Nutraceutical Properties of Syringic Acid in Civilization Diseases—Review
Abstract
:1. Introduction
2. Civilization Diseases
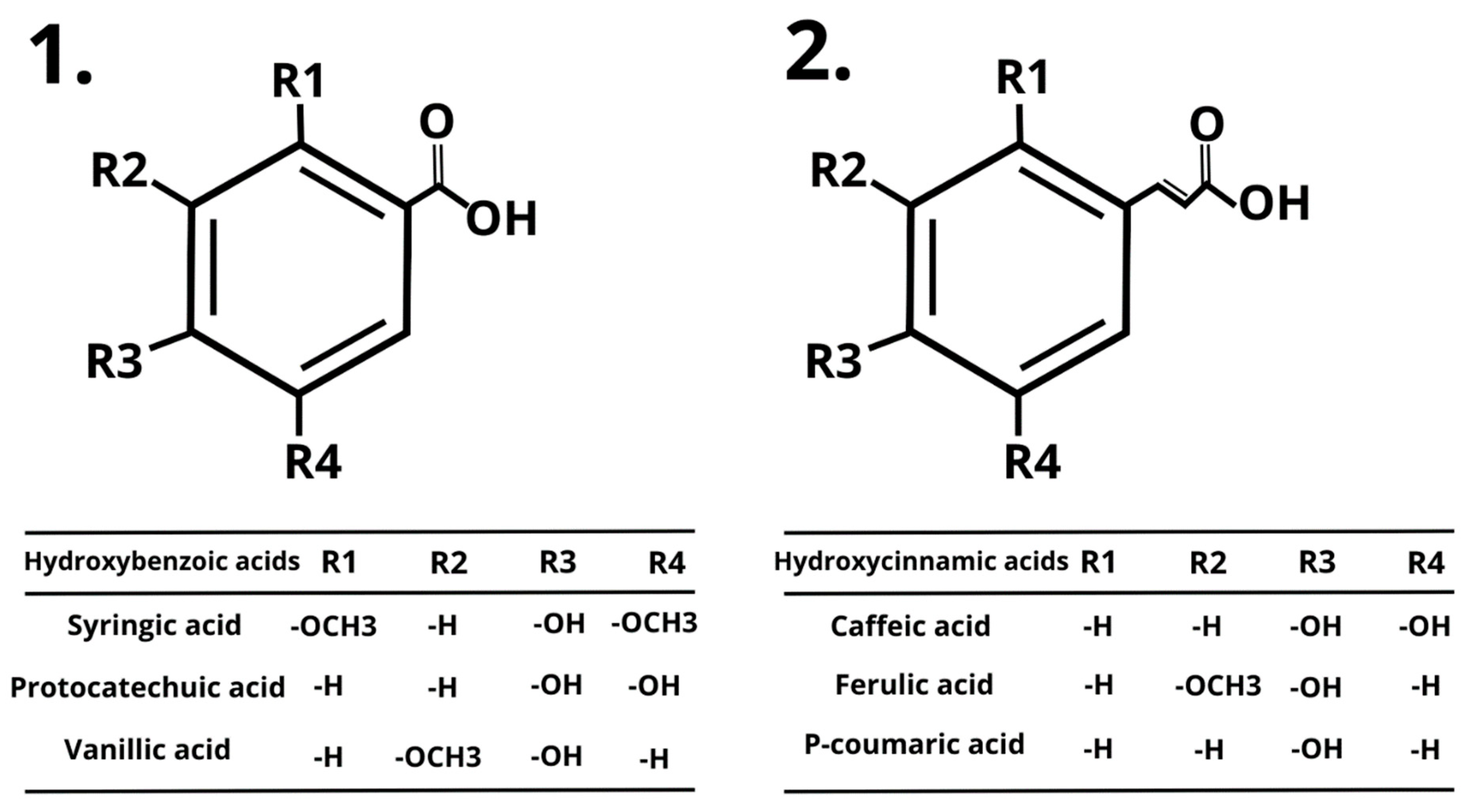
3. Polyphenolic Compounds
4. Syringic Acid (SA)
5. Cardioprotective Effects of SA
6. Anti-Cancer Properties of SA
7. Anti-Diabetic Effects of SA
8. Anti-Inflammatory Effects of SA
9. Hepatoprotective Effects of SA
10. Neuroprotective Effects of SA
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loos, R.J. From Nutrigenomics to Personalizing Diets: Are We Ready for Precision Medicine? Am. J. Clin. Nutr. 2019, 109, 1–2. [Google Scholar] [CrossRef]
- Noncommunicable Diseases. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 6 November 2023).
- Kopp, W. How Western Diet And Lifestyle Drive The Pandemic of Obesity And Civilization Diseases. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 2221–2236. [Google Scholar] [CrossRef]
- Lojanica, V.; Colic-Damjanovic, V.-M.; Jankovic, N. Housing of the Future: Housing Design of the Fourth Industrial Revolution. In Proceedings of the 2018 5th International Symposium on Environment-Friendly Energies and Applications (EFEA), Rome, Italy, 24–26 September 2018; pp. 1–4. [Google Scholar]
- Cetin, M.; Bakırtaş, İ. Does Urbanization Induce the Health Expenditures? A Dynamic Macro-Panel Analysis for Developing Countries. Dumlupınar Üniversitesi Sos. Bilim. Derg. 2019, 61, 208–222. [Google Scholar]
- Chen, W.; Zheng, R.; Zhang, S.; Zeng, H.; Zuo, T.; Xia, C.; Yang, Z.; He, J.; National Central Cancer Registry; National Cancer Center/Cancer Hospital. Cancer Incidence and Mortality in China in 2013: An Analysis Based on Urbanization Level. Chin. J. Cancer Res. 2017, 29, 1–10. [Google Scholar] [CrossRef]
- Hales, C.M.; Fryar, C.D.; Carroll, M.D.; Freedman, D.S.; Aoki, Y.; Ogden, C.L. Differences in Obesity Prevalence by Demographic Characteristics and Urbanization Level Among Adults in the United States, 2013–2016. JAMA 2018, 319, 2419. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Wai, A.; Yeung, A.W.K.; Tewari, D.; El-Demerdash, A.; Horbańczuk, O.; Das, N.; Pirgozliev, V.; Lucarini, M.; Durazzo, A.; Souto, E.B.; et al. Quercetin: Total-Scale Literature Landscape Analysis of a Valuable Nutraceutical with Numerous Potential Applications in the Promotion of Human and Animal Health a Review. Anim. Sci. Pap. Rep. 2021, 39, 199–212. [Google Scholar]
- Heleno, S.A.; Martins, A.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. Bioactivity of Phenolic Acids: Metabolites versus Parent Compounds: A Review. Food Chem. 2015, 173, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tzvetkov, N.; El-Demerdash, A.; Horbańczuk, O.; Das, N.; Pirgozliev, V.; Lucarini, M.; Durazzo, A.; Souto, E.B.; Santini, A.; et al. Apple Polyphenols in Human and Animal Health. Anim. Sci. Pap. Rep. 2021, 39, 105–118. [Google Scholar]
- D’Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, Dietary Sources and Bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348–361. [Google Scholar] [PubMed]
- Bohn, T. Dietary Factors Affecting Polyphenol Bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef] [PubMed]
- Kluska, M.; Woźniak, K. Natural Polyphenols as Modulators of Etoposide Anti-Cancer Activity. Int. J. Mol. Sci. 2021, 22, 6602. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.-P.; Li, S.; Chen, Y.-M.; Li, H.-B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of Polyphenols and Its Anticancer Properties in Biomedical Research: A Narrative Review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef] [PubMed]
- Jantan, I.; Haque, M.A.; Arshad, L.; Harikrishnan, H.; Septama, A.W.; Mohamed-Hussein, Z.-A. Dietary Polyphenols Suppress Chronic Inflammation by Modulation of Multiple Inflammation-Associated Cell Signaling Pathways. J. Nutr. Biochem. 2021, 93, 108634. [Google Scholar] [CrossRef]
- Mamirovna, T.O.; Komiljonovich, P.M.; Rasuljonovich, M.R. Hepatoprotective Potential of Polyphenols in CCL4-Induced Hepatic Damage. Eur. Sci. Rev. 2020, 11–12, 3–8. [Google Scholar]
- Sun, C.; Zhao, C.; Guven, E.C.; Paoli, P.; Simal-Gandara, J.; Ramkumar, K.M.; Wang, S.; Buleu, F.; Pah, A.; Turi, V.; et al. Dietary Polyphenols as Antidiabetic Agents: Advances and Opportunities. Food Front. 2020, 1, 18–44. [Google Scholar] [CrossRef]
- Truong, V.-L.; Jeong, W.-S. Antioxidant and Anti-Inflammatory Roles of Tea Polyphenols in Inflammatory Bowel Diseases. Food Sci. Hum. Wellness 2022, 11, 502–511. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Tewari, D.; El-Demerdash, A.; Tomczyk, M.; Das, N.; Pirgozliev, V.; Lucarini, M.; Durazzo, A.; Souto, E.B.; Santini, A.; et al. Lycopene: Total-Scale Literature Landscape Analysis of a Valuable Nutraceutical with Numerous Potential Applications in the Promotion of Human and Animal Health. Anim. Sci. Pap. Rep. 2022, 40, 119–134. [Google Scholar]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Żbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant Activity of Selected Phenolic Acids-Ferric Reducing Antioxidant Power Assay and QSAR Analysis of the Structural Features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From Chemistry to Biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Parus, A. Przeciwutleniające i farmakologiczne właściwości kwasów fenolowych. Postępy Fitoter. 2013, 1, 48–54. [Google Scholar]
- Deotale, S.M.; Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Coffee Oil as a Natural Surfactant. Food Chem. 2019, 295, 180–188. [Google Scholar] [CrossRef]
- Figat, R. Kwasy Fenolowe Jako Związki o Potencjale Antygenotoksycznym Występujące w Roślinach Leczniczych i Jadalnych. Prospect. Pharm. Sci. 2021, 19, 28–41. [Google Scholar] [CrossRef]
- Souza, M.C.; Santos, M.P.; Sumere, B.R.; Silva, L.C.; Cunha, D.T.; Martínez, J.; Barbero, G.F.; Rostagno, M.A. Isolation of Gallic Acid, Caffeine and Flavonols from Black Tea by on-Line Coupling of Pressurized Liquid Extraction with an Adsorbent for the Production of Functional Bakery Products. LWT 2020, 117, 108661. [Google Scholar] [CrossRef]
- Zhang, Y.-R.; Li, Y.-Y.; Wang, J.-Y.; Wang, H.-W.; Wang, H.-N.; Kang, X.-M.; Xu, W.-Q. Synthesis and Characterization of a Rosmarinic Acid Derivative That Targets Mitochondria and Protects against Radiation-Induced Damage In Vitro. Radiat. Res. 2017, 188, 264–275. [Google Scholar] [CrossRef]
- Vo, Q.V.; Bay, M.V.; Nam, P.C.; Quang, D.T.; Flavel, M.; Hoa, N.T.; Mechler, A. Theoretical and Experimental Studies of the Antioxidant and Antinitrosant Activity of Syringic Acid. J. Org. Chem. 2020, 85, 15514–15520. [Google Scholar] [CrossRef]
- Mittelstädt, G.; Negron, L.; Schofield, L.R.; Marsh, K.; Parker, E.J. Biochemical and Structural Characterisation of Dehydroquinate Synthase from the New Zealand Kiwifruit Actinidia Chinensis. Arch. Biochem. Biophys. 2013, 537, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Tzin, V.; Galili, G. New Insights into the Shikimate and Aromatic Amino Acids Biosynthesis Pathways in Plants. Mol. Plant 2010, 3, 956–972. [Google Scholar] [CrossRef] [PubMed]
- Santos Sánchez, N.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019; ISBN 978-1-78984-033-9. [Google Scholar]
- Srinivasulu, C.; Ramgopal, M.; Ramanjaneyulu, G.; Anuradha, C.M.; Suresh Kumar, C. Syringic Acid (SA)−A Review of Its Occurrence, Biosynthesis, Pharmacological and Industrial Importance. Biomed. Pharmacother. 2018, 108, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.C.; Panchal, S.S. Safety Evaluation of Syringic Acid: Subacute Oral Toxicity Studies in Wistar Rats. Heliyon 2019, 5, e02129. [Google Scholar] [CrossRef] [PubMed]
- Kapolou, A.; Karantonis, H.C.; Rigopoulos, N.; Koutelidakis, A.E. Association of Mean Daily Polyphenols Intake with Mediterranean Diet Adherence and Anthropometric Indices in Healthy Greek Adults: A Retrospective Study. Appl. Sci. 2021, 11, 4664. [Google Scholar] [CrossRef]
- Radtke, J.; Linseisen, J.; Wolfram, G. Phenolic acid intake of adults in a Bavarian subgroup of the national food consumption survey. Z. Ernahrungswiss. 1998, 37, 190–197. [Google Scholar] [CrossRef]
- Ovaskainen, M.-L.; Törrönen, R.; Koponen, J.M.; Sinkko, H.; Hellström, J.; Reinivuo, H.; Mattila, P. Dietary Intake and Major Food Sources of Polyphenols in Finnish Adults. J. Nutr. 2008, 138, 562–566. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Fezeu, L.; Touvier, M.; Arnault, N.; Manach, C.; Hercberg, S.; Galan, P.; Scalbert, A. Dietary Intake of 337 Polyphenols in French Adults. Am. J. Clin. Nutr. 2011, 93, 1220–1228. [Google Scholar] [CrossRef]
- Nascimento-Souza, M.A.; de Paiva, P.G.; Pérez-Jiménez, J.; do Carmo Castro Franceschini, S.; Ribeiro, A.Q. Estimated Dietary Intake and Major Food Sources of Polyphenols in Elderly of Viçosa, Brazil: A Population-Based Study. Eur. J. Nutr. 2018, 57, 617–627. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Rothwell, J.A.; Scalbert, A.; Knaze, V.; Romieu, I.; Slimani, N.; Fagherazzi, G.; Perquier, F.; Touillaud, M.; Molina-Montes, E.; et al. Dietary Intakes and Food Sources of Phenolic Acids in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Br. J. Nutr. 2013, 110, 1500–1511. [Google Scholar] [CrossRef]
- Hayes, P.J.; Smyth, M.R.; McMurrough, I. Comparison of Electrochemical and Ultraviolet Detection Methods in High-Performance Liquid Chromatography for the Determination of Phenolic Compounds Commonly Found in Beers. Part 2. Analysis of Beers. Analyst 1987, 112, 1205. [Google Scholar] [CrossRef] [PubMed]
- Jakopic, J.; Colaric, M.; Veberic, R.; Hudina, M.; Solar, A.; Stampar, F. How Much Do Cultivar and Preparation Time Influence on Phenolics Content in Walnut Liqueur? Food Chem. 2007, 104, 100–105. [Google Scholar] [CrossRef]
- Minussi, R.C.; Rossi, M.; Bologna, L.; Cordi, L.; Rotilio, D.; Pastore, G.M.; Durán, N. Phenolic Compounds and Total Antioxidant Potential of Commercial Wines. Food Chem. 2003, 82, 409–416. [Google Scholar] [CrossRef]
- Betés-Saura, C.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Phenolics in White Free Run Juices and Wines from Penedès by High-Performance Liquid Chromatography: Changes during Vinification. J. Agric. Food Chem. 1996, 44, 3040–3046. [Google Scholar] [CrossRef]
- Budak, H.N.; Guzel-Seydim, Z.B. Antioxidant Activity and Phenolic Content of Wine Vinegars Produced by Two Different Techniques. J. Sci. Food Agric. 2010, 90, 2021–2026. [Google Scholar] [CrossRef]
- Kivilompolo, M.; Obůrka, V.; Hyötyläinen, T. Comparison of GC-MS and LC-MS Methods for the Analysis of Antioxidant Phenolic Acids in Herbs. Anal. Bioanal. Chem. 2007, 388, 881–887. [Google Scholar] [CrossRef]
- Proestos, C.; Komaitis, M. Ultrasonically Assisted Extraction of Phenolic Compounds from Aromatic Plants: Comparison with Conventional Extraction Technics. J. Food Qual. 2006, 29, 567–582. [Google Scholar] [CrossRef]
- Variyar, P.S.; Bandyopadhyay, T. Effect of Gamma-irradiation on the Phenolic Acids of Some Indian Spices. Int. J. Food Sci. Technol. 1998, 33, 533–537. [Google Scholar] [CrossRef]
- Colaric, M.; Veberic, R.; Solar, A.; Hudina, M.; Stampar, F. Phenolic Acids, Syringaldehyde, and Juglone in Fruits of Different Cultivars of Juglans regia L. J. Agric. Food Chem. 2005, 53, 6390–6396. [Google Scholar] [CrossRef]
- Bianco, A.; Buiarelli, F.; Cartoni, G.; Coccioli, F.; Jasionowska, R.; Margherita, P. Analysis by Liquid Chromatography-tandem Mass Spectrometry of Biophenolic Compounds in Olives and Vegetation Waters, Part I. J. Sep. Sci. 2003, 26, 409–416. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Alasalvar, C.; Morris, A.; Baron, M.; Shahidi, F. Comparison of Antioxidant Activity, Anthocyanins, Carotenoids, and Phenolics of Three Native Fresh and Sun-Dried Date (Phoenix Dactylifera L.) Varieties Grown in Oman. J. Agric. Food Chem. 2005, 53, 7592–7599. [Google Scholar] [CrossRef] [PubMed]
- Chiou, A.; Karathanos, V.T.; Mylona, A.; Salta, F.N.; Preventi, F.; Andrikopoulos, N.K. Currants (Vitis Vinifera L.) Content of Simple Phenolics and Antioxidant Activity. Food Chem. 2007, 102, 516–522. [Google Scholar] [CrossRef]
- Tița, O.; Lengyel, E.; Stegăruș, D.I.; Săvescu, P.; Ciubara, A.B.; Constantinescu, M.A.; Tița, M.A.; Rață, D.; Ciubara, A. Identification and Quantification of Valuable Compounds in Red Grape Seeds. Appl. Sci. 2021, 11, 5124. [Google Scholar] [CrossRef]
- Stryjecka, M.; Krochmal-Marczak, B.; Cebulak, T.; Kiełtyka-Dadasiewicz, A. Assessment of Phenolic Acid Content and Antioxidant Properties of the Pulp of Five Pumpkin Species Cultivated in Southeastern Poland. Int. J. Mol. Sci. 2023, 24, 8621. [Google Scholar] [CrossRef] [PubMed]
- Cikman, O.; Soylemez, O.; Ozkan, O.F.; Kiraz, H.A.; Sayar, I.; Ademoglu, S.; Taysi, S.; Karaayvaz, M. Antioxidant Activity of Syringic Acid Prevents Oxidative Stress in L-Arginine-Induced Acute Pancreatitis: An Experimental Study on Rats. Int. Surg. 2015, 100, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Isoda, K.; Kondoh, M.; Kawase, M.; Watari, A.; Kobayashi, M.; Tamesada, M.; Yagi, K. Hepatoprotective Effect of Syringic Acid and Vanillic Acid on CCl4-Induced Liver Injury. Biol. Pharm. Bull. 2010, 33, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Wang, X.; Wu, W.; Qin, R. Effect of Syringic Acid on Antioxidant Biomarkers and Associated Inflammatory Markers in Mice Model of Asthma. Drug Dev. Res. 2019, 80, 253–261. [Google Scholar] [CrossRef]
- Mihanfar, A.; Darband, S.G.; Sadighparvar, S.; Kaviani, M.; Mirza-Aghazadeh-Attari, M.; Yousefi, B.; Majidinia, M. In Vitro and in Vivo Anticancer Effects of Syringic Acid on Colorectal Cancer: Possible Mechanistic View. Chem.-Biol. Interact. 2021, 337, 109337. [Google Scholar] [CrossRef]
- Mishra, V.; Jana, A.K.; Jana, M.M.; Gupta, A. Improvement of Selective Lignin Degradation in Fungal Pretreatment of Sweet Sorghum Bagasse Using Synergistic CuSO4-Syringic Acid Supplements. J. Environ. Manag. 2017, 193, 558–566. [Google Scholar] [CrossRef]
- Brauer, G.M.; Stansbury, J.W. Materials Science Cements Containing Syringic Acid Esters- o-Ethoxybenzoic Acid and Zinc Oxide. J. Dent. Res. 1984, 63, 137–140. [Google Scholar] [CrossRef]
- Bortolomeazzi, R.; Sebastianutto, N.; Toniolo, R.; Pizzariello, A. Comparative Evaluation of the Antioxidant Capacity of Smoke Flavouring Phenols by Crocin Bleaching Inhibition, DPPH Radical Scavenging and Oxidation Potential. Food Chem. 2007, 100, 1481–1489. [Google Scholar] [CrossRef]
- Baker, C.J.; Mock, N.M.; Whitaker, B.D.; Roberts, D.P.; Rice, C.P.; Deahl, K.L.; Aver’yanov, A.A. Involvement of Acetosyringone in Plant–Pathogen Recognition. Biochem. Biophys. Res. Commun. 2005, 328, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wu, F.; Xiang, W.S. Syringic Acid Inhibited Cucumber Seedling Growth and Changed Rhizosphere Microbial Communities. Plant Soil Environ. 2014, 60, 158–164. [Google Scholar] [CrossRef]
- Gimeno, O.; Fernandez, L.A.; Carbajo, M.; Beltran, F.; Rivas, J. Photocatalytic Ozonation of Phenolic Wastewaters: Syringic Acid, Tyrosol and Gallic Acid. J. Environ. Sci. Health Part A 2008, 43, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zhang, Y.; Ning, S.; Li, Y.; Ye, M.; Yu, Y.; Duan, G. Automated On-Line SPE/Multi-Stage Column-Switching and Benzoic Acid-Based QAMS/RODWs-HPLC for Oral Pharmacokinetics of Syringic Acid and Salicylic Acid in Rats. Chromatographia 2012, 75, 883–892. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, X.; Liu, Y.; Zhao, H.; Li, F. Dietary Addition of Garlic Straw Improved the Intestinal Barrier in Rabbits1. J. Anim. Sci. 2019, 97, 4248–4255. [Google Scholar] [CrossRef] [PubMed]
- Kfoury, M.; Lounès-Hadj Sahraoui, A.; Bourdon, N.; Laruelle, F.; Fontaine, J.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Solubility, Photostability and Antifungal Activity of Phenylpropanoids Encapsulated in Cyclodextrins. Food Chem. 2016, 196, 518–525. [Google Scholar] [CrossRef]
- Yu, J.; Zhu, Y.; Wang, L.; Peng, M.; Tong, S.; Cao, X.; Qiu, H.; Xu, X. Enhancement of Oral Bioavailability of the Poorly Water-Soluble Drug Silybin by Sodium Cholate/Phospholipid-Mixed Micelles. Acta Pharmacol. Sin. 2010, 31, 759–764. [Google Scholar] [CrossRef]
- Sabahi, Z.; Khoshnoud, M.J.; Hosseini, S.; Khoshraftar, F.; Rashedinia, M. Syringic Acid Attenuates Cardiomyopathy in Streptozotocin-Induced Diabetic Rats. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 5018092. [Google Scholar] [CrossRef]
- Kumar, S.; Prahalathan, P.; Raja, B. Syringic Acid Ameliorates L-NAME-Induced Hypertension by Reducing Oxidative Stress. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012, 385, 1175–1184. [Google Scholar] [CrossRef]
- Jalili, T.; Carlstrom, J.; Kim, S.; Freeman, D.; Jin, H.; Wu, T.-C.; Litwin, S.; Symons, J. Quercetin-Supplemented Diets Lower Blood Pressure and Attenuate Cardiac Hypertrophy in Rats With Aortic Constriction. J. Cardiovasc. Pharmacol. 2006, 47, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Isoda, K.; Kondoh, M.; Kawase, M.; Kobayashi, M.; Tamesada, M.; Yagi, K. Hepatoprotective Effect of Syringic Acid and Vanillic Acid on Concanavalin A-Induced Liver Injury. Biol. Pharm. Bull. 2009, 32, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Ferah Okkay, I.; Okkay, U.; Gundogdu, O.L.; Bayram, C.; Mendil, A.S.; Ertugrul, M.S.; Hacimuftuoglu, A. Syringic Acid Protects against Thioacetamide-Induced Hepatic Encephalopathy: Behavioral, Biochemical, and Molecular Evidence. Neurosci. Lett. 2022, 769, 136385. [Google Scholar] [CrossRef] [PubMed]
- Sammeturi, M.; Shaik, A.H.; Maruthi Prasad, E.; Mohammad, A.; Kodidhela, L.D. Cardioprotective Molecular Mechanism of Syringic Acid against Isoproterenol Induced Post- Myocardial Toxicity in Male Albino Wistar Rats. J. King Saud Univ.-Sci. 2020, 32, 1375–1381. [Google Scholar] [CrossRef]
- Han, X.; Bai, L.; Kee, H.J.; Jeong, M.H. Syringic Acid Mitigates Isoproterenol-Induced Cardiac Hypertrophy and Fibrosis by Downregulating Ereg. J. Cell Mol. Med. 2022, 26, 4076–4086. [Google Scholar] [CrossRef] [PubMed]
- Velu, P.; Vinothkumar, V.; Babukumar, S.; Ramachandhiran, D. Chemopreventive Effect of Syringic Acid on 7,12-Dimethylbenz(a)Anthracene Induced Hamster Buccal Pouch Carcinogenesis. Toxicol. Mech. Methods 2017, 27, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Lavanya, M.; Srinivasan, P.; Padmini, R. Unveiling the Anticancer Effect of Syringic Acid and Its Derivatives in Hepatocellular Carcinoma. Int. J. App. Pharm. 2023, 15, 114–124. [Google Scholar] [CrossRef]
- Muthukumaran, J.; Srinivasan, S.; Venkatesan, R.S.; Ramachandran, V.; Muruganathan, U. Syringic Acid, a Novel Natural Phenolic Acid, Normalizes Hyperglycemia with Special Reference to Glycoprotein Components in Experimental Diabetic Rats. J. Acute Dis. 2013, 2, 304–309. [Google Scholar] [CrossRef]
- Rashedinia, M.; Khoshnoud, M.J.; Fahlyan, B.K.; Hashemi, S.-S.; Alimohammadi, M.; Sabahi, Z. Syringic Acid: A Potential Natural Compound for the Management of Renal Oxidative Stress and Mitochondrial Biogenesis in Diabetic Rats. Curr. Drug Discov. Technol. 2021, 18, 405–413. [Google Scholar] [CrossRef]
- Srinivasan, S.; Muthukumaran, J.; Muruganathan, U.; Venkatesan, R.S.; Jalaludeen, A.M. Antihyperglycemic Effect of Syringic Acid on Attenuating the Key Enzymes of Carbohydrate Metabolism in Experimental Diabetic Rats. Biomed. Prev. Nutr. 2014, 4, 595–602. [Google Scholar] [CrossRef]
- Mirza, A.C.; Panchal, S.S.; Allam, A.A.; Othman, S.I.; Satia, M.; Mandhane, S.N. Syringic Acid Ameliorates Cardiac, Hepatic, Renal and Neuronal Damage Induced by Chronic Hyperglycaemia in Wistar Rats: A Behavioural, Biochemical and Histological Analysis. Molecules 2022, 27, 6722. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, D.; Yi, Y.; Qi, H.; Gao, X.; Fang, H.; Gu, Q.; Wang, L.; Gu, L. Syringic Acid Extracted from Herba Dendrobii Prevents Diabetic Cataract Pathogenesis by Inhibiting Aldose Reductase Activity. Evid. Based Complement. Alternat. Med. 2012, 2012, 426537. [Google Scholar] [CrossRef] [PubMed]
- Bhattacherjee, A.; Datta, A. Mechanism of Antiglycating Properties of Syringic and Chlorogenic Acids in in Vitro Glycation System. Food Res. Int. 2015, 77, 540–548. [Google Scholar] [CrossRef]
- Wu, Q.; Li, S.; Li, X.; Fu, X.; Sui, Y.; Guo, T.; Xie, B.; Sun, Z. A Significant Inhibitory Effect on Advanced Glycation End Product Formation by Catechin as the Major Metabolite of Lotus Seedpod Oligomeric Procyanidins. Nutrients 2014, 6, 3230–3244. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-H.; Kang, H.; Lee, K.; Yang, G.; Ham, I.; Bu, Y.; Kim, H.; Choi, H.-Y. The Aerial Part of Taraxacum Coreanum Extract Has an Anti-Inflammatory Effect on Peritoneal Macrophages in Vitro and Increases Survival in a Mouse Model of Septic Shock. J. Ethnopharmacol. 2013, 146, 1–8. [Google Scholar] [CrossRef]
- Ham, J.R.; Lee, H.-I.; Choi, R.-Y.; Sim, M.-O.; Seo, K.-I.; Lee, M.-K. Anti-Steatotic and Anti-Inflammatory Roles of Syringic Acid in High-Fat Diet-Induced Obese Mice. Food Funct. 2016, 7, 689–697. [Google Scholar] [CrossRef]
- Patra, A.; Jha, S.; Pn, M.; Aher, V.; Chattopadhyay, P.; Roy, D. Anti-Inflammatory and Antipyretic Activities of Hygrophilaspinosa T. Anders Leaves (Acanthaceae). Trop. J. Pharm. Res. 2009, 8, 133–137. [Google Scholar] [CrossRef]
- Ramachandran, V.; Raja, B. Protective Effects of Syringic Acid against Acetaminophen-Induced Hepatic Damage in Albino Rats. J. Basic Clin. Physiol. Pharmacol. 2010, 21, 369–385. [Google Scholar] [CrossRef]
- Somade, O.T.; Oyinloye, B.E.; Ajiboye, B.O.; Osukoya, O.A. Methyl Cellosolve-Induced Hepatic Oxidative Stress: The Modulatory Effect of Syringic Acid on Nrf2-Keap1-Hmox1-NQO1 Signaling Pathway in Rats. Phytomed. Plus 2023, 3, 100434. [Google Scholar] [CrossRef]
- Rashedinia, M.; Alimohammadi, M.; Shalfroushan, N.; Khoshnoud, M.J.; Mansourian, M.; Azarpira, N.; Sabahi, Z. Neuroprotective Effect of Syringic Acid by Modulation of Oxidative Stress and Mitochondrial Mass in Diabetic Rats. Biomed. Res. Int. 2020, 2020, 8297984. [Google Scholar] [CrossRef]
- Güven, M.; Aras, A.B.; Topaloğlu, N.; Özkan, A.; Şen, H.M.; Kalkan, Y.; Okuyucu, A.; Akbal, A.; Gökmen, F.; Coşar, M. The Protective Effect of Syringic Acid on Ischemia Injury in Rat Brain. Turk. J. Med. Sci. 2015, 45, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Tokmak, M.; Yuksel, Y.; Sehitoglu, M.H.; Guven, M.; Akman, T.; Aras, A.B.; Cosar, M.; Abbed, K.M. The Neuroprotective Effect of Syringic Acid on Spinal Cord Ischemia/Reperfusion Injury in Rats. Inflammation 2015, 38, 1969–1978. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.H.; Yan, B.C.; Park, J.H.; Yeun, G.H.; Yim, Y.; Ahn, J.H.; Lee, J.-C.; Hwang, I.K.; Cho, J.H.; Kim, Y.-M.; et al. Neuroprotection of a Novel Synthetic Caffeic Acid-Syringic Acid Hybrid Compound against Experimentally Induced Transient Cerebral Ischemic Damage. Planta Med. 2013, 79, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Rekha, K.R.; Selvakumar, G.P.; Sivakamasundari, R.I. Effects of Syringic Acid on Chronic MPTP/Probenecid Induced Motor Dysfunction, Dopaminergic Markers Expression and Neuroinflammation in C57BL/6 Mice. Biomed. Aging Pathol. 2014, 4, 95–104. [Google Scholar] [CrossRef]
- Pei, J.; Velu, P.; Zareian, M.; Feng, Z.; Vijayalakshmi, A. Effects of Syringic Acid on Apoptosis, Inflammation, and AKT/mTOR Signaling Pathway in Gastric Cancer Cells. Front. Nutr. 2021, 8, 788929. [Google Scholar] [CrossRef] [PubMed]
- Yeung, A.W.K.; Tzvetkov, N.T.; Durazzo, A.; Lucarini, M.; Souto, E.B.; Santini, A.; Gan, R.-Y.; Jozwik, A.; Grzybek, W.; Horbańczuk, J.O.; et al. Natural Products in Diabetes Research: Quantitative Literature Analysis. Nat. Prod. Res. 2021, 35, 5813–5827. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Tajima, T.; Yabushita, Y.; Nakamura, A.; Nezu, U.; Takahashi, M.; Kimura, M.; Terauchi, Y. A Novel Initial Codon Mutation of the Thiazide-Sensitive Na-Cl Cotransporter Gene in a Japanese Patient with Gitelman’s Syndrome. Endocr. J. 2008, 55, 557–560. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.-Y. Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef]
- Costa, M.F.; Jesus, T.I.; Lopes, B.R.P.; Angolini, C.F.F.; Montagnolli, A.; Gomes, L.d.P.; Pereira, G.S.; Ruiz, A.L.T.G.; Carvalho, J.E.; Eberlin, M.N.; et al. Eugenia Aurata and Eugenia Punicifolia HBK Inhibit Inflammatory Response by Reducing Neutrophil Adhesion, Degranulation and NET Release. BMC Complement. Altern. Med. 2016, 16, 403. [Google Scholar] [CrossRef]
- Gierlikowska, B.; Stachura, A.; Gierlikowski, W.; Demkow, U. Phagocytosis, Degranulation and Extracellular Traps Release by Neutrophils-The Current Knowledge, Pharmacological Modulation and Future Prospects. Front. Pharmacol. 2021, 12, 666732. [Google Scholar] [CrossRef]
- Dileep, K.V.; Remya, C.; Cerezo, J.; Fassihi, A.; Pérez-Sánchez, H.; Sadasivan, C. Comparative Studies on the Inhibitory Activities of Selected Benzoic Acid Derivatives against Secretory Phospholipase A2, a Key Enzyme Involved in the Inflammatory Pathway. Mol. Biosyst. 2015, 11, 1973–1979. [Google Scholar] [CrossRef] [PubMed]
- Chopyk, D.M.; Grakoui, A. Contribution of the Intestinal Microbiome and Gut Barrier to Hepatic Disorders. Gastroenterology 2020, 159, 849–863. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, J.M.; Reinus, J.F. Prevalence and Natural History of Alcoholic Liver Disease. Clin. Liver Dis. 2012, 16, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Safari, Z.; Gérard, P. The Links between the Gut Microbiome and Non-Alcoholic Fatty Liver Disease (NAFLD). Cell Mol. Life Sci. 2019, 76, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-Alcoholic Fatty Liver Disease—A Global Public Health Perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef] [PubMed]
- García-Cortés, M.; Ortega-Alonso, A.; Lucena, M.I.; Andrade, R.J. Spanish Group for the Study of Drug-Induced Liver Disease Drug-Induced Liver Injury: A Safety Review. Expert Opin. Drug Saf. 2018, 17, 795–804. [Google Scholar] [CrossRef]
- Iorga, A.; Dara, L.; Kaplowitz, N. Drug-Induced Liver Injury: Cascade of Events Leading to Cell Death, Apoptosis or Necrosis. Int. J. Mol. Sci. 2017, 18, 1018. [Google Scholar] [CrossRef]
- Komori, A. Recent Updates on the Management of Autoimmune Hepatitis. Clin. Mol. Hepatol. 2021, 27, 58–69. [Google Scholar] [CrossRef]
- Ogut, E.; Armagan, K.; Gül, Z. The Role of Syringic Acid as a Neuroprotective Agent for Neurodegenerative Disorders and Future Expectations. Metab. Brain Dis. 2022, 37, 859–880. [Google Scholar] [CrossRef]
- Jokioja, J.; Linderborg, K.M.; Kortesniemi, M.; Nuora, A.; Heinonen, J.; Sainio, T.; Viitanen, M.; Kallio, H.; Yang, B. Anthocyanin-Rich Extract from Purple Potatoes Decreases Postprandial Glycemic Response and Affects Inflammation Markers in Healthy Men. Food Chem. 2020, 310, 125797. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Szabo, L.; Molnar, R.; Tomesz, A.; Deutsch, A.; Darago, R.; Nowrasteh, G.; Varjas, T.; Nemeth, B.; Budan, F.; Kiss, I. The Effects of Flavonoids, Green Tea Polyphenols and Coffee on DMBA Induced LINE-1 DNA Hypomethylation. PLoS ONE 2021, 16, e0250157. [Google Scholar] [CrossRef] [PubMed]
- Molnar, R.; Szabo, L.; Tomesz, A.; Deutsch, A.; Darago, R.; Raposa, B.L.; Ghodratollah, N.; Varjas, T.; Nemeth, B.; Orsos, Z.; et al. The Chemopreventive Effects of Polyphenols and Coffee, Based upon a DMBA Mouse Model with microRNA and mTOR Gene Expression Biomarkers. Cells 2022, 11, 1300. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador-Palmer, R.; Dellinger, R. Polyphenolic Phytochemicals in Cancer Prevention and Therapy: Bioavailability versus Bioefficacy. J. Med. Chem. 2017, 60, 9413–9436. [Google Scholar] [CrossRef] [PubMed]
- Arfaoui, L. Dietary Plant Polyphenols: Effects of Food Processing on Their Content and Bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, M.; Xiao, Z.; Daglia, M.; Dragan, S.; Delmas, D.; Vong, C.T.; Wang, Y.; Zhao, Y.; Shen, J.; et al. Dietary Polyphenols for Managing Cancers: What Have We Ignored? Trends Food Sci. Technol. 2020, 101, 150–164. [Google Scholar] [CrossRef]
- Na, H.-K.; Kim, E.-H.; Jung, J.-H.; Lee, H.-H.; Hyun, J.-W.; Surh, Y.-J. (-)-Epigallocatechin Gallate Induces Nrf2-Mediated Antioxidant Enzyme Expression via Activation of PI3K and ERK in Human Mammary Epithelial Cells. Arch. Biochem. Biophys. 2008, 476, 171–177. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Ulrih, N.P.; Sengupta, P.K.; Xiao, J. Plasma Protein Binding of Dietary Polyphenols to Human Serum Albumin: A High Performance Affinity Chromatography Approach. Food Chem. 2019, 270, 257–263. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in Obesity, Diabetes, and Related Disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef]

| Product | Content | References |
|---|---|---|
| Alcoholic and non-alcoholic beverage [mg/100 mL] | ||
| Dark beer | 0.02 ± 0.02 | [44] |
| Walnut liquor | 3.12 ± 0.58 | [45] |
| Red wine | 0.27 ± 0.47 | [46] |
| Green grape juice | 0.05 ± 0.00 | [47] |
| Traditional vinegar | 0.70 ± 0.36 | [48] |
| Food products [mg/100 g] | ||
| Thyme | 11.70 ± 0.42 | [49] |
| Oregano | 3.75 ± 5.30 | [49] |
| Sage | 3.35 ± 4.74 | [49] |
| Rosemary | 1.03 ± 1.79 | [50] |
| Cloves | 0.79 ± 0.00 | [51] |
| Walnut | 33.83 ± 13.96 | [52] |
| Black olive | 33.10 ± 32.13 | [53] |
| Green olive | 6.00 ± 8.49 | [52] |
| Cauliflower | 1.13 ± 0.02 | [52] |
| Date (dried) | 6.06 ± 3.81 | [54] |
| Date (fresh) | 2.45 ± 4.10 | [54] |
| Currant | 0.34 ± 0.13 | [55] |
| Grape seed (Cabernet Sauvignon) | 122.87 ± 0.25 | [56] |
| Pumpkin pulp (C. maxima ‘Bambino’) | 2.67 ± 0.05 | [57] |
| Disease | Model | Dosage and Duration Period of Study | Effect of SA | References |
|---|---|---|---|---|
| CVDs | rats | 100 mg/kg for 6 weeks | ↓LDH, ↓CK-MB | [72] |
| 50 and 100 mg/kg for 6 weeks | ↓cardiac TBARS, ↓carbonylated protein | [72] | ||
| rats | 50 mg/kg for 7 days | ↓CK-MB, ↓LDH, ↓GGT, ↓hs-CRP, ↓SOD, ↓CAT, ↑NF-κB, ↑TNF-α | [77] | |
| mice | 100 mg/kg/for 7 days | ↓Ereg | [78] | |
| H9c2 cells | 10 µL for 24 h | ↓Nppa, ↓Nppb↓, ↓Col1a1 mRNA levels | [78] | |
| cancers | rats | 50 mg/kg for 15 weeks | ↓tumor incidence, volume, and weight; ↓Gobblet cells | [79] |
| SW-480 cells | 1000–1200 µL for 48 h | ↓CAT, ↓SOD, ↓GR, ↓PGx, ↓GST, ↓ERK1/2, ↓PI3K, ↓AKT, ↓NF-κB, ↓LC3, ↓BECLIN1, ↓ATG-3, ↑p53, ↑ROS, ↑apoptosis | [61] | |
| hamsters | 50 and 100 mg/kg for 14 weeks | ↓PCNA, ↓Cyclin D1, ↓mutant p53, ↓tumor incidence, Volume, and weight, ↑LPO, ↑CAT, ↑SOD, ↑GPx | [80] | |
| rats | 25 mg/kg for 14 weeks | ↓AFP, ↓AST, ↓ALT, ↓cellular expansion, nodules and hyperplasia, ↓BCL2, ↑Bax, ↑caspase 3, ↑cytochrome C | [80] | |
| diabetes | rats | 50 mg/kg for 30 days | ↓plasma glucose, ↑plasma insulin, ↑C-peptide | [81] |
| rats | 50 mg/kg for weeks | ↓blood glucose, ↓ALP, ↓TBARS, ↑GSH, ↑PGC-1α, ↑NRF1, ↑mtDNA/nDNA ratio | [82] | |
| rats | 50 mg/kg for 30 days | ↑insulin, ↑Hb, ↑glycogen, ↓glucose, ↓HbA1c | [83] | |
| rats | 25 and 59 mg/kg for 10 weeks | ↓hyperglycaemia, ↓polydipsia, ↓polyphagia, ↓polyuria, ↓relative organ weight, ↓cardiac hypertrophic indices, ↓inflammatory markers, ↓cell injury markers, ↓HbA1c, ↓ROS, ↓histopathological score, ↑Na/K ATPase activity | [84] | |
| rats | 200 µg/mL for 60 days | ↓AR | [85] | |
| BSA glucose model system | 50, 100 and 150 µg | ↓structural alterations of BSA | [86] | |
| rats | 300 mg/kg (LSOPC) for 1 day | ↓AG | [87] | |
| inflammation | mice | 50, 100 and 200 mg/kg of TCC | ↓TNF-α, ↓IL-6, ↓IL-1β | [88] |
| mouse peritoneal macrophages | 50, 100 and 200 µg/mL | ↓iNOS, ↓COX-2, ↓NO, ↓PGE2, ↓IκBα, ↓MAPKs, ↓STAT1, ↓1KK phosphorylation | [88] | |
| Mice | HFD-SA diet 0.5g/kg for 16 weeks | ↓body weight, ↓visceral fat mass, ↓serum levels of leptin, ↓TNF-α, ↓IFN-γ, ↓IL-6, ↓MCP-1, ↓insulin resistance, ↓hepatic lipid content, ↓droplets, ↓early fibrosis, ↓Cidea, ↓Pparγ, ↓Srebp-1c, ↓Srebp-2, ↓Hmgcr, ↓Fasn, ↓Tlr4, ↓Myd88, ↓NF-κB, ↑Pparα, ↑Acsl, ↑Cpt1, ↑Cpt2 ↑circulation of adiponectin | [89] | |
| rats | 200–400 mg/kg one dose of chloroform and alcoholic extracts of Hygrophilia Spinosa | ↑anti-inflammatory activity | [90] | |
| hepatic disorders | mice | 10 mg/kg 2×/week for 4 weeks | ↓MDA, ↓hepatic hydroxyproline content, ↓collagen accumulation, ↓ALT, ↓AST | [59] |
| HSC | 0.5 mg/mL | ↓cells activation, ↓collagen genes, ↓α-SMA | [59] | |
| liver parenchymal hepatocytes | 24 h long incubation in presence of SA | ↑viability of hepatocytes | [59] | |
| mice | 20 mg/kg, intraperitoneal administration | ↓AST, ↓ALT | [75] | |
| 10 mg/kg, intraperitoneal administration | ↓TNF-α, ↓IL-6, ↓IFN-γ | [75] | ||
| rats | 25, 50, and 100 mg/kg for 6 days | ↓AST, ↓ALT, ↓ALP, ↓GGT, ↓TBARS, ↓lipid peroxides, ↓SOD, ↓CAT, ↓GPx, ↑vitamin E, ↑vitamin C, ↑GSH | [91] | |
| rats | 50 and 100 mg/kg for 14 days | ↓AST, ↓ALT, ↓ALP, ↓LDH, ↓MDA, ↓ROS, ↑SOD, ↑GSH, ↓TNF-α, ↓IL-1β, ↓NF-κB, ↓IL-10, ↓iNOS, ↓8-OHdG, ↓GFAP, ↓ammonia concentration, preserved astrocyte and hepatocye structure | [76] | |
| rats | 25, 50, and 75 mg/kg for 30 days | ↓Hmox1, ↓NQO1, ↑SOD, ↑GST, ↑PGx, ↑NO, ↑CAT | [92] | |
| 50 and 75 mg/kg for 30 days | ↓MDA, ↓Nrf2, ↓Keap1 | [92] | ||
| neurodegenerative diseases | rats | 100 mg/kg for 6 weeks | ↑learning, ↑memory, ↑motor coordination, ↑PGC-1α, ↑NRF1 | [93] |
| 50 and 100 mg/kg for 6 weeks | ↑mtDNA/nDNA, ↓lipid peroxidation, ↓inflammation, ↓demyelination in sciatic nerves, | [93] | ||
| rats | 10 mg/kg, intraperitoneal administration | ↓MDA, ↓caspase-3-immunipositive neurons, ↓BECN1, ↓apoptotic neurons ↑SOD, ↑NRF1 | [94] | |
| rats | 10 mg/kg, intraperitoneal administration | ↑SOD, ↑NRF1, ↓MDA, ↓caspase-3, ↓caspase-9 | [95] | |
| Mongolian gerbils | 20 mg/kg of CA-SA for 3 days | ↓IL-1β, ↓5-LOX | [96] | |
| mice | 20 mg/kg for 3.5 days | ↑motor coordination, ↑neurochemicals, ↑TH, ↑DAT, ↑VMAT2, ↓IL-1β, ↓TNF-α, ↓COX-2 | [97] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartel, I.; Mandryk, I.; Horbańczuk, J.O.; Wierzbicka, A.; Koszarska, M. Nutraceutical Properties of Syringic Acid in Civilization Diseases—Review. Nutrients 2024, 16, 10. https://doi.org/10.3390/nu16010010
Bartel I, Mandryk I, Horbańczuk JO, Wierzbicka A, Koszarska M. Nutraceutical Properties of Syringic Acid in Civilization Diseases—Review. Nutrients. 2024; 16(1):10. https://doi.org/10.3390/nu16010010
Chicago/Turabian StyleBartel, Iga, Izabela Mandryk, Jarosław O. Horbańczuk, Agnieszka Wierzbicka, and Magdalena Koszarska. 2024. "Nutraceutical Properties of Syringic Acid in Civilization Diseases—Review" Nutrients 16, no. 1: 10. https://doi.org/10.3390/nu16010010
APA StyleBartel, I., Mandryk, I., Horbańczuk, J. O., Wierzbicka, A., & Koszarska, M. (2024). Nutraceutical Properties of Syringic Acid in Civilization Diseases—Review. Nutrients, 16(1), 10. https://doi.org/10.3390/nu16010010






