 ULN n = 105 (48.6%) | LT Patients with Ab < 10 ULN n = 111 (51.4%) | p Value | |
|---|---|---|---|
| Females | 76.2% | 65.8% | 0.101 |
| Median months of GFD (range) | 14 (12–36) | 14 (12–36) | 0.435 |
| Adequate GFD | 95% | 91.8% | 0.414 |
| Median BMI kg/m2 (range) | 22.4 (16.3–32.8) | 23 (20.5–36.6) | 0.284 |
| Underweight (BMI < 18 kg/m2) | 5.3% | 3.9% | 0.739 |
| GI symptoms Total pts with GI symptoms Nausea/Vomit Heartburn Regurgitation Dysphagia Postprandial fullness/early satiety Abdominal pain Abdominal bloating Constipation Diarrhoea | 24.3% 1% 5.8% 2.9% 0 3.9% 9.6% 11.6% 5.8% 4.8% | 38.2% 3.7% 9.2% 5.6% 1.8% 7.3% 18.3% 21.1% 10.1% 8.2% | 0.038 0.370 0.439 0.499 0.376 0.047 0.067 0.315 0.410 |
| Antibodies positivity | 34.3% | 14.4% | 0.001 |
| Signs of malabsorption Total pts with signs of malabsorption Anaemia Hypoferritinaemia Hypocholesterolaemia Hypotriglyceridaemia | 29.1% 13.7% 28.4% 2% 11.6% | 33.9% 11.2% 28.1% 3.9% 8% | 0.464 1 1 0.684 0.472 |
| Hystological control Marsh III (atrophic disease) Marsh 0 score | 79% 33.3% 54.7% | 89.1% 24.2% 58.6% | 0.060 0.191 0.654 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galli, G.; Carabotti, M.; Conti, L.; Scalamonti, S.; Annibale, B.; Lahner, E. Comparison of Clinical, Biochemical and Histological Features between Adult Celiac Patients with High and Low Anti-Transglutaminase IgA Titer at Diagnosis and Follow-Up. Nutrients 2023, 15, 2151. https://doi.org/10.3390/nu15092151
Galli G, Carabotti M, Conti L, Scalamonti S, Annibale B, Lahner E. Comparison of Clinical, Biochemical and Histological Features between Adult Celiac Patients with High and Low Anti-Transglutaminase IgA Titer at Diagnosis and Follow-Up. Nutrients. 2023; 15(9):2151. https://doi.org/10.3390/nu15092151
Chicago/Turabian StyleGalli, Gloria, Marilia Carabotti, Laura Conti, Silvia Scalamonti, Bruno Annibale, and Edith Lahner. 2023. "Comparison of Clinical, Biochemical and Histological Features between Adult Celiac Patients with High and Low Anti-Transglutaminase IgA Titer at Diagnosis and Follow-Up" Nutrients 15, no. 9: 2151. https://doi.org/10.3390/nu15092151
APA StyleGalli, G., Carabotti, M., Conti, L., Scalamonti, S., Annibale, B., & Lahner, E. (2023). Comparison of Clinical, Biochemical and Histological Features between Adult Celiac Patients with High and Low Anti-Transglutaminase IgA Titer at Diagnosis and Follow-Up. Nutrients, 15(9), 2151. https://doi.org/10.3390/nu15092151







