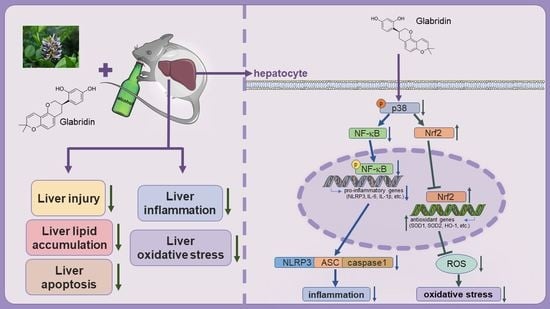
Glabridin Ameliorates Alcohol-Caused Liver Damage by Reducing Oxidative Stress and Inflammation via p38 MAPK/Nrf2/NF-κB Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
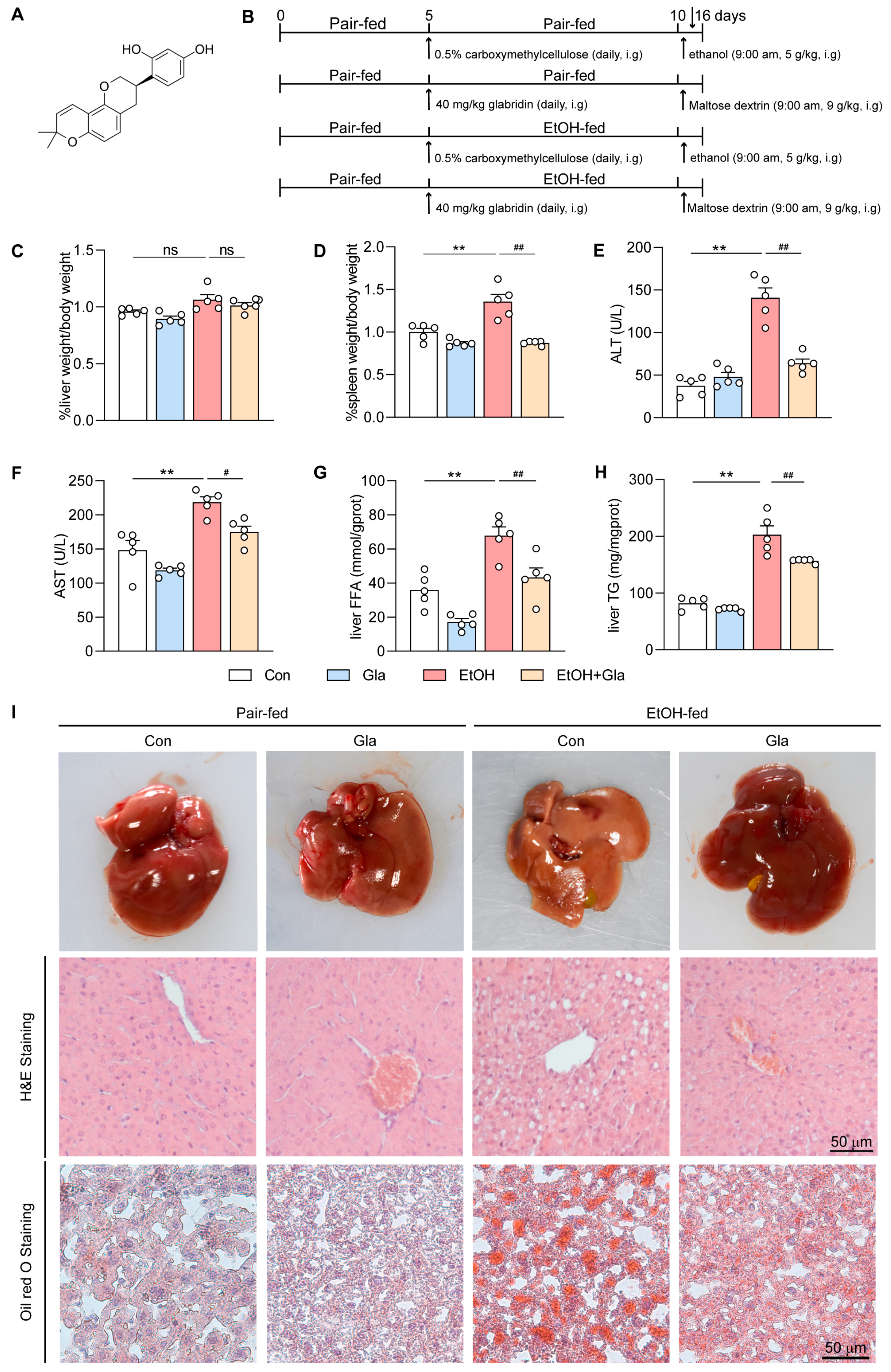
2.2. Animals
2.3. Histopathologic Assay
2.4. Determination of Liver TG, FFA, GSH, SOD, and CAT Levels
2.5. Assessment of Serum Parameters
2.6. Reactive Oxygen Species (ROS) Level Determination
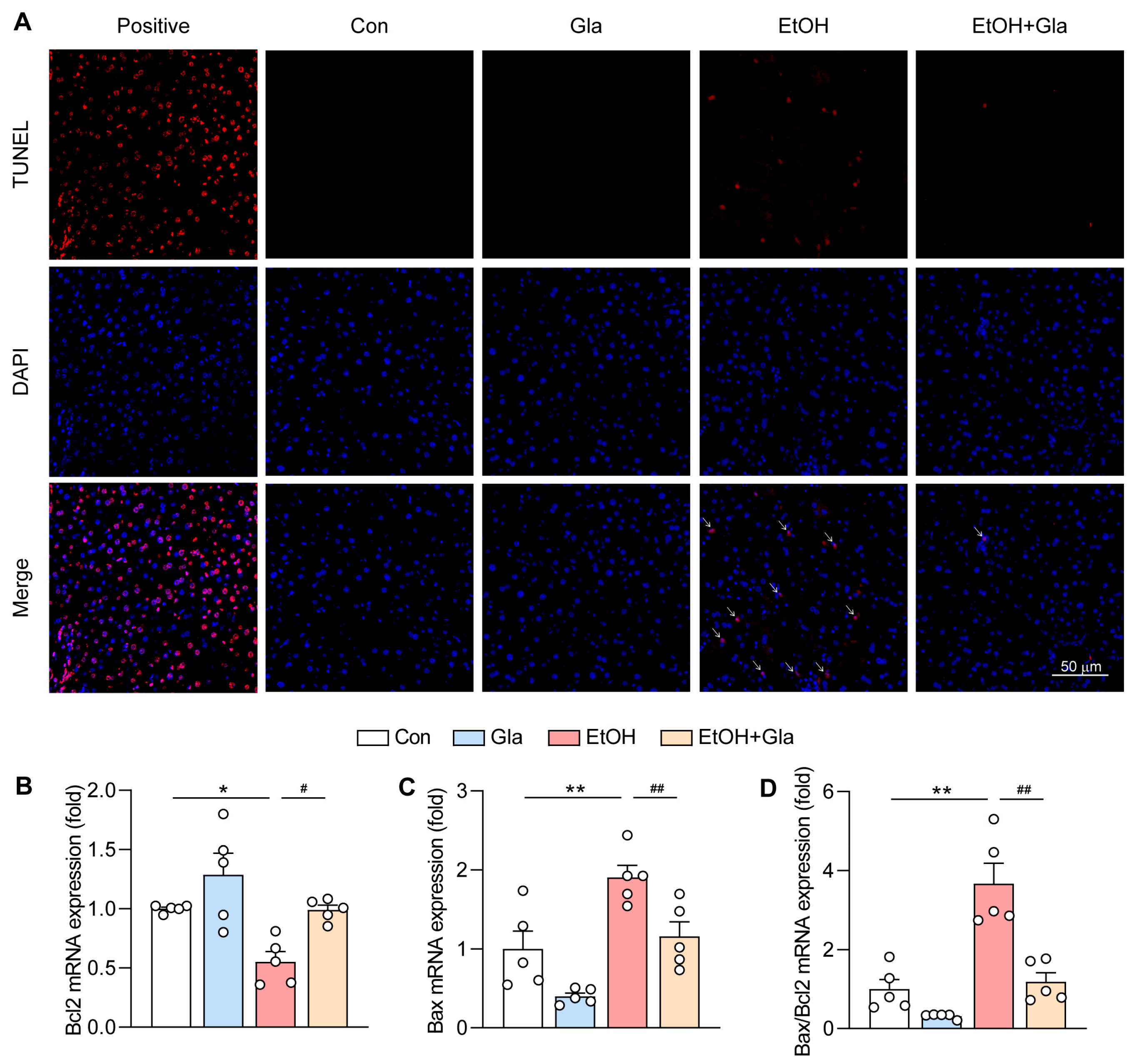
2.7. TUNEL Staining
2.8. Western Blot and Quantitative Real-Time PCR (qPCR)
2.9. Cell Culture
2.10. Cell Viability Detection
2.11. Statistical Analysis
3. Results
3.1. Gla Ameliorated Liver Injury in Ethanol-Induced Mice
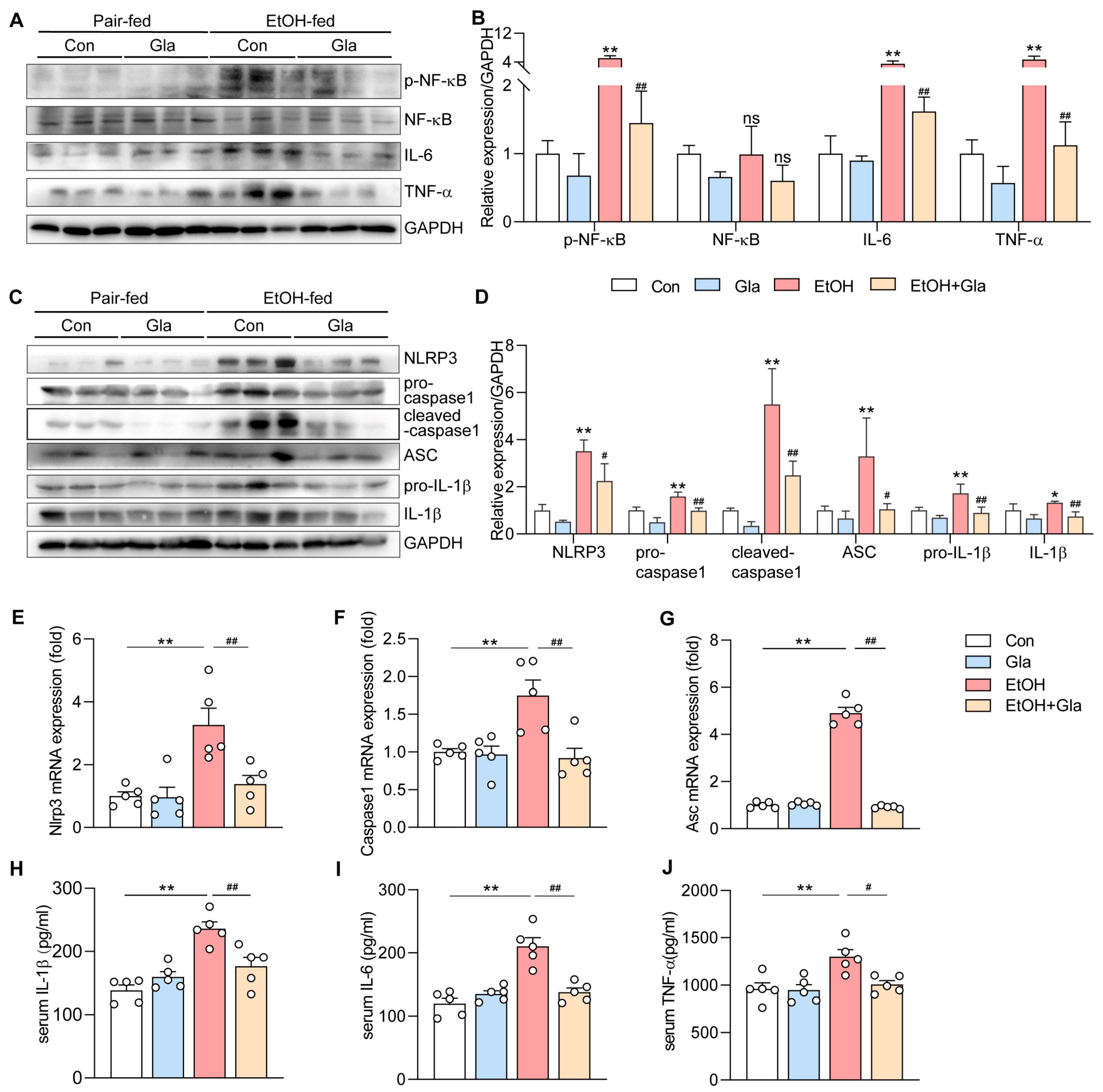
3.2. Effect of Gla on Inflammation of Ethanol-Induced Mice
3.3. Effect of Gla on Oxidative Stress and Apoptosis of Ethanol-Induced Mice
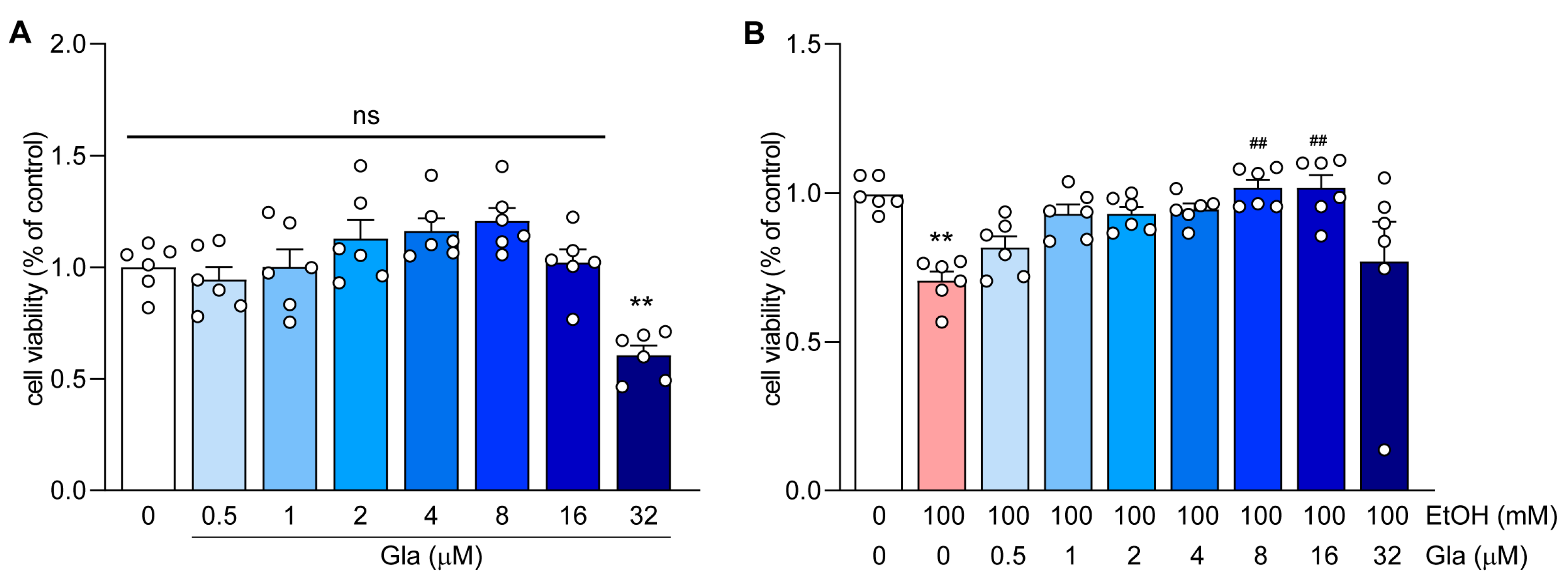
3.4. Effect of Gla on HepG2 Cell Viability
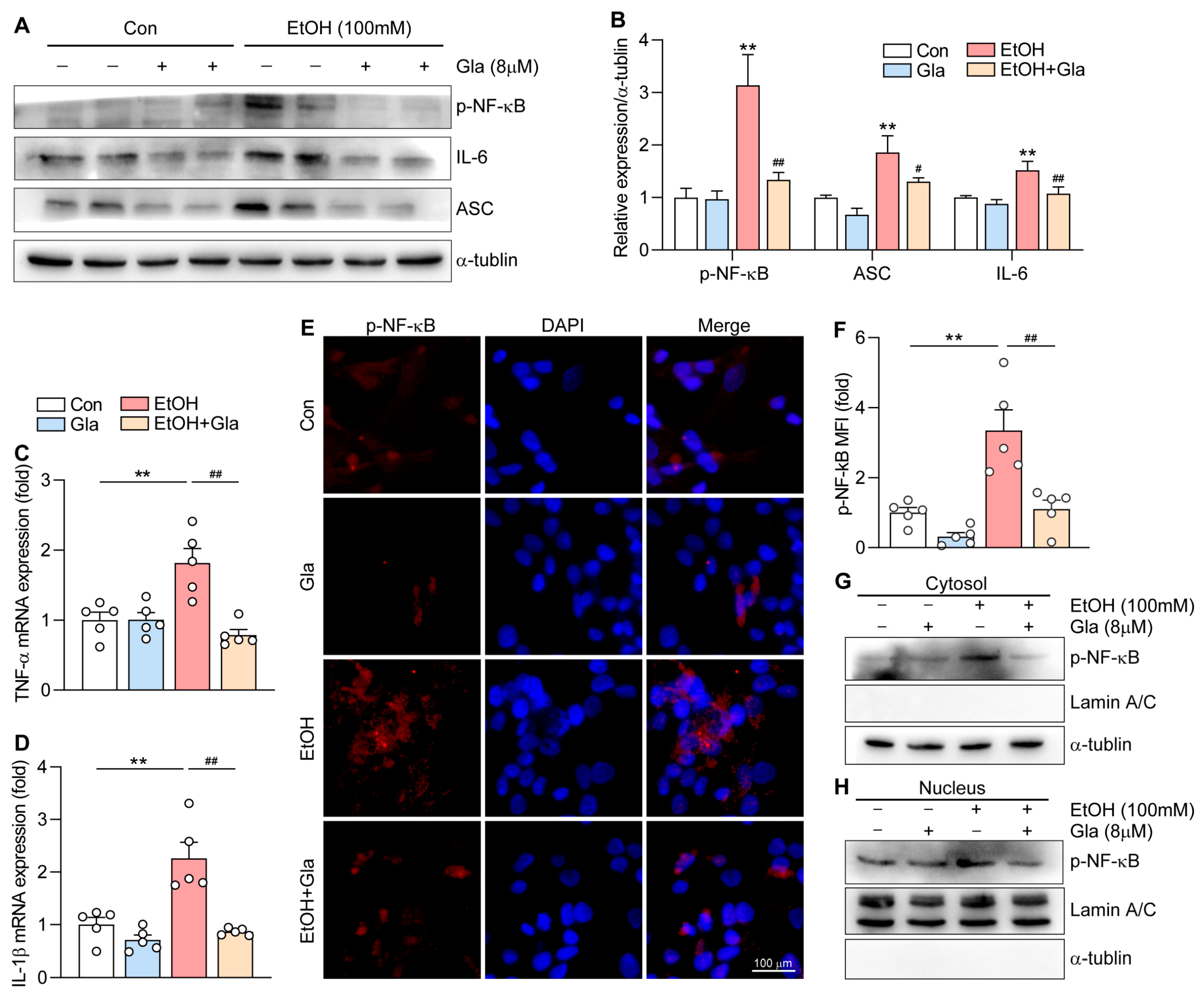
3.5. Gla Attenuated the NF-κB-Mediated Inflammation in Ethanol-Induced HepG2 Cells
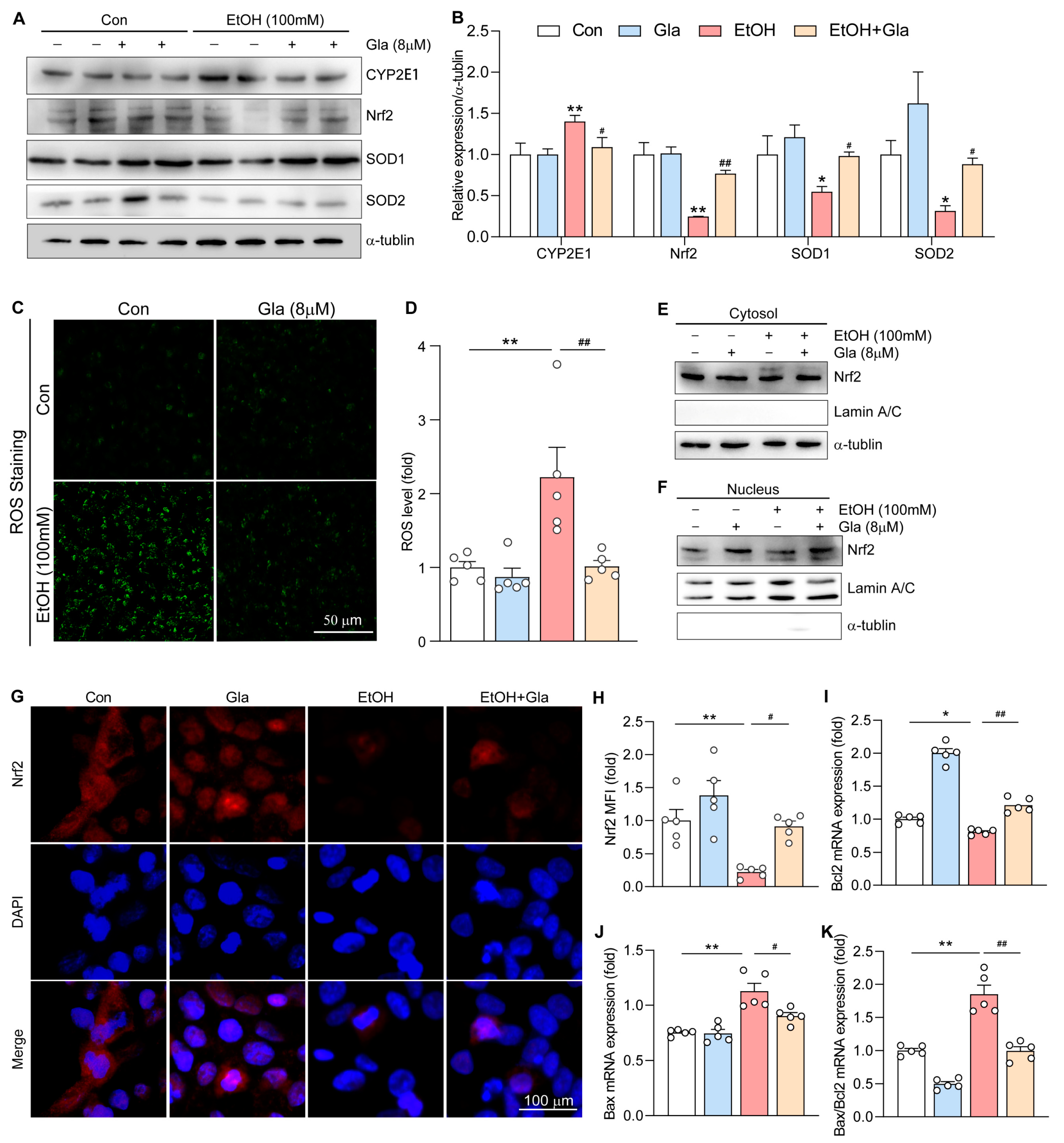
3.6. Gla Promoted the Nrf2-Mediated Antioxidant Responses in Ethanol-Induced HepG2 Cells
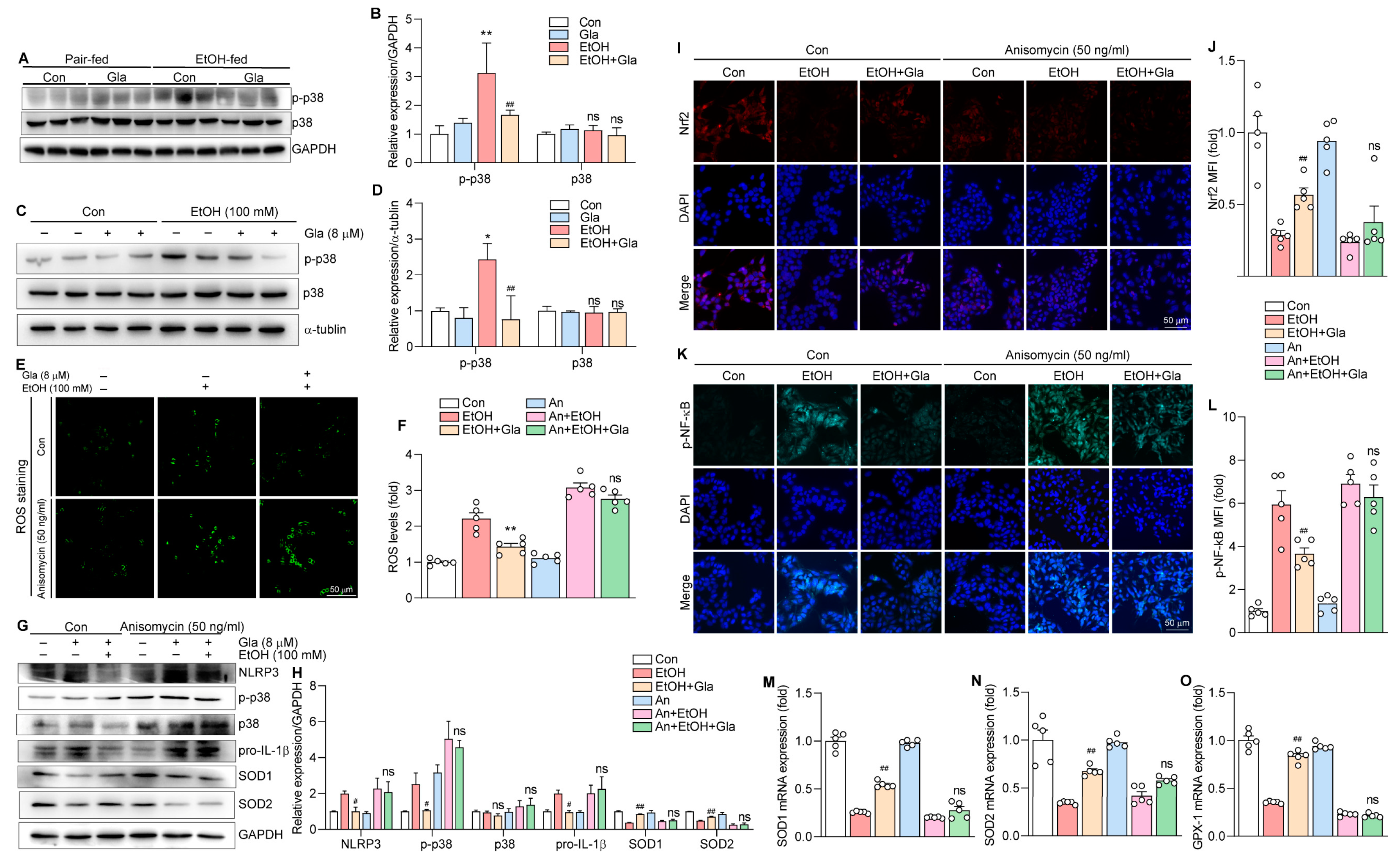
3.7. Gla May Ameliorate Ethanol-Induced Oxidative Stress and Inflammation via the p38 MAPK/Nrf2/NF-κB Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hendriks, H.F.J. Alcohol and Human Health: What Is the Evidence? Annu. Rev. Food Sci. Technol. 2020, 11, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Alcohol, G.B.D.; Drug Use, C. The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry 2018, 5, 987–1012. [Google Scholar]
- Altamirano, J.; Bataller, R. Alcoholic liver disease: Pathogenesis and new targets for therapy. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef] [PubMed]
- Gramenzi, A.; Caputo, F.; Biselli, M.; Kuria, F.; Loggi, E.; Andreone, P.; Bernardi, M. Review article: Alcoholic liver disease—Pathophysiological aspects and risk factors. Aliment. Pharmacol. Ther. 2006, 24, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Louvet, A.; Mathurin, P. Alcoholic liver disease: Mechanisms of injury and targeted treatment. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Seitz, H.K.; Bataller, R.; Cortez-Pinto, H.; Gao, B.; Gual, A.; Lackner, C.; Mathurin, P.; Mueller, S.; Szabo, G.; Tsukamoto, H. Alcoholic liver disease. Nat. Rev. Dis. Prim. 2018, 4, 16. [Google Scholar] [CrossRef]
- Cederbaum, A.I. Cytochrome P450 2E1-dependent oxidant stress and upregulation of anti-oxidant defense in liver cells. J. Gastroenterol. Hepatol. 2006, 21 (Suppl. 3), S22–S25. [Google Scholar] [CrossRef]
- Leung, T.M.; Nieto, N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J. Hepatol. 2013, 58, 395–398. [Google Scholar] [CrossRef]
- Mandrekar, P.; Szabo, G. Signalling pathways in alcohol-induced liver inflammation. J. Hepatol. 2009, 50, 1258–1266. [Google Scholar] [CrossRef]
- Singal, A.K.; Mathurin, P. Diagnosis and Treatment of Alcohol-Associated Liver Disease: A Review. JAMA 2021, 326, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Dastagir, G.; Rizvi, M.A. Review—Glycyrrhiza glabra L. (Liquorice). Pak. J. Pharm. Sci. 2016, 29, 1727–1733. [Google Scholar]
- Tang, B.; Qiao, H.; Meng, F.; Sun, X. Glycyrrhizin attenuates endotoxin-induced acute liver injury after partial hepatectomy in rats. Braz. J. Med. Biol. Res. 2007, 40, 1637–1646. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tang, X.; Mao, B.; Zhang, Q.; Zhao, J.; Cui, S.; Chen, W. Ethanol Extract of Licorice Alleviates HFD-Induced Liver Fat Accumulation in Association with Modulation of Gut Microbiota and Intestinal Metabolites in Obesity Mice. Nutrients 2022, 14, 4180. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kong, L.; Xu, S.; Wang, X.; Huang, K.; Wang, S.; Wu, J.; Wang, C.; Sun, H.; Liu, K.; et al. Isoliquiritigenin-mediated miR-23a-3p inhibition activates PGC-1α to alleviate alcoholic liver injury. Phytomedicine 2022, 96, 153845. [Google Scholar] [CrossRef] [PubMed]
- Simmler, C.; Pauli, G.F.; Chen, S.N. Phytochemistry and biological properties of glabridin. Fitoterapia 2013, 90, 160–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choe, S.S.; Jang, H.; Kim, J.; Jeong, H.W.; Jo, H.; Jeong, K.H.; Tadi, S.; Park, M.G.; Kwak, T.H.; et al. AMPK activation with glabridin ameliorates adiposity and lipid dysregulation in obesity. J. Lipid Res. 2012, 53, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Dogra, A.; Gupta, D.; Bag, S.; Ahmed, I.; Bhatt, S.; Nehra, E.; Dhiman, S.; Kumar, A.; Singh, G.; Abdullah, S.T.; et al. Glabridin ameliorates methotrexate-induced liver injury via attenuation of oxidative stress, inflammation, and apoptosis. Life Sci. 2021, 278, 119583. [Google Scholar] [CrossRef]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef]
- Gong, K.; Wang, M.; Wang, D.; Gao, Y.; Ma, L.; Yang, X.; Zhu, X.; Chen, S.; Zhang, M.; Li, H.; et al. Overexpression of NgBR inhibits high-fat diet-induced atherosclerosis in ApoE-deficiency mice. Hepatol. Commun. 2023, 7, e0048. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Wang, Y.; Gong, K.; Yan, T.; Wang, D.; Meng, X.; Yang, X.; Chen, Y.; Han, J.; et al. Daidzein alleviates doxorubicin-induced heart failure via the SIRT3/FOXO3a signaling pathway. Food Funct. 2022, 13, 9576–9588. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yang, W.; Zhang, C.; Liu, T.; Zhu, J.; Wang, H.; Li, T.; Jin, A.; Ding, L.; Xian, J.; et al. Modulation of the p38 MAPK Pathway by Anisomycin Promotes Ferroptosis of Hepatocellular Carcinoma through Phosphorylation of H3S10. Oxid. Med. Cell. Longev. 2022, 2022, 6986445. [Google Scholar] [CrossRef] [PubMed]
- Dukic, M.; Radonjic, T.; Jovanovic, I.; Zdravkovic, M.; Todorovic, Z.; Kraisnik, N.; Arandelovic, B.; Mandic, O.; Popadic, V.; Nikolic, N.; et al. Alcohol, Inflammation, and Microbiota in Alcoholic Liver Disease. Int. J. Mol. Sci. 2023, 24, 3735. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shao, M.; Xiang, H.; Wang, J.; Ji, G.; Wu, T. Qinggan Huoxue Recipe Alleviates Alcoholic Liver Injury by Suppressing Endoplasmic Reticulum Stress Through LXR-LPCAT3. Front. Pharmacol. 2022, 13, 824185. [Google Scholar] [CrossRef] [PubMed]
- Coulthard, L.R.; White, D.E.; Jones, D.L.; McDermott, M.F.; Burchill, S.A. p38(MAPK): Stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 2009, 15, 369–379. [Google Scholar] [CrossRef]
- Keum, Y.S.; Yu, S.; Chang, P.P.; Yuan, X.; Kim, J.H.; Xu, C.; Han, J.; Agarwal, A.; Kong, A.N. Mechanism of action of sulforaphane: Inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 2006, 66, 8804–8813. [Google Scholar] [CrossRef]
- Pimpin, L.; Cortez-Pinto, H.; Negro, F.; Corbould, E.; Lazarus, J.V.; Webber, L.; Sheron, N.; Committee, E.H.S. Burden of liver disease in Europe: Epidemiology and analysis of risk factors to identify prevention policies. J. Hepatol. 2018, 69, 718–735. [Google Scholar] [CrossRef]
- Singal, A.K.; Bataller, R.; Ahn, J.; Kamath, P.S.; Shah, V.H. ACG Clinical Guideline: Alcoholic Liver Disease. Am. J. Gastroenterol. 2018, 113, 175–194. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, X.; Zhong, B.; Liao, Q.; Wang, X.; Xie, Y.; He, X. Review on the Diverse Biological Effects of Glabridin. Drug Des. Devel. Ther. 2023, 17, 15–37. [Google Scholar] [CrossRef]
- Liu, K.; Pi, F.; Zhang, H.; Ji, J.; Xia, S.; Cui, F.; Sun, J.; Sun, X. Metabolomics Analysis To Evaluate the Anti-Inflammatory Effects of Polyphenols: Glabridin Reversed Metabolism Change Caused by LPS in RAW 264.7 Cells. J. Agric. Food Chem. 2017, 65, 6070–6079. [Google Scholar] [CrossRef]
- Yehuda, I.; Madar, Z.; Szuchman-Sapir, A.; Tamir, S. Glabridin, a phytoestrogen from licorice root, up-regulates manganese superoxide dismutase, catalase and paraoxonase 2 under glucose stress. Phytother. Res. 2011, 25, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Atrahimovich, D.; Vaya, J.; Tavori, H.; Khatib, S. Glabridin protects paraoxonase 1 from linoleic acid hydroperoxide inhibition via specific interaction: A fluorescence-quenching study. J. Agric. Food Chem. 2012, 60, 3679–3685. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Li, Y.; Mu, J.; Hu, C.; Zhou, M.; Wang, X.; Si, L.; Ning, S.; Li, Z. Glabridin inhibits cancer stem cell-like properties of human breast cancer cells: An epigenetic regulation of miR-148a/SMAd2 signaling. Mol. Carcinog. 2016, 55, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Mu, J.; Wang, X.; Ye, X.; Si, L.; Ning, S.; Li, Z.; Li, Y. The repressive effect of miR-148a on TGF beta-SMADs signal pathway is involved in the glabridin-induced inhibition of the cancer stem cells-like properties in hepatocellular carcinoma cells. PLoS ONE 2014, 9, e96698. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Xia, M.; Liu, W.; Li, L.; Yang, J.; Mei, Y.; Meng, Q.; Xie, Y. Glabridin resensitizes p-glycoprotein-overexpressing multidrug-resistant cancer cells to conventional chemotherapeutic agents. Eur. J. Pharmacol. 2019, 852, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Gangwar, B.; Kumar, S.; Darokar, M.P. Glabridin Averts Biofilms Formation in Methicillin-Resistant Staphylococcus aureus by Modulation of the Surfaceome. Front. Microbiol. 2020, 11, 1779. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kang, J.S.; Kim, H.M.; Ryu, H.S.; Kim, H.S.; Lee, H.K.; Kim, Y.J.; Hong, J.T.; Kim, Y.; Han, S.B. Inhibition of bone marrow-derived dendritic cell maturation by glabridin. Int. Immunopharmacol. 2010, 10, 1185–1193. [Google Scholar] [CrossRef]
- Kong, L.; Chen, J.; Ji, X.; Qin, Q.; Yang, H.; Liu, D.; Li, D.; Sun, M. Alcoholic fatty liver disease inhibited the co-expression of Fmo5 and PPARα to activate the NF-κB signaling pathway, thereby reducing liver injury via inducing gut microbiota disturbance. J. Exp. Clin. Cancer Res. 2021, 40, 18. [Google Scholar] [CrossRef] [PubMed]
- Steffan, N.M.; Bren, G.D.; Frantz, B.; Tocci, M.J.; O’Neill, E.A.; Paya, C.V. Regulation of IkB alpha phosphorylation by PKC- and Ca2+-dependent signal transduction pathways. J. Immunol. 1995, 155, 4685–4691. [Google Scholar] [CrossRef]
- Sun, L.; Ma, W.; Gao, W.; Xing, Y.; Chen, L.; Xia, Z.; Zhang, Z.; Dai, Z. Propofol directly induces caspase-1-dependent macrophage pyroptosis through the NLRP3-ASC inflammasome. Cell Death Dis. 2019, 10, 542. [Google Scholar] [CrossRef]
- Li, P.; Li, Y.; Jiang, H.; Xu, Y.; Liu, X.; Che, B.; Tang, J.; Liu, G.; Tang, Y.; Zhou, W.; et al. Glabridin, an isoflavan from licorice root, ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int. Immunopharmacol. 2018, 59, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, G.; Zibrila, A.I.; Li, Y.; Liu, J.; Feng, W. Acetylcholine ameliorated hypoxia-induced oxidative stress and apoptosis in trophoblast cells via p38 MAPK/NF-κB pathway. Mol. Hum. Reprod. 2021, 27, gaab045. [Google Scholar] [CrossRef] [PubMed]
- Butura, A.; Nilsson, K.; Morgan, K.; Morgan, T.R.; French, S.W.; Johansson, I.; Schuppe-Koistinen, I.; Ingelman-Sundberg, M. The impact of CYP2E1 on the development of alcoholic liver disease as studied in a transgenic mouse model. J. Hepatol. 2009, 50, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Bardag-Gorce, F.; Yuan, Q.X.; Li, J.; French, B.A.; Fang, C.; Ingelman-Sundberg, M.; French, S.W. The effect of ethanol-induced cytochrome p4502E1 on the inhibition of proteasome activity by alcohol. Biochem. Biophys. Res. Commun. 2000, 279, 23–29. [Google Scholar] [CrossRef] [PubMed]
- He, L.; He, T.; Farrar, S.; Ji, L.; Liu, T.; Ma, X. Antioxidants Maintain Cellular Redox Homeostasis by Elimination of Reactive Oxygen Species. Cell. Physiol. Biochem. 2017, 44, 532–553. [Google Scholar] [CrossRef]
- Cao, Y.W.; Jiang, Y.; Zhang, D.Y.; Wang, M.; Chen, W.S.; Su, H.; Wang, Y.T.; Wan, J.B. Protective effects of Penthorum chinense Pursh against chronic ethanol-induced liver injury in mice. J. Ethnopharmacol. 2015, 161, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zheng, Q.; Chen, Z. The Nrf2 Pathway in Liver Diseases. Front. Cell Dev. Biol. 2022, 10, 826204. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Han, Q.; Yang, Z.; Ni, Y.; Agbana, Y.L.; Bai, H.; Yi, Z.; Yi, X.; Kuang, Y.; Zhu, Y. G6PD facilitates clear cell renal cell carcinoma invasion by enhancing MMP2 expression through ROSMAPK axis pathway. Int. J. Oncol. 2020, 57, 197–212. [Google Scholar]
- Kim, E.K.; Choi, E.J. Compromised MAPK signaling in human diseases: An update. Arch. Toxicol. 2015, 89, 867–882. [Google Scholar] [CrossRef]

| GENE | Forward Primer | Reverse Primer |
|---|---|---|
| h-GAPDH | GGTGGTCTCCTCTGACTTCAACA | GTTGCTGTAGCCAAATTCGTTGT |
| h-TNF-α | GTGACAAGCCTGTAGCCCAT | CAGACTCGGCAAAGTCGAGA |
| h-IL-1β | GACCTTCCAGGATGAGGACA | AGCTCATATGGGTCCGACAG |
| h-BCL2 | TGTGTGTGGAGAGCGTCAAC | GAAATCAAACAGAGGCCGCAT |
| h-BAX | GAAGCTGAGCGAGTGTCTC | CAGACACGTAAGGAAAACGCA |
| h-SOD1 | CCAGTGCAGGACCTCATTTT | TCATGGACCACCATTGTACG |
| h-SOD2 | TCAATGGTGGGGGACATATT | GAACCTTGGACTCCCACAGA |
| m-Gapdh | GGAGAGTGTTTCCTCGTCCC | ACTGTGCCGTTGAATTTGCC |
| m-b-actin | CCCCTGAACCCTAAGGCCA | CGGACTCATCGTACTCCTGC |
| m-Nlrp3 | CTACGGCCGTCTACGTCTTC | GGCCAAAGAGGAATCGGACA |
| m-Caspase1 | ACTGCTATGGACAAGGCACG | GCAAGACGTGTACGAGTGGT |
| m-Asc | TGAGCAGCTGCAAACGACTA | CACGAACTGCCTGGTACTGT |
| m-Ho-1 | GAAATCATCCCTTGCACGCC | TGTTTGAACTTGGTGGGGCT |
| m-Gclc | CATCTACCACGCAGTCAAGGA | GTTGGGGTTTGTCCTCTCCC |
| m-Gpx-1 | AGTCCACCGTGTATGCCTTC | TTGCCATTCTGGTGTCCGAA |
| m-Bcl2 | AGTACCTGAACCGGCATCTG | GGTATGCACCCAGAGTGATG |
| m-Bax | CACCTGAGCTGACCTTGGAG | CAGCCACCCTGGTCTTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, F.; Zhou, J.; Gong, K.; Chen, S.; Zhu, X.; Zhang, M.; Duan, Y.; Liao, C.; Han, J.; et al. Glabridin Ameliorates Alcohol-Caused Liver Damage by Reducing Oxidative Stress and Inflammation via p38 MAPK/Nrf2/NF-κB Pathway. Nutrients 2023, 15, 2157. https://doi.org/10.3390/nu15092157
Wang M, Zhang F, Zhou J, Gong K, Chen S, Zhu X, Zhang M, Duan Y, Liao C, Han J, et al. Glabridin Ameliorates Alcohol-Caused Liver Damage by Reducing Oxidative Stress and Inflammation via p38 MAPK/Nrf2/NF-κB Pathway. Nutrients. 2023; 15(9):2157. https://doi.org/10.3390/nu15092157
Chicago/Turabian StyleWang, Mengyao, Feng Zhang, Jie Zhou, Ke Gong, Shasha Chen, Xinran Zhu, Mengxue Zhang, Yajun Duan, Chenzhong Liao, Jihong Han, and et al. 2023. "Glabridin Ameliorates Alcohol-Caused Liver Damage by Reducing Oxidative Stress and Inflammation via p38 MAPK/Nrf2/NF-κB Pathway" Nutrients 15, no. 9: 2157. https://doi.org/10.3390/nu15092157