Relative Recovery of Non-Alcoholic Fatty Liver Disease (NAFLD) in Diet-Induced Obese Rats
Abstract
1. Introduction
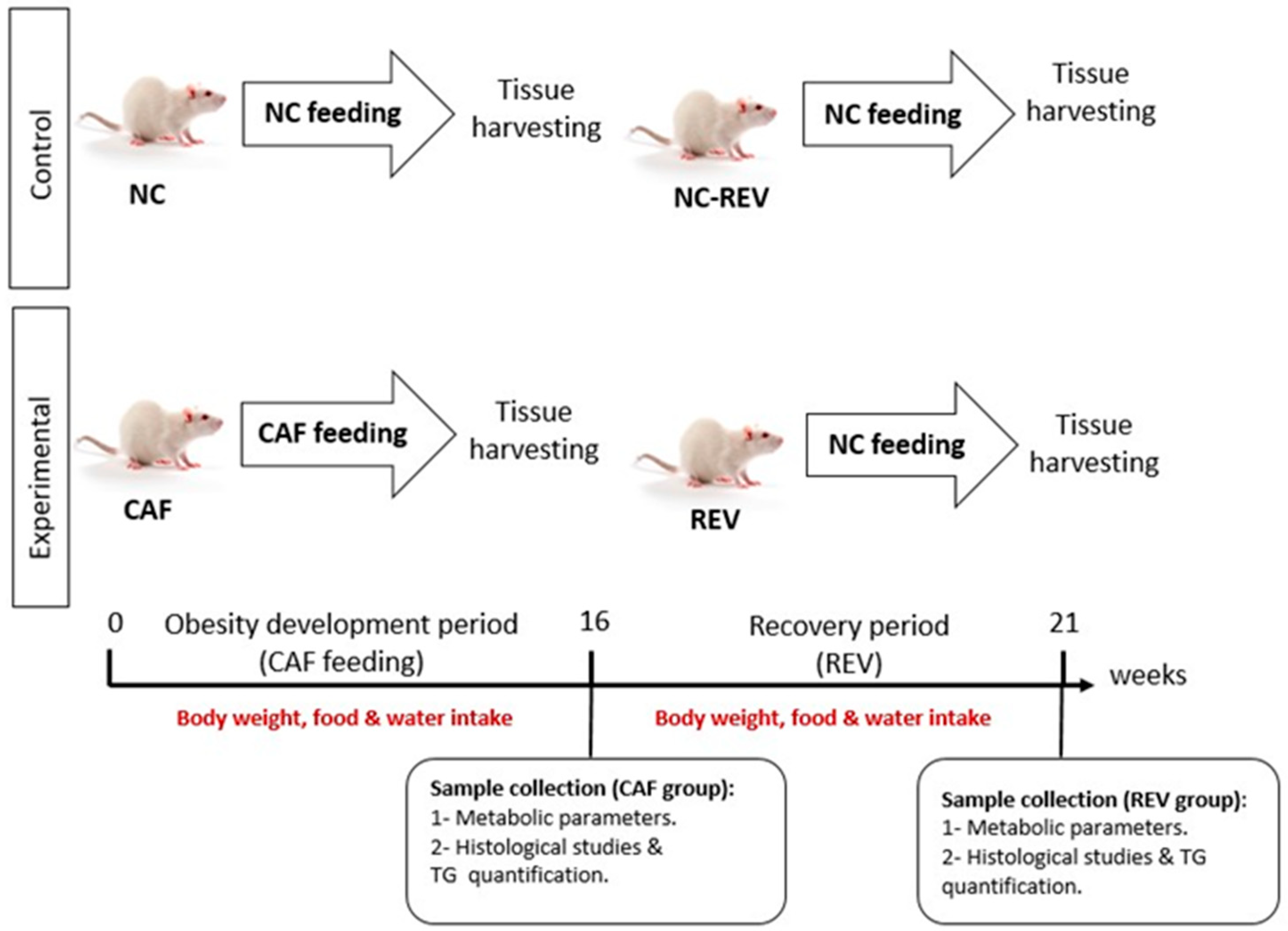
2. Materials and Methods
2.1. Animals
2.2. Tissue Dissection and Sample Collection
2.3. Metabolic Parameters
2.4. Hepatology
2.5. Statistical Analysis
3. Results
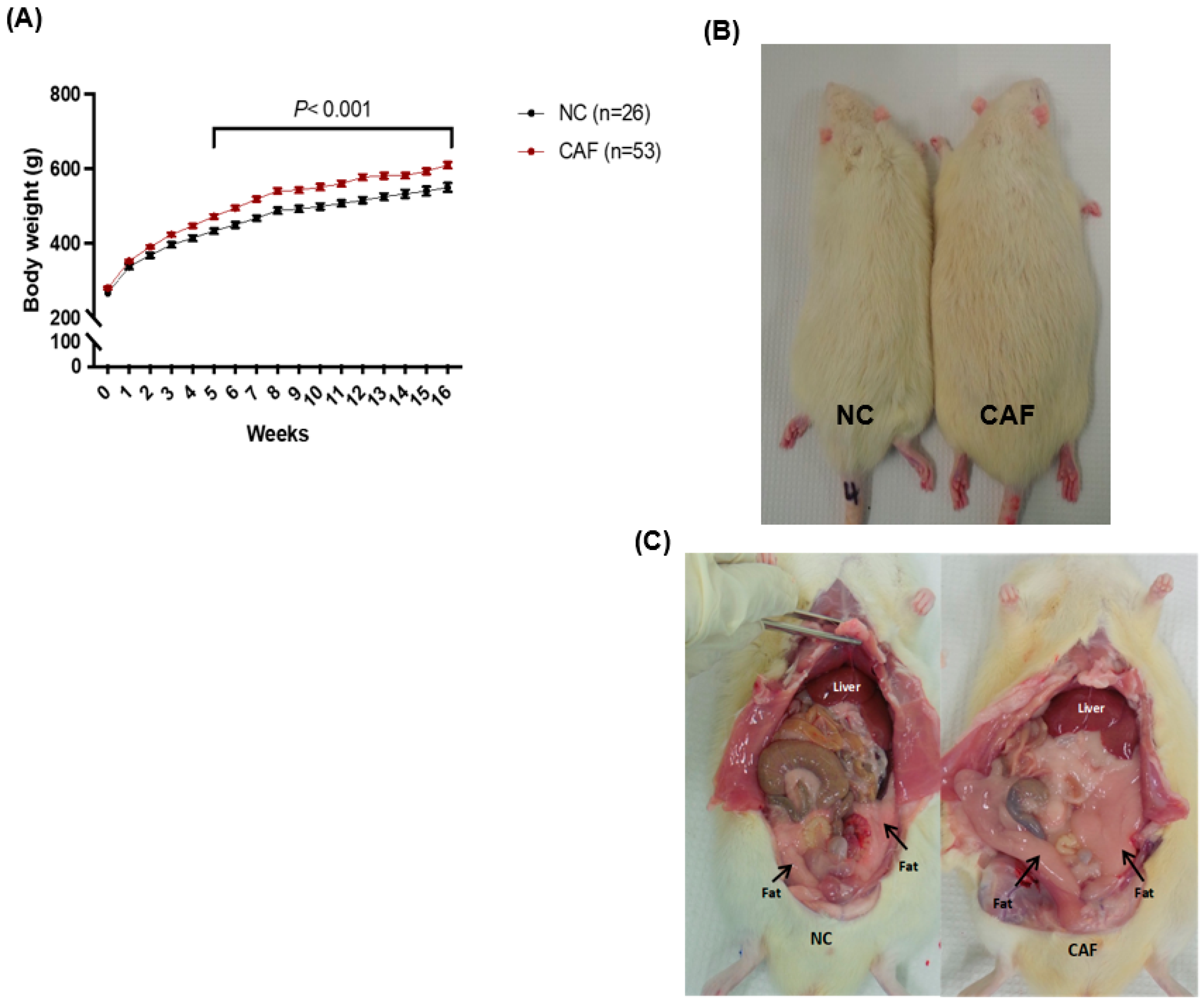
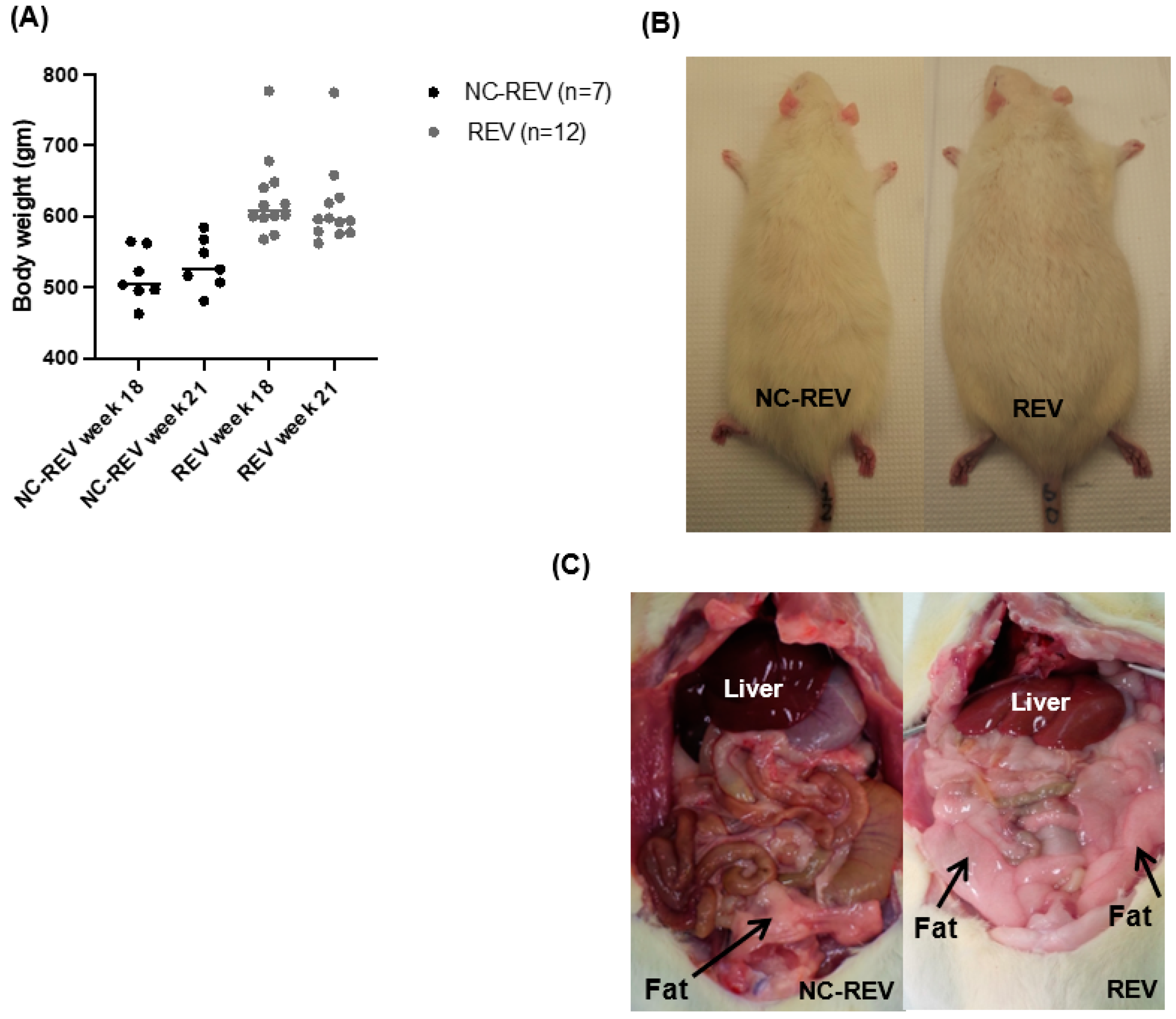
3.1. Body Weight
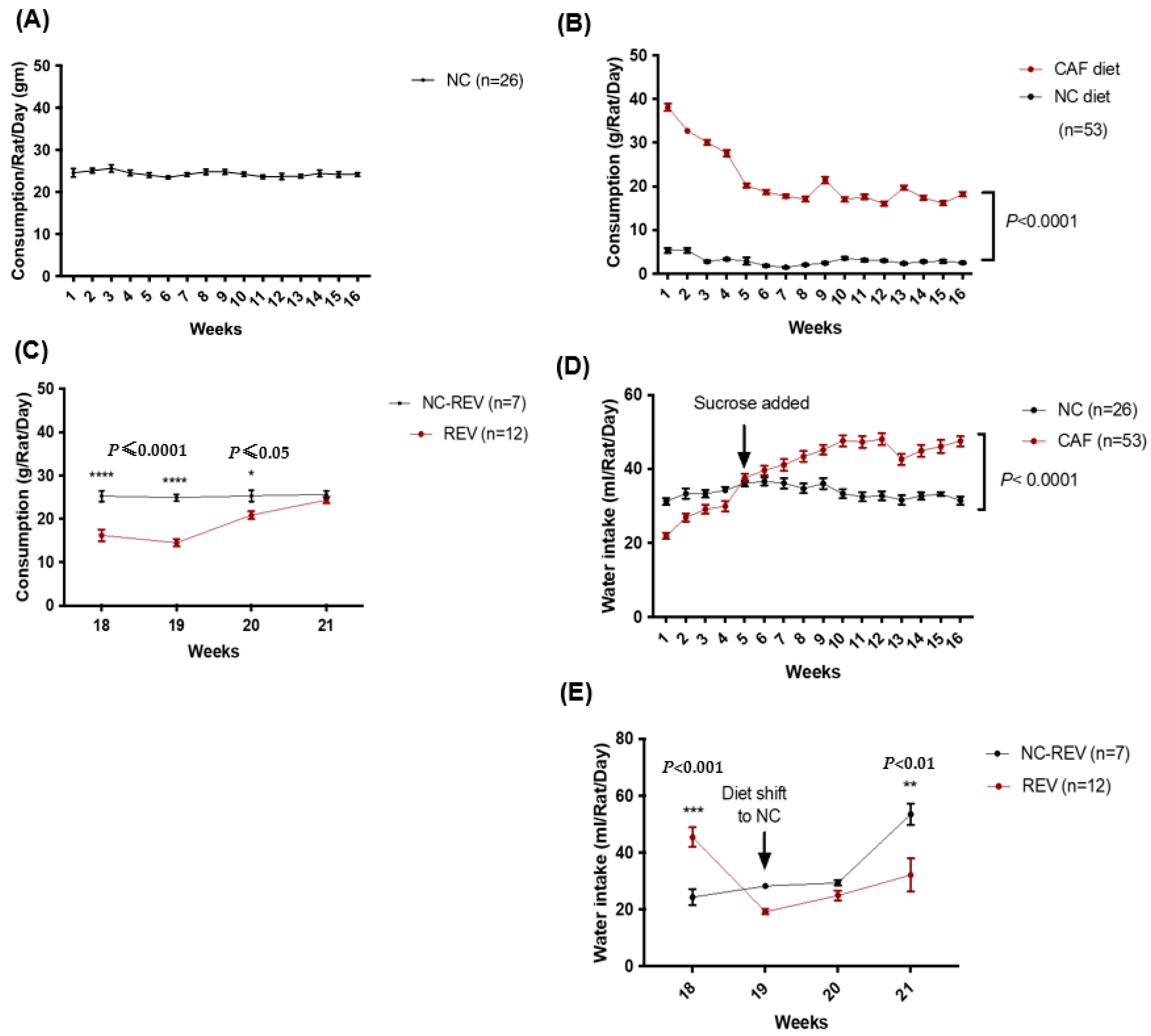
3.2. Food and Water Consumption
3.3. Metabolic Parameters
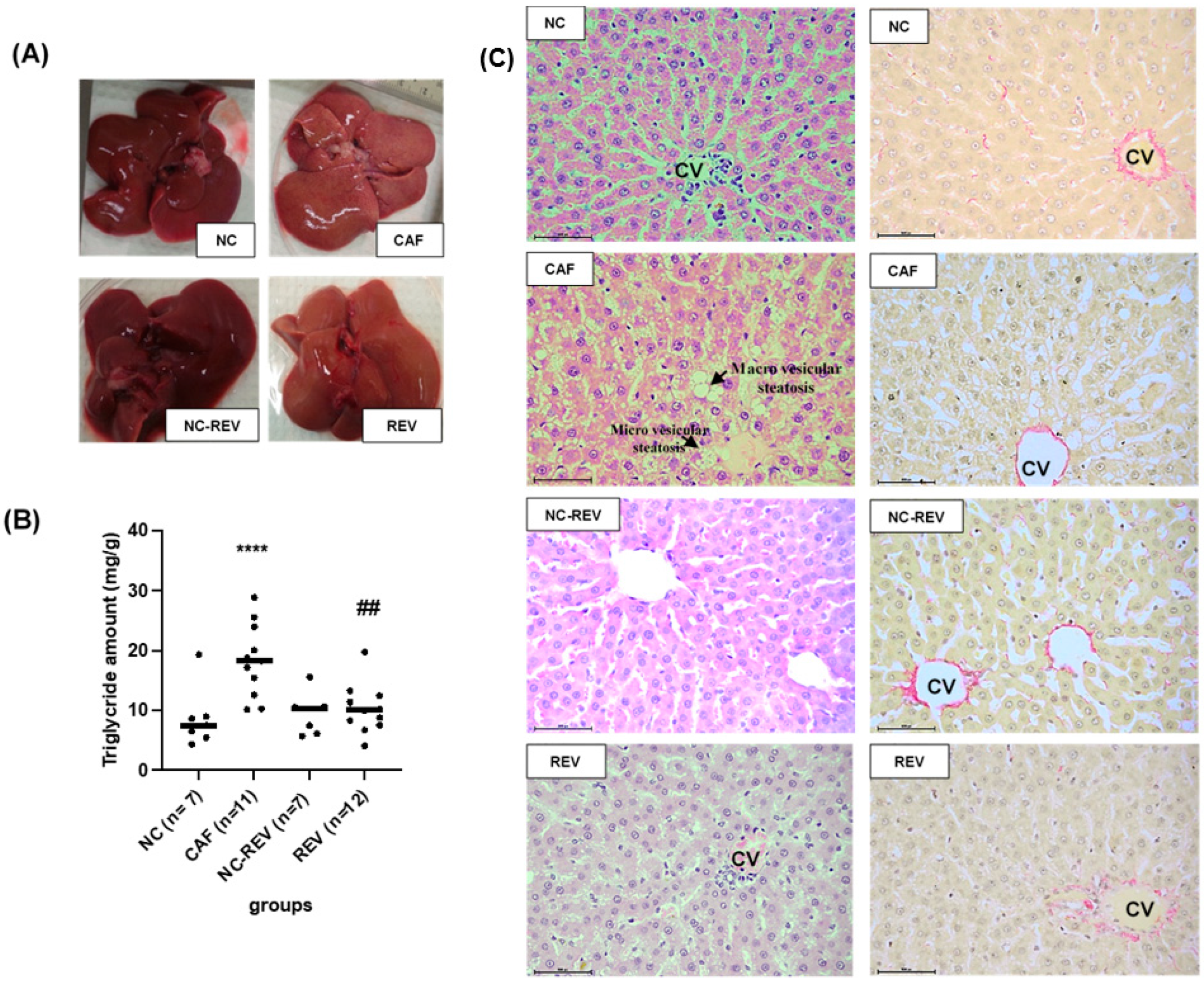
3.4. Hepatic Steatosis Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dario, A.B.; Loureiro Ferreira, M.; Refshauge, K.; Luque-Suarez, A.; Ordoñana, J.R.; Ferreira, P.H. Obesity does not increase the risk of chronic low back pain when genetics are considered. A prospective study of Spanish adult twins. Spine J. 2017, 17, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, M.A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet-induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Adedeji, T.G.; Abosede, C.O.; Dareowolabi, B.O. A high carbohydrate and soda diet influences metabolic variables in Wistar rats. Life Sci. 2022, 291, 120295. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M. Non-alcoholic fatty liver disease in 2015. World J. Hepatol. 2015, 7, 1450–1459. [Google Scholar] [CrossRef]
- Le, M.H.; Yeo, Y.H.; Li, X.; Li, J.; Zou, B.; Wu, Y.; Ye, Q.; Huang, D.Q.; Zhao, C.; Zhang, J.; et al. 2019 Global NAFLD Prevalence: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 2809–2817.e28. [Google Scholar] [CrossRef]
- Milic, S.; Lulic, D.; Stimac, D. Non-alcoholic fatty liver disease and obesity: Biochemical, metabolic and clinical presentations. World J. Gastroenterol. 2014, 20, 9330–9337. [Google Scholar]
- Stefan, N.; Kantartzis, K.; Häring, H.U. Causes and metabolic consequences of Fatty liver. Endocr. Rev. 2008, 29, 939–960. [Google Scholar] [CrossRef]
- Sackner-Bernstein, J.; Kanter, D.; Kaul, S. Dietary Intervention for Overweight and Obese Adults: Comparison of Low-Carbohydrate and Low-Fat Diets. A Meta-Analysis. PLoS ONE 2015, 10, e0139817. [Google Scholar] [CrossRef]
- O’Gorman, P.; Naimimohasses, S.; Monaghan, A.; Kennedy, M.; Melo, A.M.; Fhloinn, D.N.; Doherty, D.G.; Beddy, P.; Finn, S.P.; Moore, J.B.; et al. Improvement in histological endpoints of MAFLD following a 12-week aerobic exercise intervention. Aliment. Pharmacol. Ther. 2020, 52, 1387–1398. [Google Scholar] [CrossRef]
- Zivkovic, A.M.; German, J.B.; Sanyal, A.J. Comparative review of diets for the metabolic syndrome: Implications for nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2007, 86, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Menke, A.L.; Driessen, A.; Koek, G.H.; Lindeman, J.H.; Stoop, R.; Havekes, L.M.; Kleemann, R.; van den Hoek, A.M. Establishment of a general NAFLD scoring system for rodent models and comparison to human liver pathology. PLoS ONE 2014, 9, e115922. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, C.D.B.; Bellentani, F.F.; Fernandes, G.S.A.; Perobelli, J.E.; Favareto, A.P.A.; Nascimento, A.F.; Cicogna, A.C.; Kempinas, W.D.G. Diet-induced obesity in rats leads to a decrease in sperm motility. Reprod. Biol. Endocrinol. 2011, 9, 32. [Google Scholar] [CrossRef] [PubMed]
- Sampey, B.P.; Vanhoose, A.M.; Winfield, H.M.; Freemerman, A.J.; Muehlbauer, M.J.; Fueger, P.T.; Newgard, C.B.; Makowski, L. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: Comparison to high-fat diet. Obesity 2011, 19, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Shafat, A.; Murray, B.; Rumsey, D. Energy density in cafeteria diet induced hyperphagia in the rat. Appetite 2009, 52, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Buyukdere, Y.; Gulec, A.; Akyol, A. Cafeteria diet increased adiposity in comparison to high fat diet in young male rats. PeerJ 2019, 7, e6656. [Google Scholar] [CrossRef]
- Lindqvist, A.; Baelemans, A.; Erlanson-Albertsson, C. Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul. Pept. 2008, 150, 26–32. [Google Scholar] [CrossRef]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C., Jr.; et al. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of Dietary Fructose and Hepatic De Novo Lipogenesis in Fatty Liver Disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef]
- Strable, M.S.; Ntambi, J.M. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef]
- Francque, S.; Verrijken, A.; Mertens, I.; Hubens, G.; Van Marck, E.; Pelckmans, P.; Michielsen, P.; Van Gaal, L. Visceral adiposity and insulin resistance are independent predictors of the presence of non-cirrhotic NAFLD-related portal hypertension. Int. J. Obes. 2011, 35, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Cedo, L.; Santos, D.; Roglans, N.; Julve, J.; Pallares, V.; Rivas-Urbina, A.; Llorente-Cortes, V.; Laguna, J.C.; Blanco-Vaca, F.; Escola-Gil, J.C. Human hepatic lipase overexpression in mice induces hepatic steatosis and obesity through promoting hepatic lipogenesis and white adipose tissue lipolysis and fatty acid uptake. PLoS ONE 2017, 12, e0189834. [Google Scholar] [CrossRef]
- Zeeni, N.; Dagher-Hamalian, C.; Dimassi, H.; Faour, W.H. Cafeteria diet-fed mice is a pertinent model of obesity-induced organ damage: A potential role of inflammation. Inflamm. Res. 2015, 64, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.C.; Tsai, S.P.; Jhao, J.Y.; Jiang, W.K.; Tsao, C.K.; Chang, L.Y. Liver Fat, Hepatic Enzymes, Alkaline Phosphatase and the Risk of Incident Type 2 Diabetes: A Prospective Study of 132,377 Adults. Sci. Rep. 2017, 7, 4649. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.H.; Petroski, G.F.; Diaz-Arias, A.A.; Al Juboori, A.; Wheeler, A.A.; Ganga, R.R.; Pitt, J.B.; Spencer, N.M.; Hammoud, G.M.; Rector, R.S.; et al. A Model Incorporating Serum Alkaline Phosphatase for Prediction of Liver Fibrosis in Adults with Obesity and Nonalcoholic Fatty Liver Disease. J. Clin. Med. 2021, 10, 3311. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Fan, J.G. Diagnosis and management of non-alcoholic fatty liver disease and related metabolic disorders: Consensus statement from the Study Group of Liver and Metabolism, Chinese Society of Endocrinology. J. Diabetes 2013, 5, 406–415. [Google Scholar] [CrossRef]
- Fracanzani, A.L.; Valenti, L.; Bugianesi, E.; Andreoletti, M.; Colli, A.; Vanni, E.; Bertelli, C.; Fatta, E.; Bignamini, D.; Marchesini, G.; et al. Risk of severe liver disease in nonalcoholic fatty liver disease with normal aminotransferase levels: A role for insulin resistance and diabetes. Hepatology 2008, 48, 792–798. [Google Scholar] [CrossRef]
- Donnelly, K.L.; Smith, C.I.; Schwarzenberg, S.J.; Jessurun, J.; Boldt, M.D.; Parks, E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Investig. 2005, 115, 1343–1351. [Google Scholar] [CrossRef]
- Asghar, A.; Akhtar, T.; Batool, T.; Khawar, M.B.; Nadeem, S.; Mehmood, R.; Sheikh, N. High-fat diet-induced splenic, hepatic, and skeletal muscle architecture damage: Cellular and molecular players. Mol. Cell. Biochem. 2021, 476, 3671–3679. [Google Scholar] [CrossRef]
- Mamikutty, N.; Thent, Z.C.; Haji Suhaimi, F. Fructose-Drinking Water Induced Nonalcoholic Fatty Liver Disease and Ultrastructural Alteration of Hepatocyte Mitochondria in Male Wistar Rat. BioMed Res. Int. 2015, 2015, 895961. [Google Scholar] [CrossRef]
- Velázquez, K.T.; Enos, R.T.; Bader, J.E.; Sougiannis, A.T.; Carson, M.S.; Chatzistamou, I.; Carson, J.A.; Nagarkatti, P.S.; Nagarkatti, M.; Murphy, E.A. Prolonged high-fat-diet feeding promotes non-alcoholic fatty liver disease and alters gut microbiota in mice. World J. Hepatol. 2019, 11, 619–637. [Google Scholar] [CrossRef]
- Reynés, B.; García-Ruiz, E.; Díaz-Rúa, R.; Palou, A.; Oliver, P. Reversion to a control balanced diet is able to restore body weight and to recover altered metabolic parameters in adult rats long-term fed on a cafeteria diet. Food Res. Int. 2014, 64, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Brandt, N.; De Bock, K.; Richter, E.A.; Hespel, P. Cafeteria diet-induced insulin resistance is not associated with decreased insulin signaling or AMPK activity and is alleviated by physical training in rats. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E215–E224. [Google Scholar] [CrossRef] [PubMed]
- Lalanza, J.F.; Caimari, A.; del Bas, J.M.; Torregrosa, D.; Cigarroa, I.; Pallas, M.; Capdevila, L.; Arola, L.; Escorihuela, R.M. Effects of a post-weaning cafeteria diet in young rats: Metabolic syndrome, reduced activity and low anxiety-like behaviour. PLoS ONE 2014, 9, e85049. [Google Scholar] [CrossRef] [PubMed]
- Hubscher, S.G. Histological assessment of non-alcoholic fatty liver disease. Histopathology 2006, 49, 450–465. [Google Scholar] [CrossRef]
- Parker, B.M.; Wu, J.; You, J.; Barnes, D.S.; Yerian, L.; Kirwan, J.P.; Schauer, P.R.; Sessler, D.I. Reversal of fibrosis in patients with nonalcoholic steatohepatosis after gastric bypass surgery. BMC Obes. 2017, 4, 32. [Google Scholar] [CrossRef][Green Version]
| (A) | ||||
|---|---|---|---|---|
| Parameter | Obese | Reversible | ||
| NC (n = 11) | CAF (n = 12) | NC-REV (n = 6) | REV (n = 10) | |
| Cholesterol (mmol/L) | 1.77 ± 0.125 | 1.77 ± 0.107 | 1.88 ± 0.13 | 2.1 ± 0.15 |
| HDL (mmol/L) | 1.44 ± 0.107 | 1.11 ± 0.08 | 1.58 ± 0.13 | 1.66 ± 0.12 |
| LDL (mmol/L) | 0.29 ± 0.04 | 0.26 ± 0.04 | 0.35 ± 0.058 | 0.35 ± 0.044 |
| Triglycerides (mmol/L) | 0.904 ± 0.127 | 1.75 ± 0.19 *** | 0.82± 0.2 | 1.1 ± 0.12 ## |
| ALP (U/L) | 68.91 ± 4.57 | 89.9 ± 5.44 | 71.89 ± 7.5 | 62.03 ± 3.9 |
| ALT (U/L) | 79.66 ± 9.77 | 83.48 ± 28.77 | 123.3 ± 16.80 | 83.22 ± 9.24 |
| AST (U/L) | 362.77 ± 56.21 | 295.61 ± 52.65 | 372.7 ± 70.32 | 292.95 ± 35.24 |
| Glucose (mmol/L) | 9.79 ± 0.35 | 9.8 ± 1.1 | 12.9 ± 0.8 | 10.4 ± 0.9 |
| (B) | ||||
| Parameter | Obese | Reversible | ||
| NC (n = 7) | CAF (n = 7) | NC-REV (n = 4) | REV (n = 6) | |
| Insulin (ng/mL) | 0.58 ± 0.08 | 1.19 ± 0.2 * | 0.79 ± 0.13 | 1.0 ± 0.15 |
| CAF | REV | |||
|---|---|---|---|---|
| Parameter | NC (n = 11) | CAF (n = 15) | NC-REV (n = 7) | REV (n = 12) |
| Body wt (g) | 555.4 ± 14.34 | 646.56 ± 16.54 *** | 532.85 ± 13.66 | 612.54 ± 16.54 * |
| Liver (g) | 12.66 ± 0.42 | 17.36 ± 0.87 **** | 12.73 ± 0.8 | 15.62 ± 0.74 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboujassoum, H.M.; Mohamed-Ali, V.; Abraham, D.; Clapp, L.H.; Al-Naemi, H.A. Relative Recovery of Non-Alcoholic Fatty Liver Disease (NAFLD) in Diet-Induced Obese Rats. Nutrients 2024, 16, 115. https://doi.org/10.3390/nu16010115
Aboujassoum HM, Mohamed-Ali V, Abraham D, Clapp LH, Al-Naemi HA. Relative Recovery of Non-Alcoholic Fatty Liver Disease (NAFLD) in Diet-Induced Obese Rats. Nutrients. 2024; 16(1):115. https://doi.org/10.3390/nu16010115
Chicago/Turabian StyleAboujassoum, Hamda M., Vidya Mohamed-Ali, David Abraham, Lucie H. Clapp, and Hamda A. Al-Naemi. 2024. "Relative Recovery of Non-Alcoholic Fatty Liver Disease (NAFLD) in Diet-Induced Obese Rats" Nutrients 16, no. 1: 115. https://doi.org/10.3390/nu16010115
APA StyleAboujassoum, H. M., Mohamed-Ali, V., Abraham, D., Clapp, L. H., & Al-Naemi, H. A. (2024). Relative Recovery of Non-Alcoholic Fatty Liver Disease (NAFLD) in Diet-Induced Obese Rats. Nutrients, 16(1), 115. https://doi.org/10.3390/nu16010115






