Browning of Adipocytes: A Potential Therapeutic Approach to Obesity
Abstract
:1. Introduction
2. Adipose Tissue: Typologies, Role, Physiology
3. Distribution of Adipose Tissue
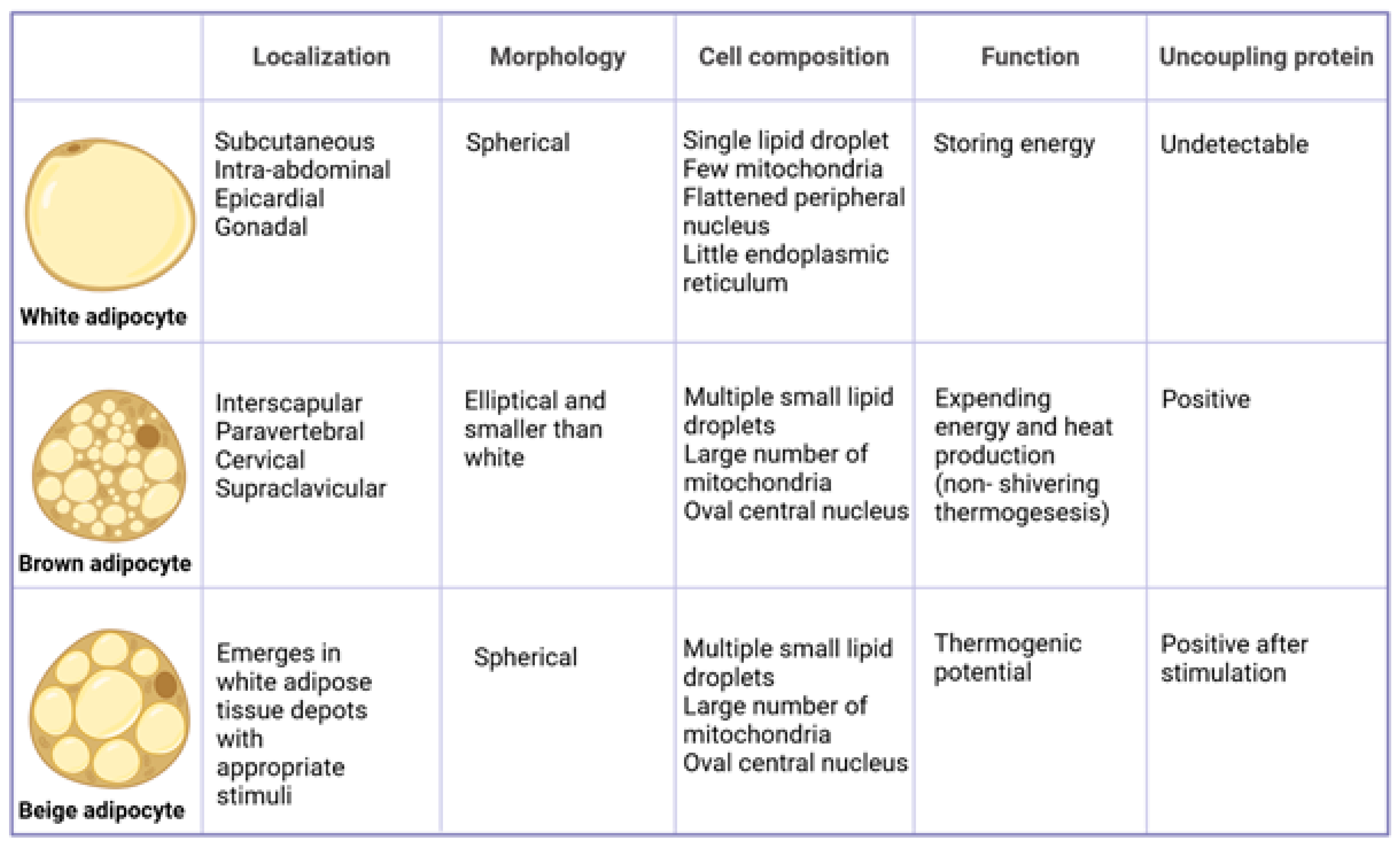
4. Differences between White and Brown Adipocytes
5. Conversion of White Adipocytes into Brown-like Adipocytes
6. Thermogenesis and Thermogenin
7. BAT-Secreted Factors with Potential Direct and/or Indirect Cardioprotective Effects
8. Browning Strategies: Cold, Physical Activity, and Adrenergic Agonists
| Intervention | Mechanism of Action | Bat Activation | Experimental Model | Ref. |
|---|---|---|---|---|
| COLD EXPOSURE | yes | |||
| UCP1 activation | human | [28,29,31] | ||
| ↑ production of noradrenergic signals | human | [30] | ||
| ↑ PGC1α | mouse human | [31] | ||
| ↑ UCP1 in BAT | human | [19] | ||
| ↑ BAT and ↑ its functionality | human | [32,33] | ||
| ADRENERGIC AGONISTS | yes | |||
| stimulate β-adrenergic receptors | human | [34] | ||
| ↑ levels of catecholamines and amplify thermogenesis | mouse | [35] | ||
| PHYSICAL ACTIVITY | yes | |||
| UCP1 activation | human | [37,38] | ||
| ↑ PGC1α | human | [37,38] | ||
| releases catecholamines | human | [6,28] | ||
| activates lipogenesis in WAT | human | [6,28] | ||
| MYOKINES | ||||
| Irisin | governs white adipocyte browning | human mouse | [29,30] | |
| β-aminoisobutyric acid (BAIBA) | governs white adipocyte browning | human mouse | [29,30] | |
| Fibroblast growth factor 21 (FGF21) | governs white adipocyte browning | human mouse | [29,30] |
9. Thermogenic Nutraceuticals
9.1. Polyunsatured Fatty Acids
9.2. Capsaicin and Capsinoids
9.3. Green Tea Catechins
9.4. Curcumin
9.5. Resveratrol
9.6. Berberine
9.7. Other Nutraceutical Compounds
9.7.1. Oleuropein
9.7.2. Anthocyanins
9.7.3. Quercetin
9.7.4. Analogues of Capsaicin
| Thermogenic Nutraceuticals | Dose | Mechanism of Action | Experimental Model | Ref. |
|---|---|---|---|---|
| EPA DHA | simulates thermogenesis in BAT | mouse human | [41,42,43,44] | |
| ↑ expression of UCP1 in BAT, | mouse | [47,48] | ||
| ↓ adipose accumulation via the induction of marked, non-shivering thermogenesis, | mouse | [36,37] | ||
| promotes the adipogenesis of mature brown adipocytes | mouse | [50] | ||
| promotes the differentiation of pre-adipocytes into beige adipocytes, particularly in the inguinal WAT | mouse | [48] | ||
| Capsaicin | activates TRPV1 channels: implements BAT function | in vitro and pre-clinical studies | [53,54] | |
| regulates the epigenetic expression of the transcription factors involved in WAT browning | in vitro and pre-clinical studies | [53,54] | ||
| Capsinoids | 9 mg/day in capsule form for 6 weeks | promotes BAT activity and reduces fat mass | human | [55] |
| 12 mg combined with exposure to cold (14.5 °C) | ↑ energy expenditure and, when combined with cold, ↑ fat oxidation, ↑ insulin sensitivity and ↑ HDL-cholesterol | human | [56,57,58] | |
| Catechins | for 8 weeks | ↓ mass of perirenal WAT, ↑ expression of mRNA coding for UCP1 in BAT | rat | [62] |
| 100 mg/kg body weight for 4 weeks | ↓ total fat mass (subcutaneous and visceral) and liver size, fatty acid oxidation in the BAT increased twofold | rat | [63] | |
| >300 mg catechins/day | ↓ body weight and prevents weight regain | human | [64] | |
| inhibits catechol-O-methyltransferases | human | [69] | ||
| Curcumin | 20 μM for 6–8 days | ↑ in thermogenic markers of BAT and in hormone-sensitive lipase (HSL), | isolated WAT cells of obese rats | [71] |
| 45 mg/kg of body weight | ↑ energy expenditure via the induction of mitochondrial biogenesis | mouse | [73] | |
| 500 mg (in bioavailable form) daily for 10 weeks | ↓ in body mass index, waist circumference and hip circumference, and triglyceride/HDL ratio, and ↑ HDL cholesterol | human | [73,74] | |
| Resveratrol | 30 mg/kg of body weight for 8 weeks | ↓ fat mass, plasma glucose concentrations and total cholesterol | mouse | [76] |
| ↑ UCP1 expression | mouse | [77] | ||
| activates upstream AMPK, which promotes the production of PGC1α, and SIRT1, which promotes mitochondrial biogenesis and WAT browning | mouse | [78] | ||
| up-regulates the expression of genes coding for proteins involved in WAT adipogenesis (FAS, SREBP1, LPL and HSL) | human | [81] | ||
| Berberine | 5 mg/kg/day for 4 weeks | ↑ energy expenditure and the mobilization of lipids | mouse | [84] |
| stimulates BAT activity, and induces browning of the inguinal WAT | mouse | [85] | ||
| Oleuropein | 3 mg injected intravenously for 7 weeks | ↑ UCP1 content in BAT by activating SIRT1, PPARγ and PGC1α, stimulates the secretion of adrenalin and noradrenalin via the activation of TRP channels | mouse | [88] |
| Oleuropein aglycone (the absorbed form of oleuropein) | attenuates diet-induced obesity by supporting the expression of thermogenic genes and genes related to mitochondrial biogenesis in the BAT of overfed mice | mouse | [89] | |
| promotes the browning of adipose tissue from mesenchymal stem cells in humans | in vitro | [90] | ||
| Anthocyanins | 150 mg/day of an extract of Aronia melanocarpa | ↑ surface body temperature and plasma adrenalin levels, suggesting that it has stimulating effect on the SNS | human | [91] |
| long-term treatment with cyanidin-3-glucoside, such as raspberry and mulberry extract | ↑ UCP1 expression and mitochondrial biogenesis during adipogenic differentiation of brown and white preadipocytes | rat | [92,93] | |
| black soybean peel extract | ↑ the expression of thermogenic genes in BAT, induces WAT browning and increases the lipid respiration quotient, preventing visceral fat accumulation on a hyperlipidic diet | mouse | [94] | |
| Quercetin | HFD supplemented with 1% quercetin for 16 weeks | reduces plasma cholesterol levels | mouse | [95] |
| ↑ the expression of thermogenic genes in BAT (UCP1, PGC1α, FGF21) and genes coding for β-adrenergic receptors and AMPK | in vitro | [96] | ||
| Menthol | activates TRPM8 receptors | in vitro | [98] | |
| Cinnamaldehyde | ↑ UCP1 expression | in vitro | [97] | |
| Gingerol, Shogaol, 6-Paradol | activates TRPV1 channels | in vitro mouse | [102,103,104] | |
| 100 mg/day of Kaempferia parviflora | ↑ energy expenditure in BAT-positive subjects; activates brown adipocytes | human | [107] |
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADRB3 | β-3 adrenoceptor |
| AMP | Adenosine monophosphate |
| AMPK | adenosine monophosphate-activated protein kinase |
| ATP | adenosine triphosphate |
| BAIBA | β-aminoisobutyric acid |
| BAT | brown adipose tissue |
| cAMP | cyclic adenosine monophosphate |
| COMT | catechol-O-methyltransferase |
| CREB | cAMP response element binding protein |
| CVD | cardiovascular disease |
| DHA | docosahexaenoic acid |
| DIT | diet-induced thermogenesis |
| EGCG | epigallocatechin gallate |
| EPA | eicosapentaenoic acid |
| ERK | extracellular signal-regulated kinase |
| FAS | fatty acid synthase |
| FGF21 | fibroblast growth factor 21 |
| FT3 | free triiodothyronine |
| FT4 | free thyroxine |
| GLUT | glucose transporter |
| HFD | high fat diet |
| HSL | lipase sensitive hormone |
| IL-6 | interleukin 6 |
| LPL | lipoprotein lipase |
| MSCs | mesenchymal stem cells |
| Myf5 | myogenic factor 5 |
| NPY | neuropeptide Y |
| NST | non-shivering thermogenesis |
| OA | oleuropein |
| PET | positron emission tomography |
| PGC1α | peroxisome proliferator–activated receptor gamma coactivator 1-alpha |
| PKA | protein kinase A |
| PPAR | peroxisome proliferator-activated receptor |
| PRDM16 | proline rich domain-containing protein 16 |
| SIRT1 | Sirtuin 1 |
| SREBP1 | sterol response element-binding protein |
| SNS | sympathetic nervous system |
| TIF | cold-induced thermogenesis |
| TLR | toll-like receptor |
| TNFα | tumour necrosis factor alpha |
| TRPA1 | transient receptor potential cation channel subfamily A member 1 |
| TRPM8 | transient receptor potential cation channel subfamily M member 8 |
| TRPV1 | transient receptor potential vanilloid 1 |
| UCP1 | uncoupling protein 1 |
| VEGF | vascular endothelial growth factor |
| WAT | white adipose tissue |
References
- Golden, A. Obesity’s Impact. Nurs. Clin. N. Am. 2021, 4, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Blüher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 5, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Safaei, M.; Sundararajan, E.A.; Driss, M.; Boulila, W.; Shapi’I, A. A systematic literature review on obesity: Understanding the causes & consequences of obesity and reviewing various machine learning approaches used to predict obesity. Comput. Biol. Med. 2021, 136, 104754. [Google Scholar]
- Gavaldà-Navarro, A.; Villarroya, J.; Cereijo, R.; Giralt, M.; Villarroya, F. The endocrine role of brown adipose tissue: An update on actors and actions. Rev. Endocr. Metab. Disord. 2022, 1, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Kuryłowicz, A.; Puzianowska-Kuźnicka, M. Induction of Adipose Tissue Browning as a Strategy to Combat Obesity. Int. J. Mol. Sci. 2020, 21, 6241. [Google Scholar] [CrossRef] [PubMed]
- Armani, A.; Feraco, A.; Camajani, E.; Gorini, S.; Lombardo, M.; Caprio, M. Nutraceuticals in Brown Adipose Tissue Activation. Cells 2022, 11, 3996. [Google Scholar] [CrossRef]
- Martins, F.F.; Souza-Mello, V.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. Brown adipose tissue as an endocrine organ: Updates on the emerging role of batokines. Horm. Mol. Biol. Clin. Investig. 2022, 10, 22–44. [Google Scholar] [CrossRef]
- Negrea, M.O.; Neamtu, B.; Dobrotă, I.; Sofariu, C.R.; Crisan, R.M.; Ciprian, B.I.; Domnariu, C.D.; Teodoru, M. Causative Mechanisms of Childhood and Adolescent Obesity Leading to Adult Cardiometabolic Disease: A Literature Review. Appl. Sci. 2021, 11, 11565. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Zhang, F.; Hao, G.; Shao, M.; Nham, K.; An, Y.; Wang, Q.; Zhu, Y.; Kusminski, C.M.; Hassan, G.; Gupta, R.K.; et al. An Adipose Tissue Atlas: An Image-Guided Identification of Human-like BAT and Beige Depots in Rodents. Cell Metab. 2018, 27, 252–262.e3. [Google Scholar] [CrossRef]
- Srivastava, S.; Veech, R.L. Brown and Brite: The Fat Soldiers in the Anti-obesity Fight. Front. Physiol. 2019, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Gesta, S.; Tseng, Y.-H.; Kahn, C.R. Developmental Origin of Fat: Tracking Obesity to Its Source. Cell 2007, 131, 242–256. [Google Scholar] [CrossRef] [PubMed]
- Long, J.Z.; Svensson, K.J.; Tsai, L.; Zeng, X.; Roh, H.C.; Kong, X.; Rao, R.R.; Lou, J.; Lokurkar, I.; Baur, W.; et al. A Smooth Muscle-Like Origin for Beige Adipocytes. Cell Metab. 2014, 19, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Rosenwald, M.; Wolfrum, C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte 2013, 3, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Bartness, T.J.; Vaughan, C.H.; Song, C.K. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. 2010, 34, S36–S42. [Google Scholar] [CrossRef]
- Cinti, S. La Transdifferenziazione Dell’organo Adiposo; Società Italiana dell’Obesità (SIO), Istituto di Morfologia Umana Normale, Università Politecnica delle Marche: Ancona, Italy, 2008. [Google Scholar]
- Widmaier, E.; Raff, H.; Strang, T.; Vander, J. Vander’s Human Physiology: The Mechanisms of Body Function; New York McGraw-Hill Education: New York, NY, USA, 2016. [Google Scholar]
- Perez, L.; Perez, L.; Nene, Y.; Umpierrez, G. Interventions associated with brown adipose tissue activation and the impact on energy expenditure and weight loss. Front. Endocrinol. 2022, 13, 1037458. [Google Scholar] [CrossRef]
- Van Der Lans, A.A.J.J.; Hoeks, J.; Brans, B.; Vijgen, G.H.E.J.; Visser, M.G.W.; Vosselman, M.J.; Hansen, J.; Jörgensen, J.A.; Wu, J.; Mottaghy, F.M.; et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Investig. 2013, 123, 3395–3403. [Google Scholar] [CrossRef]
- Cannon, B.; Nedergaard, J. Metabolic consequences of the presence or absence of the thermogenic capacity of brown adipose tissue in mice (and probably in humans). Int. J. Obes. 2010, 34, S7–S16. [Google Scholar] [CrossRef]
- Wankhade, U.D.; Shen, M.; Yadav, H.; Thakali, K.M. Novel Browning Agents, Mechanisms, and Therapeutic Potentials of Brown Adipose Tissue. BioMed Res. Int. 2016, 2016, 65–69. [Google Scholar] [CrossRef]
- Morrison, S.F.; Madden, C.J.; Tupone, D. Central Control of Brown Adipose Tissue Thermogenesis. Front. Endocrinol. 2012, 3, 33–39. [Google Scholar] [CrossRef]
- Pereira, R.O.; McFarlane, S.I. The Role of Brown Adipose Tissue in Cardiovascular Disease Protection: Current Evidence and Future Directions. Int. J. Clin. Res. Trials 2019, 4, 136. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.-H.; Cypess, A.M.; Kahn, C.R. Cellular bioenergetics as a target for obesity therapy. Nat. Rev. Drug Discov. 2010, 9, 465–482. [Google Scholar] [CrossRef] [PubMed]
- Preusch, M.R.; Baeuerle, M.; Albrecht, C.; Blessing, E.; Bischof, M.; A Katus, H.; Bea, F. GDF-15 protects from macrophage accumulation in a mousemodel of advanced atherosclerosis. Eur. J. Med. Res. 2013, 18, 19. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.; Middelbeek, R.; Townsend, R.; An, D.; Nygaard, E. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Aldiss, P.; Davies, G.; Woods, R.; Budge, H.; Sacks, H.S.; Symonds, M.E. ‘Browning’ the cardiac and peri-vascular adipose tissues to modulate cardiovascular risk. Int. J. Cardiol. 2016, 228, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Goh, H.J.; Verma, S.; Govindharajulu, P.; Sadananthan, S.A.; Michael, N.; Jadegoud, Y.; Henry, C.J.; Velan, S.S.; Yeo, P.S.; et al. Metabolic effects of brown fat in transitioning from hyperthyroidism to euthyroidism. Eur. J. Endocrinol. 2021, 185, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Loeliger, R.C.; Maushart, C.I.; Gashi, G.; Senn, J.R.; Felder, M.; Becker, A.S.; Müller, J.; Balaz, M.; Wolfrum, C.; Burger, I.A.; et al. Relation of diet-induced thermogenesis to brown adipose tissue activity in healthy men. Am. J. Physiol. Metab. 2021, 320, E93–E101. [Google Scholar] [CrossRef]
- Van Marken Lichtenbelt, W.D.; Vanhommerig, J.W.; Smulders, N.M.; Drossaerts, J.M.A.F.L.; Kemerink, G.J.; Bouvy, N.D.; Schrauwen, P.; Teule, G.J.J. Cold-Activated Brown Adipose Tissue in Healthy Men. N. Engl. J. Med. 2009, 360, 1500–1508. [Google Scholar] [CrossRef]
- Labbé, S.M.; Mouchiroud, M.; Caron, A.; Secco, B.; Freinkman, E.; Lamoureux, G.; Gélinas, Y.; Lecomte, R.; Bossé, Y.; Chimin, P.; et al. mTORC1 is Required for Brown Adipose Tissue Recruitment and Metabolic Adaptation to Cold. Sci. Rep. 2016, 6, 37223. [Google Scholar] [CrossRef]
- Singh, R.; Barrios, A.; Dirakvand, G.; Pervin, S. Human Brown Adipose Tissue and Metabolic Health: Potential for Therapeutic Avenues. Cells 2021, 10, 3030. [Google Scholar] [CrossRef]
- Orava, J.; Nuutila, P.; Noponen, T.; Parkkola, R.; Viljanen, T.; Enerbäck, S.; Rissanen, A.; Pietiläinen, K.; Virtanen, K.A. Blunted metabolic responses to cold and insulin stimulation in brown adipose tissue of obese humans. Obesity 2013, 21, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Vosselman, M.J.; van der Lans, A.A.; Brans, B.; Wierts, R.; van Baak, M.A.; Schrauwen, P.; Lichtenbelt, W.D.V.M. Systemic β-Adrenergic Stimulation of Thermogenesis Is Not Accompanied by Brown Adipose Tissue Activity in Humans. Diabetes 2012, 61, 3106–3113. [Google Scholar] [CrossRef] [PubMed]
- Chitraju, C.; Fischer, A.; Farese, R.V.; Walther, T.C. Lipid Droplets in Brown Adipose Tissue Are Dispensable for Cold-Induced Thermogenesis. Cell Rep. 2020, 5, 108–134. [Google Scholar] [CrossRef] [PubMed]
- Scheele, C.; Wolfrum, C. Brown Adipose Crosstalk in Tissue Plasticity and Human Metabolism. Endocr. Rev. 2019, 41, 53–65. [Google Scholar] [CrossRef]
- Sanchez-Delgado, G.; Martinez-Tellez, B.; Olza, J.; Aguilera, C.M.; Gil, Á.; Ruiz, J.R. Role of Exercise in the Activation of Brown Adipose Tissue. Ann. Nutr. Metab. 2015, 67, 21–32. [Google Scholar] [CrossRef]
- Ruiz, J.; Tellez, B.; Delgado, G.; Aguilera, C. Regulation of energy balance by brown adipose tissue: At least three potential roles for physical activity. Br. J. Sport Med. 2015, 49, 37–45. [Google Scholar] [CrossRef]
- Roberts, L.D.; Boström, P.; O’sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.-K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. β-Aminoisobutyric Acid Induces Browning of White Fat and Hepatic β-Oxidation and Is Inversely Correlated with Cardiometabolic Risk Factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Minj, K.; Tsuyoshi, G.; Rina, Y. Fish oil intake induces UCP1 upregulation in brown and white adipose tissue via the sympathetic nervous system. Sci. Rep. 2016, 5, 186–197. [Google Scholar]
- Matta, J.A.; Miyares, R.L.; Ahern, G.P.; Brothwell, S.L.C.; Barber, J.L.; Monaghan, D.T.; Jane, D.E.; Gibb, A.J.; Jones, S. TRPV1 is a novel target for omega-3 polyunsaturated fatty acids. J. Physiol. 2007, 578, 397–411. [Google Scholar] [CrossRef]
- Ming, Z.; XIaoli, C. Eicosapentaenoic acid promotes thermogenic and fatty acid storage capacity in mouse subcutaneous adipocytes. Biochem. Biophys. Res. Commun. 2014, 4, 1446–1461. [Google Scholar]
- Tsuboyama, N.; Mayumi, K.; Hyounjulu, T. Up-Regulation of Liver Uncoupling Protein-2 mRNA by either Fish Oil Feeding or Fibrate Administration in Mice. Biochem. Biophys. Res. Commun. 2002, 3, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Ide, T. Dietary n-3 fatty acids affect mRNA level of brown adipose tissue uncoupling protein 1, and white adipose tissue leptin and glucose transporter 4 in the rat. Br. J. Nutr. 2000, 2, 175–184. [Google Scholar] [CrossRef]
- Oudart, H.; Groscolas, R.; Calgari, C.; Nibbelink, M.; Leray, C.; Le Maho, Y.; Malan, A. Brown fat thermogenesis in rats fed high-fat diets enriched with n-3 polyunsaturated fatty acids. Int. J. Obes. 1997, 21, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Sadurskis, A.; Dicker, A.; Cannon, B.; Nedergaard, J. Polyunsaturated fatty acids recruit brown adipose tissue: Increased UCP content and NST capacity. Am. J. Physiol. Metab. 1995, 269, E351–E360. [Google Scholar] [CrossRef] [PubMed]
- Bargut, T.C.L.; Silva-E-Silva, A.C.A.G.; Souza-Mello, V.; Mandarim-De-Lacerda, C.A.; Aguila, M.B. Mice fed fish oil diet and upregulation of brown adipose tissue thermogenic markers. Eur. J. Nutr. 2016, 55, 159–169. [Google Scholar] [CrossRef]
- Eckel, M.; Fleckenstein-Elsen, D.; Dennis, T. Eicosapentaenoic acid and arachidonic acid differentially regulate adipogenesis, acquisition of a brite phenotype and mitochondrial function in primary human adipocytes. Mol. Nutr. Food Res. 2016, 9, 2065–2075. [Google Scholar]
- Kim, J.; Okla, M.; Erickson, A.; Carr, T.; Natarajan, S.K.; Chung, S. Eicosapentaenoic Acid Potentiates Brown Thermogenesis through FFAR4-dependent Up-regulation of miR-30b and miR-378. J. Biol. Chem. 2016, 291, 20551–20562. [Google Scholar] [CrossRef]
- Laiglesia, L.; Lorente-Cebrián, S.; Prieto-Hontoria, P.; Fernández-Galilea, M.; Ribeiro, S.; Sáinz, N.; Martínez, J.; Moreno-Aliaga, M. Eicosapentaenoic acid promotes mitochondrial biogenesis and beige-like features in subcutaneous adipocytes from overweight subjects. J. Nutr. Biochem. 2016, 37, 76–82. [Google Scholar] [CrossRef]
- Ludy, M.-J.; Moore, G.E.; Mattes, R.D. The Effects of Capsaicin and Capsiate on Energy Balance: Critical Review and Meta-analyses of Studies in Humans. Chem. Senses 2011, 37, 103–121. [Google Scholar] [CrossRef]
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in Metabolic Syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, P.; Krishnan, V.; Ren, J.; Thyagarajan, B. Capsaicin induces browning of white adipose tissue and counters obesity by activating TRPV1 channel-dependent mechanisms. Br. J. Pharmacol. 2016, 173, 2369–2389. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Aita, S.; Matsushita, M.; Kayahara, T.; Kameya, T.; Kawai, Y.; Iwanaga, T.; Saito, M. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Investig. 2013, 123, 3404–3408. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Camps, S.G.; Goh, H.J.; Govindharajulu, P.; Schaefferkoetter, J.D.; Townsend, D.W.; Verma, S.K.; Velan, S.S.; Sun, L.; Sze, S.K.; et al. Capsinoids activate brown adipose tissue (BAT) with increased energy expenditure associated with subthreshold 18-fluorine fluorodeoxyglucose uptake in BAT-positive humans confirmed by positron emission tomography scan. Am. J. Clin. Nutr. 2018, 107, 62–70. [Google Scholar] [CrossRef]
- Ohyama, K.; Nogusa, Y.; Shinoda, K.; Suzuki, K.; Bannai, M.; Kajimura, S. A synergistic anti-obesity effect by a combination of capsinoids and cold temperature through promoting beige adipocyte biogenesis. Diabetes 2016, 1, 1410–1423. [Google Scholar] [CrossRef]
- Maliszewska, K.; Kretowski, A. Brown Adipose Tissue and Its Role in Insulin and Glucose Homeostasis. Int. J. Mol. Sci. 2021, 22, 1530. [Google Scholar] [CrossRef]
- Schaik, L.; Kettle, C.; Green, R. Both caffeine and Capsicum annuum fruit powder lower blood glucose levels and increase brown adipose tissue temperature in healthy adult males. Front. Physiol. 2022, 13, 20–27. [Google Scholar]
- Gosselin, C.; Haman, F. Effects of green tea extracts on non-shivering thermogenesis during mild cold exposure in young men. Br. J. Nutr. 2012, 110, 282–288. [Google Scholar] [CrossRef]
- Kurogi, M.; Kawai, Y.; Nagatomo, K.; Tateyama, M.; Kubo, Y.; Saitoh, O. Auto-oxidation Products of Epigallocatechin Gallate Activate TRPA1 and TRPV1 in Sensory Neurons. Chem. Senses 2014, 40, 27–46. [Google Scholar] [CrossRef]
- Nomura, S.; Ichinose, T.; Jinde, M.; Kawashima, Y.; Tachiyashiki, K.; Imaizumi, K. Tea catechins enhance the mRNA expression of uncoupling protein 1 in rat brown adipose tissue. J. Nutr. Biochem. 2008, 19, 840–847. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Y.; Zhao, B. Green tea catechins prevent obesity through modulation of peroxisome proliferator-activated receptors. Sci. China Life Sci. 2013, 56, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Hursel, R.; Viechtbauer, W.; Dulloo, A.; Tremblay, A.; Tappy, L.; Rumpler, W.; Westerterp-Plantenga, M.S. The effects of catechin rich teas and caffeine on energy expenditure and fat oxidation: A meta-analysis. Obes. Rev. 2011, 12, e573–e581. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Sanchez, K.; Leyva, M.J.; Wu, M.; Betts, N.M.; E Aston, C.; Lyons, T.J. Green Tea Supplementation Affects Body Weight, Lipids, and Lipid Peroxidation in Obese Subjects with Metabolic Syndrome. J. Am. Coll. Nutr. 2010, 29, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Van Schaik, L.; Kettle, C.; Green, R.; Irving, H.R.; Rathner, J.A. Effects of Caffeine on Brown Adipose Tissue Thermogenesis and Metabolic Homeostasis: A Review. Front. Neurosci. 2021, 15, 56–62. [Google Scholar] [CrossRef]
- Thielecke, F.; Rahn, G.; Böhnke, J.; Adams, F.; Birkenfeld, A.L.; Jordan, J.; Boschmann, M. Epigallocatechin-3-gallate and postprandial fat oxidation in overweight/obese male volunteers: A pilot study. Eur. J. Clin. Nutr. 2010, 64, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Matsushita, M.; Hibi, M.; Tone, H.; Takeshita, M.; Yasunaga, K.; Katsuragi, Y.; Kameya, T.; Sugie, H.; Saito, M. Tea catechin and caffeine activate brown adipose tissue and increase cold-induced thermogenic capacity in humans. Am. J. Clin. Nutr. 2017, 105, 873–881. [Google Scholar] [CrossRef]
- Akhtar, J.; Yar, M.S.; Grover, G.; Nath, R. Neurological and psychiatric management using COMT inhibitors: A review. Bioorganic Chem. 2019, 94, 103418. [Google Scholar] [CrossRef]
- Di Pierro, F.; Bressan, A.; Ranaldi, D.; Rapacioli, G.; Giacomelli, L.; Bertuccioli, A. Potential role of bioavailable curcumin in weight loss and omental adipose tissue decrease: Preliminary data of a randomized, controlled trial in overweight people with metabolic syndrome. Preliminary study. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 4195–4202. [Google Scholar]
- Kim, S.W.; Choi, J.H.; Mukherjee, R.; Hwang, K.-C.; Yun, J.W. Proteomic identification of fat-browning markers in cultured white adipocytes treated with curcumin. Mol. Cell. Biochem. 2016, 415, 51–66. [Google Scholar] [CrossRef]
- Nishikawa, S.; Kamiya, M.; Aoyama, H.; Nomura, M. Highly Dispersible and Bioavailable Curcumin but not Native Curcumin Induces Brown-Like Adipocyte Formation in Mice. Mol. Nutr. Food. Res. 2018, 5, 700–731. [Google Scholar]
- Nishikawa, S.; Kamiya, M.; Aoyama, H.; Yoshimura, K.; Miyata, R.; Kumazawa, S.; Tsuda, T. Co-Administration of Curcumin and Artepillin C Induces Development of Brown-Like Adipocytes in Association with Local Norepinephrine Production by Alternatively Activated Macrophages in Mice. J. Nutr. Sci. Vitaminol. 2019, 65, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of curcumin on cardiovascular risk factors in obese and overweight adolescent girls: A randomized clinical trial. Sao Paulo Med. J. 2019, 137, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Glinjak, S.; Aebisher, D.; Bartusik, B. Health benefits of resveratrol administration. Acta Biochim. Pol. 2019, 1, 13–21. [Google Scholar]
- Andrade, J.M.O.; Frade, A.C.M.; Guimarães, J.B.; Freitas, K.M.; Lopes, M.T.P.; Guimaraes, A.; De Paula, A.M.B.; Coimbra, C.C.; Santos, S.H.S. Resveratrol increases brown adipose tissue thermogenesis markers by increasing SIRT1 and energy expenditure and decreasing fat accumulation in adipose tissue of mice fed a standard diet. Eur. J. Nutr. 2014, 53, 1503–1510. [Google Scholar] [CrossRef] [PubMed]
- Alberdi, G.; Rodríguez, V.M.; Miranda, J.; Macarulla, M.T.; Churruca, I.; Portillo, M.P. Thermogenesis is involved in the body-fat lowering effects of resveratrol in rats. Food Chem. 2013, 141, 1530–1535. [Google Scholar] [CrossRef]
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int. J. Obes. 2015, 39, 967–976. [Google Scholar] [CrossRef]
- Um, J.-H.; Park, S.-J.; Kang, H.; Yang, S.; Foretz, M.; McBurney, M.W.; Kim, M.K.; Viollet, B.; Chung, J.H. AMP-Activated Protein Kinase–Deficient Mice Are Resistant to the Metabolic Effects of Resveratrol. Diabetes 2010, 59, 554–563. [Google Scholar] [CrossRef]
- Milton-Laskíbar, I.; Gómez-Zorita, S.; Arias, N.; Romo-Miguel, N.; González, M.; Fernández-Quintela, A.; Portillo, M.P. Effects of resveratrol and its derivative pterostilbene on brown adipose tissue thermogenic activation and on white adipose tissue browning process. J. Physiol. Biochem. 2020, 76, 269–278. [Google Scholar] [CrossRef]
- Scarano, F.; Gliozzi, M.; Zito, M.C.; Guarnieri, L.; Carresi, C.; Macrì, R.; Nucera, S.; Scicchitano, M.; Bosco, F.; Ruga, S.; et al. Potential of Nutraceutical Supplementation in the Modulation of White and Brown Fat Tissues in Obesity-Associated Disorders: Role of Inflammatory Signalling. Int. J. Mol. Sci. 2021, 22, 3351. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Pinto-Garcia, L.; Efferth, T.; Torres, A.; Hoheisel, J.D.; Youns, M. Berberine Inhibits Cell Growth and Mediates Caspase-Independent Cell Death in Human Pancreatic Cancer Cells. Planta Medica 2010, 76, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Li, B.; Meng, X.; Wang, J.; Zhang, Y.; Yao, S.; Ma, Q.; Jin, L.; Yang, J.; et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat. Commun. 2014, 5, 5493. [Google Scholar] [CrossRef] [PubMed]
- van Dam, A.D.; Kooijman, S.; Schilperoort, M.; Rensen, P.C.; Boon, M.R. Regulation of brown fat by AMP-activated protein kinase. Trends Mol. Med. 2015, 21, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xia, M.; Duan, Y.; Zhang, L.; Jiang, H.; Hu, X.; Yan, H.; Zhang, Y.; Gu, Y.; Shi, H.; et al. Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 2019, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yang, M.; Han, Y.; Zhao, H.; Sun, L. PRDM16 Regulating Adipocyte Transformation and Thermogenesis: A Promising Therapeutic Target for Obesity and Diabetes. Front. Pharmacol. 2022, 13, 25–47. [Google Scholar] [CrossRef]
- Oi-Kano, Y.; Iwasaki, Y.; Nakamura, T.; Watanabe, T.; Goto, T.; Kawada, T.; Watanabe, K.; Iwai, K. Oleuropein aglycone enhances UCP1 expression in brown adipose tissue in high-fat-diet-induced obese rats by activating β-adrenergic signaling. J. Nutr. Biochem. 2017, 40, 209–218. [Google Scholar] [CrossRef]
- Oi-Kano, Y.; Kawada, T.; Watanabe, T.; Koyama, F. Oleuropein, a phenolic compound in extra virgin olive oil, increases uncoupling protein 1 content in brown adipose tissue and enhances noradrenaline and adrenaline secretions in rats. J Nutr Sci Vitaminol 2018, 5, 363–370. [Google Scholar] [CrossRef]
- Palmeri, R.; Monteleone, J.I.; Spagna, G.; Restuccia, C.; Raffaele, M.; Vanella, L.; Volti, G.L.; Barbagallo, I. Olive Leaf Extract from Sicilian Cultivar Reduced Lipid Accumulation by Inducing Thermogenic Pathway during Adipogenesis. Front. Pharmacol. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Pei, L.; Wan, T.; Wang, S.; Ye, M.; Qiu, Y.; Jiang, R.; Pang, N.; Huang, Y.; Zhou, Y.; Jiang, X.; et al. Cyanidin-3-O-β-glucoside regulates the activation and the secretion of adipokines from brown adipose tissue and alleviates diet induced fatty liver. Biomed. Pharmacother. 2018, 105, 625–632. [Google Scholar] [CrossRef]
- Chen, K.; Kortesniemi, M.K.; Linderborg, K.M.; Yang, B. Anthocyanins as Promising Molecules Affecting Energy Homeostasis, Inflammation, and Gut Microbiota in Type 2 Diabetes with Special Reference to Impact of Acylation. J. Agric. Food Chem. 2022, 71, 1002–1017. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Wei, Y.; Hao, J. The polyphenol-rich extract from chokeberry (Aronia melanocarpa L.) modulates gut microbiota and improves lipid metabolism in diet-induced obese rats. Nutr. Metab. 2020, 17, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Matsukawa, T.; Villareal, M.O.; Motojima, H.; Isoda, H. Increasing cAMP levels of preadipocytes by cyanidin-3-glucoside treatment induces the formation of beige phenotypes in 3T3-L1 adipocytes. J. Nutr. Biochem. 2017, 40, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Liu, J.; Zheng, S.; Liang, F.; Luo, Y.; Huang, K.; Xu, W.; He, X. Mulberry leaves ameliorate obesity through enhancing brown adipose tissue activity and modulating gut microbiota. Food Funct. 2019, 10, 4771–4781. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; My, L. The Effects of C3G and D3G Anthocyanin-Rich Black Soybean on Energy Metabolism in Beige-like Adipocytes. J. Agric Food Chem. 2020, 43, 12011–12018. [Google Scholar] [CrossRef]
- Pei, Y.; Otieno, D.; Gu, I.; Lee, S.-O.; Parks, J.S.; Schimmel, K.; Kang, H.W. Effect of quercetin on nonshivering thermogenesis of brown adipose tissue in high-fat diet-induced obese mice. J. Nutr. Biochem. 2020, 88, 108532. [Google Scholar] [CrossRef]
- Gil Lee, S.; Parks, J.S.; Kang, H.W. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J. Nutr. Biochem. 2017, 42, 62–71. [Google Scholar]
- Typolt, O.; Filingeri, D. Evidence for the involvement of peripheral cold-sensitive TRPM8 channels in human cutaneous hygrosensation. Am. J. Physiol. Integr. Comp. Physiol. 2020, 318, R579–R589. [Google Scholar] [CrossRef]
- Vizin, R.C.L.; Motzko-Soares, A.C.P.; Armentano, G.M.; Ishikawa, D.T.; Cruz-Neto, A.P.; Carrettiero, D.C.; Almeida, M.C.; Motzko-Soares, A.C.P. Short-term menthol treatment promotes persistent thermogenesis without induction of compensatory food consumption in Wistar rats: Implications for obesity control. J. Appl. Physiol. 2018, 124, 672–683. [Google Scholar] [CrossRef]
- Jiang, J.; Emont, M.; Jun, H.; Qiao, X.; Liao, J.; Kim, D.-I.; Wu, J. Cinnamaldehyde induces fat cell-autonomous thermogenesis and metabolic reprogramming. Metabolism 2017, 77, 58–64. [Google Scholar] [CrossRef]
- Young, Y.; Eun, J.; Kima, D. Thiacremonone, a sulfur compound isolated from garlic, attenuates lipid accumulation partially mediated via AMPK activation in 3T3-L1 adipocytes. J. Nutr. Biochem. 2012, 12, 1552–1558. [Google Scholar]
- Kaur, J.; Singh, D.P.; Kumar, V.; Kaur, S.; Bhunia, R.K.; Kondepudi, K.K.; Kuhad, A.; Bishnoi, M. Transient Receptor Potential (TRP) based polypharmacological combination stimulates energy expending phenotype to reverse HFD-induced obesity in mice. Life Sci. 2023, 12, 1704. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, S.; Wu, J.; Sun, X. Promotion of Mitochondrial Biogenesis via Activation of AMPK-PGC1α Signaling Pathway by Ginger (Zingiber officinale Roscoe) Extract, and Its Major Active Component 6-Gingerol. J. Food Sci. 2019, 84, 2101–2111. [Google Scholar] [CrossRef] [PubMed]
- Konstantinidi, M.; Koutelidakis, A.E. Functional Foods and Bioactive Compounds: A Review of Its Possible Role on Weight Management and Obesity’s Metabolic Consequences. Medicines 2019, 6, 94. [Google Scholar] [CrossRef] [PubMed]
- Hattori, H.; Yamauchi, K.; Onwona-Agyeman, S.; Mitsunaga, T. Identification of vanilloid compounds in grains of paradise and their effects on sympathetic nerve activity. J. Sci. Food Agric. 2018, 98, 4742–4748. [Google Scholar] [CrossRef]
- Matsushita, M.; Yoneshiro, T.; Aita, S.; Kamiya, T.; Kusaba, N.; Yamaguchi, K.; Takagaki, K.; Kameya, T.; Sugie, H.; Saito, M. Kaempferia parviflora Extract Increases Whole-Body Energy Expenditure in Humans: Roles of Brown Adipose Tissue. J. Nutr. Sci. Vitaminol. 2015, 61, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Saito, M.; Okamatsu-Ogura, Y.; Matsushita, M.; Watanabe, K. Human brown adipose tissue: Regulation and anti-obesity potential. Endocr. J. 2014, 2, 15–27. [Google Scholar] [CrossRef]
- Poekes, L.; Lanthier, N.; Leclercq, I.A. Brown adipose tissue: A potential target in the fight against obesity and the metabolic syndrome. Clin. Sci. 2015, 129, 933–949. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Li, Y. Adipose Tissue Aging and Metabolic Disorder, and the Impact of Nutritional Interventions. Nutrients 2022, 14, 3134. [Google Scholar] [CrossRef]

| UCP1 activation | Cold Exposure | [6,28,29] |
| Physical Activity | ||
| Fasting | ||
| Nutraceutical Foods | ||
| Amino Acids such as Tyrosine (Noradrenaline precursor) | ||
| Triiodothyronine (Ft3), Thyroxine (Ft4) | ||
| Molecules that stimulate β-adrenergic receptors and drugs that inhibit noradrenaline reuptake |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schirinzi, V.; Poli, C.; Berteotti, C.; Leone, A. Browning of Adipocytes: A Potential Therapeutic Approach to Obesity. Nutrients 2023, 15, 2229. https://doi.org/10.3390/nu15092229
Schirinzi V, Poli C, Berteotti C, Leone A. Browning of Adipocytes: A Potential Therapeutic Approach to Obesity. Nutrients. 2023; 15(9):2229. https://doi.org/10.3390/nu15092229
Chicago/Turabian StyleSchirinzi, Vittoria, Carolina Poli, Chiara Berteotti, and Alessandro Leone. 2023. "Browning of Adipocytes: A Potential Therapeutic Approach to Obesity" Nutrients 15, no. 9: 2229. https://doi.org/10.3390/nu15092229
APA StyleSchirinzi, V., Poli, C., Berteotti, C., & Leone, A. (2023). Browning of Adipocytes: A Potential Therapeutic Approach to Obesity. Nutrients, 15(9), 2229. https://doi.org/10.3390/nu15092229







