Dietary Macronutrient Intake and Cardiovascular Disease Risk and Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Methods for Identification of Studies
2.2. Eligibility Criteria for Study Selection
2.3. Data Extraction and Management
2.4. Assessment of Risk of Bias in Included Studies
2.5. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Characteristics of Included Studies
3.3. Study Quality Assessment
3.4. Systematic Review
3.4.1. Systematic Review for All-Cause Mortality and Cause-Specific Mortality
3.4.2. Systematic Review for Cardiovascular Disease Events
3.5. Meta-Analysis on Dietary Macronutrient Intake and CVD Morbidity and Mortality
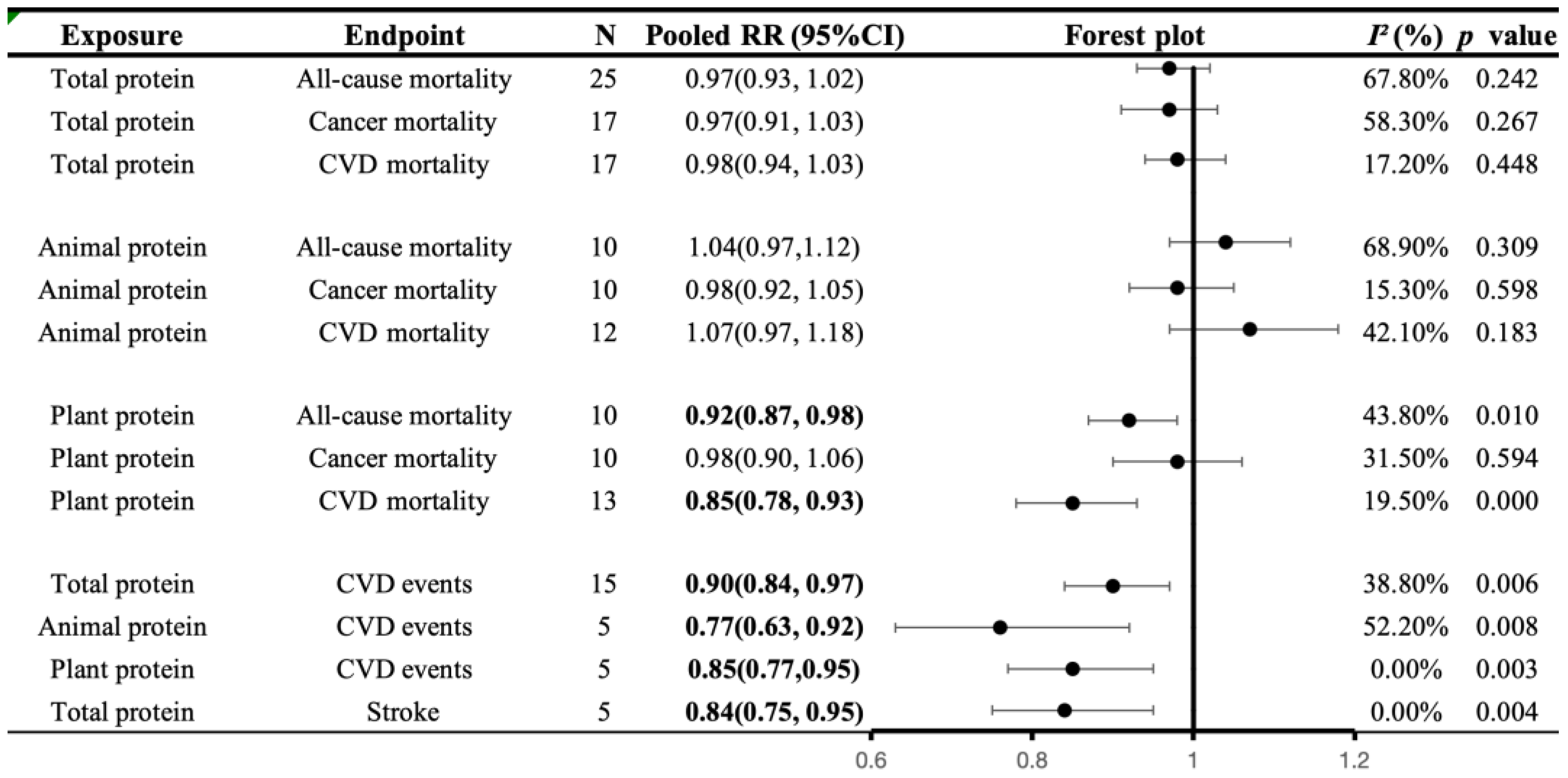
3.5.1. Associations of Protein Intake with All-Cause and Cause-Specific Mortality
3.5.2. Associations of Protein Intake with CVD Morbidity
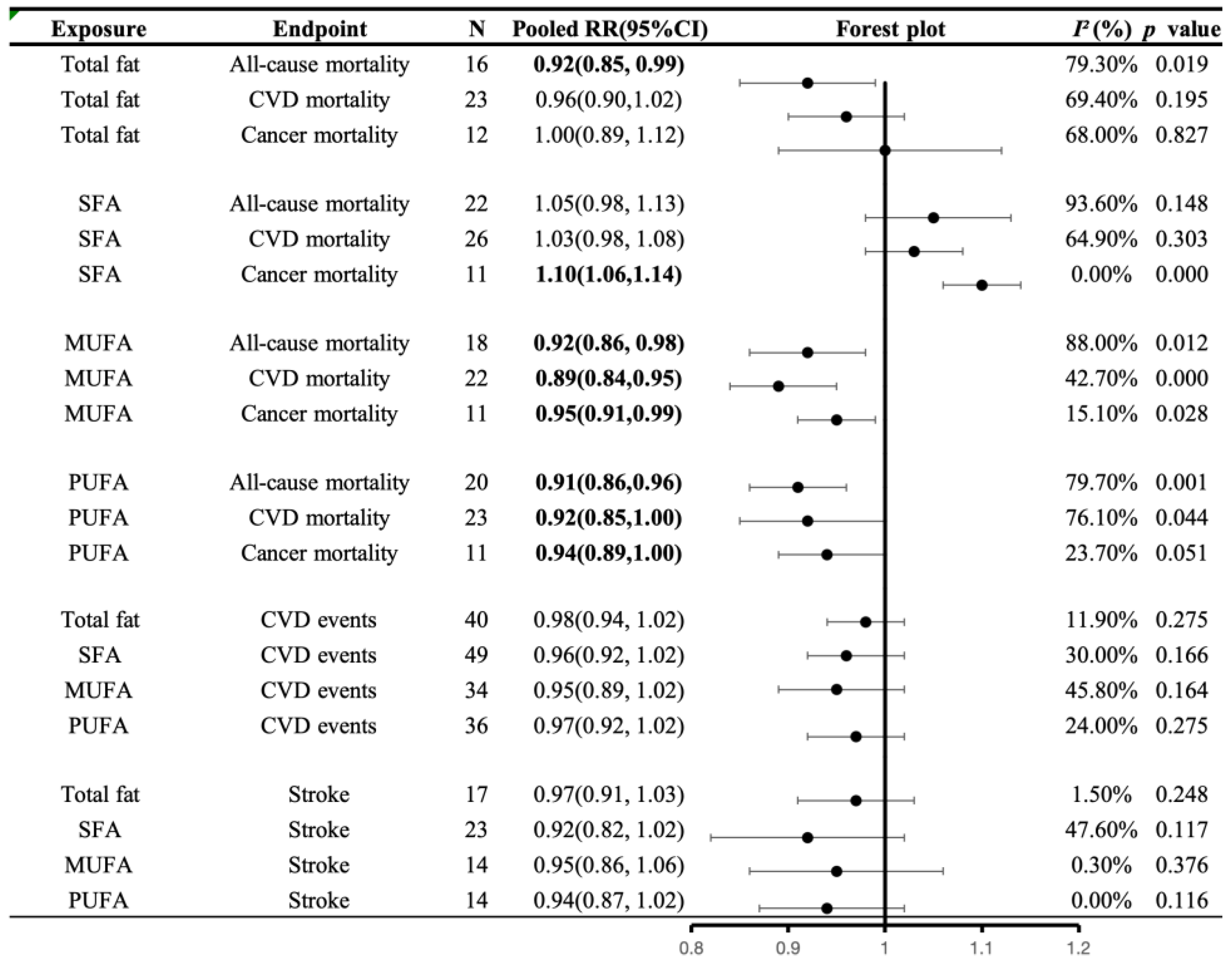
3.5.3. Associations of Fat Intake with All-Cause and Cause-Specific Mortality
3.5.4. Associations of Fat Intake with CVD Morbidity
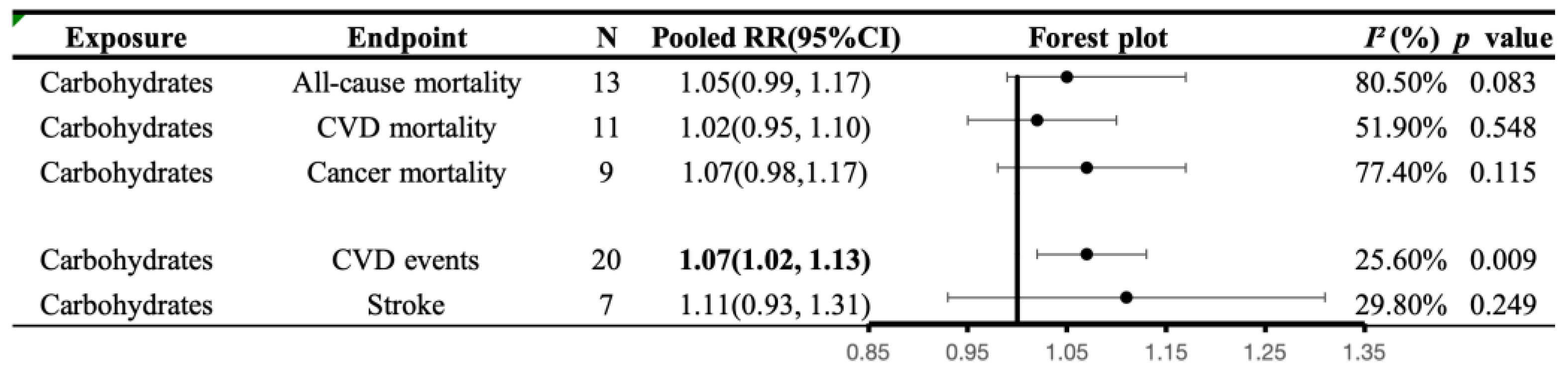
3.5.5. Associations of Carbohydrates Intake with All-Cause and Cause-Specific Mortality
3.5.6. Associations of Carbohydrates Intake with CVD Morbidity
3.6. Non-Linear Dose-Response Analysis
3.6.1. Non-Linear Dose-Response Analysis with Mortality
3.6.2. Non-Linear Dose-Response Analysis with CVD Morbidity
3.7. Secondary Analyses and Publication Bias
3.7.1. Subgroup Analyses
3.7.2. Sensitivity Analysis
3.7.3. Publication Bias
4. Discussion
4.1. Principal Findings
4.2. Protein and Health Outcomes
4.3. Fat and Health Outcomes
4.4. Carbohydrate and Health Outcomes
4.5. Strengths and Weaknesses of the Study
4.6. Practical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Forouzanfar, M.H.; Afshin, A.; Alexander, L.T.; Anderson, H.R.; Bhutta, Z.A.; Biryukov, S.; Brauer, M.; Burnett, R.; Cercy, K.; Charlson, F.J.; et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Grodstein, F.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Trends in the incidence of coronary heart disease and changes in diet and lifestyle in women. N. Engl. J. Med. 2000, 343, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M.J.; Hu, F.B.; Manson, J.E.; Rimm, E.B.; Willett, W.C. Primary prevention of coronary heart disease in women through diet and lifestyle. N. Engl. J. Med. 2000, 343, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Knoops, K.T.; de Groot, L.C.; Kromhout, D.; Perrin, A.E.; Moreiras-Varela, O.; Menotti, A.; van Staveren, W.A. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: The HALE project. JAMA 2004, 292, 1433–1439. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Health and Human Services; the U.S. Department of Agriculture. 2020–2025 Dietary Guidelines for Americans, 9th ed.; December 2020. Available online: www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf (accessed on 19 March 2023).
- WHO. Healthy Diet Fact Sheet. 14 September 2015. Available online: http://www.who.int/mediacentre/factsheets/fs394/en/ (accessed on 7 August 2018).
- Nilsson, L.M.; Winkvist, A.; Eliasson, M.; Jansson, J.H.; Hallmans, G.; Johansson, I.; Lindahl, B.; Lenner, P.; Van Guelpen, B. Low-carbohydrate, high-protein score and mortality in a northern Swedish population-based cohort. Eur. J. Clin. Nutr. 2012, 66, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.T.; van Dam, R.M.; Hankinson, S.E.; Stampfer, M.; Willett, W.C.; Hu, F.B. Low-carbohydrate diets and all-cause and cause-specific mortality: Two cohort studies. Ann. Intern. Med. 2010, 153, 289–298. [Google Scholar] [CrossRef]
- Lagiou, P.; Sandin, S.; Weiderpass, E.; Lagiou, A.; Mucci, L.; Trichopoulos, D.; Adami, H.O. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J. Intern. Med. 2007, 261, 366–374. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Psaltopoulou, T.; Orfanos, P.; Hsieh, C.C.; Trichopoulos, D. Low-carbohydrate-high-protein diet and long-term survival in a general population cohort. Eur. J. Clin. Nutr. 2007, 61, 575–581. [Google Scholar] [CrossRef]
- Noto, H.; Goto, A.; Tsujimoto, T.; Noda, M. Low-carbohydrate diets and all-cause mortality: A systematic review and meta-analysis of observational studies. PLoS ONE 2013, 8, e55030. [Google Scholar] [CrossRef]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): A prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Seidelmann, S.B.; Claggett, B.; Cheng, S.; Henglin, M.; Shah, A.; Steffen, L.M.; Folsom, A.R.; Rimm, E.B.; Willett, W.C.; Solomon, S.D. Dietary carbohydrate intake and mortality: A prospective cohort study and meta-analysis. Lancet Public Health 2018, 3, e419–e428. [Google Scholar] [CrossRef] [PubMed]
- Naghshi, S.; Sadeghi, O.; Willett, W.C.; Esmaillzadeh, A. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: Systematic review and dose-response meta-analysis of prospective cohort studies. BMJ 2020, 370, m2412. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, B.; Snetselaar, L.; Wallace, R.; Shadyab, A.; Kroenke, C.; Haring, B.; Howard, B.; Shikany, J.; Valdiviezo, C.; et al. Association of Major Dietary Protein Sources with All-cause and Cause-specific Mortality: The Women’s Health Initiative (FS03-08-19). Curr. Dev. Nutr. 2019, 3, e015553. [Google Scholar] [CrossRef]
- Virtanen, H.E.K.; Voutilainen, S.; Koskinen, T.T.; Mursu, J.; Kokko, P.; Ylilauri, M.P.T.; Tuomainen, T.P.; Salonen, J.T.; Virtanen, J.K. Dietary proteins and protein sources and risk of death: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2019, 109, 1462–1471. [Google Scholar] [CrossRef] [PubMed]
- Budhathoki, S.; Sawada, N.; Iwasaki, M.; Yamaji, T.; Goto, A.; Kotemori, A.; Ishihara, J.; Takachi, R.; Charvat, H.; Mizoue, T.; et al. Association of Animal and Plant Protein Intake with All-Cause and Cause-Specific Mortality in a Japanese Cohort. JAMA Intern. Med. 2019, 179, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Papanikolaou, Y.; Fulgoni, V., III. Animal and Plant Protein Usual Intakes Are Not Associated with Mortality in US Adults (P18-037-19). Curr. Dev. Nutr. 2019, 3, nzz039.P18-037-19. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://traiing.cochrane.org/handbook (accessed on 17 November 2023).
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Wells, G.; Wells, G.; Shea, B.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P.; Wells, G.S.; et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality if Nonrandomized Studies in Meta-Analyses. Available online: https://www.semanticscholar.org/paper/The-Newcastle-Ottawa-Scale-(NOS)-for-Assessing-the-Wells-Wells/c293fb316b6176154c3fdbb8340a107d9c8c82bf (accessed on 17 November 2023).
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Valentine, J.C.; Cooper, H. The Handbook of Research Synthesis and Meta-Analysis. In Sensitivity Analysis and Diagnostics; Cooper, H.M., Hedges, L.V., Valentine, J.C., Eds.; The Handbook of Research Synthesis and Meta-Analysis on JSTOR; Available online: https://www.daneshnamehicsa.ir/userfiles/files/1/9-%20The%20Handbook%20of%20Research%20Synthesis%20and%20Meta-Analysis.pdf (accessed on 17 November 2023).
- Chen, Z.; Glisic, M.; Song, M.; Aliahmad, H.A.; Zhang, X.; Moumdjian, A.C.; Gonzalez-Jaramillo, V.; van der Schaft, N.; Bramer, W.M.; Ikram, M.A.; et al. Dietary protein intake and all-cause and cause-specific mortality: Results from the Rotterdam Study and a meta-analysis of prospective cohort studies. Eur. J. Epidemiol. 2020, 35, 411–429. [Google Scholar] [CrossRef]
- Miller, V.; Mente, A.; Dehghan, M.; Rangarajan, S.; Zhang, X.; Swaminathan, S.; Dagenais, G.; Gupta, R.; Mohan, V.; Lear, S.; et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): A prospective cohort study. Lancet 2017, 390, 2037–2049. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: A systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016, 14, 207. [Google Scholar] [CrossRef]
- Wang, D.D.; Li, Y.; Chiuve, S.E.; Stampfer, M.J.; Manson, J.E.; Rimm, E.B.; Willett, W.C.; Hu, F.B. Association of Specific Dietary Fats with Total and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1134–1145. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef]
- Lin, Y.; Bolca, S.; Vandevijvere, S.; De Vriese, S.; Mouratidou, T.; De Neve, M.; Polet, A.; Van Oyen, H.; Van Camp, J.; De Backer, G.; et al. Plant and animal protein intake and its association with overweight and obesity among the Belgian population. Br. J. Nutr. 2011, 105, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Cross, A.J.; Graubard, B.I.; Leitzmann, M.F.; Schatzkin, A. Meat intake and mortality: A prospective study of over half a million people. Arch. Intern. Med. 2009, 169, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, A.M.; Sun, Q.; Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Willett, W.C. Major dietary protein sources and risk of coronary heart disease in women. Circulation 2010, 122, 876–883. [Google Scholar] [CrossRef] [PubMed]
- McGee, D.; Reed, D.; Stemmerman, G.; Rhoads, G.; Yano, K.; Feinleib, M. The relationship of dietary fat and cholesterol to mortality in 10 years: The Honolulu Heart Program. Int. J. Epidemiol. 1985, 14, 97–105. [Google Scholar] [CrossRef]
- Goldbourt, U.; Yaari, S.; Medalie, J.H. Factors predictive of long-term coronary heart disease mortality among 10,059 male Israeli civil servants and municipal employees. A 23-year mortality follow-up in the Israeli Ischemic Heart Disease Study. Cardiology 1993, 82, 100–121. [Google Scholar] [CrossRef]
- Ascherio, A.; Rimm, E.B.; Giovannucci, E.L.; Spiegelman, D.; Stampfer, M.; Willett, W.C. Dietary fat and risk of coronary heart disease in men: Cohort follow up study in the United States. BMJ 1996, 313, 84–90. [Google Scholar] [CrossRef]
- Esrey, K.L.; Joseph, L.; Grover, S.A. Relationship between dietary intake and coronary heart disease mortality: Lipid research clinics prevalence follow-up study. J. Clin. Epidemiol. 1996, 49, 211–216. [Google Scholar] [CrossRef]
- Mann, J.I.; Appleby, P.N.; Key, T.J.; Thorogood, M. Dietary determinants of ischaemic heart disease in health conscious individuals. Heart 1997, 78, 450–455. [Google Scholar] [CrossRef]
- Pietinen, P.; Ascherio, A.; Korhonen, P.; Hartman, A.M.; Willett, W.C.; Albanes, D.; Virtamo, J. Intake of fatty acids and risk of coronary heart disease in a cohort of Finnish men. The Alpha-Tocopherol, Beta-Carotene Cancer Prevention Study. Am. J. Epidemiol. 1997, 145, 876–887. [Google Scholar] [CrossRef]
- Boniface, D.R.; Tefft, M.E. Dietary fats and 16-year coronary heart disease mortality in a cohort of men and women in Great Britain. Eur. J. Clin. Nutr. 2002, 56, 786–792. [Google Scholar] [CrossRef]
- Trichopoulou, A.; Costacou, T.; Bamia, C.; Trichopoulos, D. Adherence to a Mediterranean diet and survival in a Greek population. N. Engl. J. Med. 2003, 348, 2599–2608. [Google Scholar] [CrossRef]
- Sauvaget, C.; Nagano, J.; Hayashi, M.; Yamada, M. Animal protein, animal fat, and cholesterol intakes and risk of cerebral infarction mortality in the adult health study. Stroke 2004, 35, 1531–1537. [Google Scholar] [CrossRef]
- Leosdottir, M.; Nilsson, P.M.; Nilsson, J.A.; Månsson, H.; Berglund, G. Dietary fat intake and early mortality patterns—Data from The Malmö Diet and Cancer Study. J. Intern. Med. 2005, 258, 153–165. [Google Scholar] [CrossRef]
- Solfrizzi, V.; D’Introno, A.; Colacicco, A.M.; Capurso, C.; Palasciano, R.; Capurso, S.; Torres, F.; Capurso, A.; Panza, F. Unsaturated fatty acids intake and all-causes mortality: A 8.5-year follow-up of the Italian Longitudinal Study on Aging. Exp. Gerontol. 2005, 40, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Orfanos, P.; Norat, T.; Bueno-de-Mesquita, B.; Ocké, M.C.; Peeters, P.H.; van der Schouw, Y.T.; Boeing, H.; Hoffmann, K.; Boffetta, P.; et al. Modified Mediterranean diet and survival: EPIC-elderly prospective cohort study. BMJ 2005, 330, 991. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Hallfrisch, J.; Qiao, N.; Muller, D.; Andres, R.; Fleg, J.L. The combination of high fruit and vegetable and low saturated fat intakes is more protective against mortality in aging men than is either alone: The Baltimore Longitudinal Study of Aging. J. Nutr. 2005, 135, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Eilat-Adar, S.; Loria, C.; Goldbourt, U.; Howard, B.V.; Fabsitz, R.R.; Zephier, E.M.; Mattil, C.; Lee, E.T. Dietary fat intake and risk of coronary heart disease: The Strong Heart Study. Am. J. Clin. Nutr. 2006, 84, 894–902. [Google Scholar] [CrossRef]
- Dilis, V.; Katsoulis, M.; Lagiou, P.; Trichopoulos, D.; Naska, A.; Trichopoulou, A. Mediterranean diet and CHD: The Greek European Prospective Investigation into Cancer and Nutrition cohort. Br. J. Nutr. 2012, 108, 699–709. [Google Scholar] [CrossRef]
- Kim, Y.; Je, Y.; Giovannucci, E.L. Association between dietary fat intake and mortality from all-causes, cardiovascular disease, and cancer: A systematic review and meta-analysis of prospective cohort studies. Clin. Nutr. 2021, 40, 1060–1070. [Google Scholar] [CrossRef]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Mazidi, M.; Mikhailidis, D.P.; Sattar, N.; Toth, P.P.; Judd, S.; Blaha, M.J.; Hernandez, A.V.; Penson, P.E.; Banach, M. Association of types of dietary fats and all-cause and cause-specific mortality: A prospective cohort study and meta-analysis of prospective studies with 1,164,029 participants. Clin. Nutr. 2020, 39, 3677–3686. [Google Scholar] [CrossRef]
- Giovannucci, E.; Rimm, E.B.; Stampfer, M.J.; Colditz, G.A.; Ascherio, A.; Willett, W.C. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994, 54, 2390–2397. [Google Scholar]
- Brennan, S.F.; Woodside, J.V.; Lunny, P.M.; Cardwell, C.R.; Cantwell, M.M. Dietary fat and breast cancer mortality: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2017, 57, 1999–2008. [Google Scholar] [CrossRef]
- Cao, Y.; Hou, L.; Wang, W. Dietary total fat and fatty acids intake, serum fatty acids and risk of breast cancer: A meta-analysis of prospective cohort studies. Int. J. Cancer 2016, 138, 1894–1904. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Cheng, L.; Wang, J.; Zhang, Y.; Jiao, J. Saturated Fatty Acid Intake Is Associated with Total Mortality in a Nationwide Cohort Study. J. Nutr. 2019, 149, 68–77. [Google Scholar] [CrossRef]
- Larsson, S.C.; Orsini, N. Red meat and processed meat consumption and all-cause mortality: A meta-analysis. Am. J. Epidemiol. 2014, 179, 282–289. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, T.A.; Hafekost, K.; Mitrou, F.; Lawrence, D. Food sources of saturated fat and the association with mortality: A meta-analysis. Am. J. Public Health 2013, 103, e31–e42. [Google Scholar] [CrossRef] [PubMed]
- Rohrmann, S.; Overvad, K.; Bueno-de-Mesquita, H.B.; Jakobsen, M.U.; Egeberg, R.; Tjønneland, A.; Nailler, L.; Boutron-Ruault, M.C.; Clavel-Chapelon, F.; Krogh, V.; et al. Meat consumption and mortality—Results from the European Prospective Investigation into Cancer and Nutrition. BMC Med. 2013, 11, 63. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yang, X.; Zhang, C.; Zhu, C.; Tao, G.; Zhao, L.; Tang, S.; Shu, Z.; Cai, J.; Dai, S.; et al. Red and processed meat intake is associated with higher gastric cancer risk: A meta-analysis of epidemiological observational studies. PLoS ONE 2013, 8, e70955. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Song, S.; Song, Y.; Lee, J.E. Consumption of red and processed meat and esophageal cancer risk: Meta-analysis. World J. Gastroenterol. 2013, 19, 1020–1029. [Google Scholar] [CrossRef]
- Aune, D.; Chan, D.S.; Vieira, A.R.; Navarro Rosenblatt, D.A.; Vieira, R.; Greenwood, D.C.; Kampman, E.; Norat, T. Red and processed meat intake and risk of colorectal adenomas: A systematic review and meta-analysis of epidemiological studies. Cancer Causes Control 2013, 24, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Virtamo, J.; Wolk, A. Dietary fats and dietary cholesterol and risk of stroke in women. Atherosclerosis 2012, 221, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Ferré, M.; Babio, N.; Martínez-González, M.A.; Corella, D.; Ros, E.; Martín-Peláez, S.; Estruch, R.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am. J. Clin. Nutr. 2015, 102, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Zhang, Y.; He, W.; Chen, X.; Chen, J.; He, L.; Mao, L.; Wu, F.; Jiao, J. Dietary Fats in Relation to Total and Cause-Specific Mortality in a Prospective Cohort of 521 120 Individuals with 16 Years of Follow-Up. Circ. Res. 2019, 124, 757–768. [Google Scholar] [CrossRef]
- GISSI-Prevenzione Investigators. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: Results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto miocardico. Lancet 1999, 354, 447–455. [Google Scholar] [CrossRef]
- Tavazzi, L.; Maggioni, A.P.; Marchioli, R.; Barlera, S.; Franzosi, M.G.; Latini, R.; Lucci, D.; Nicolosi, G.L.; Porcu, M.; Tognoni, G. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): A randomised, double-blind, placebo-controlled trial. Lancet 2008, 372, 1223–1230. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef] [PubMed]
- Wakai, K.; Naito, M.; Date, C.; Iso, H.; Tamakoshi, A. Dietary intakes of fat and total mortality among Japanese populations with a low fat intake: The Japan Collaborative Cohort (JACC) Study. Nutr. Metab. 2014, 11, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Wang, W.; Wang, J.; Zhang, Y.; Jiao, J. Polyunsaturated fatty acids intake, omega-6/omega-3 ratio and mortality: Findings from two independent nationwide cohorts. Clin. Nutr. 2019, 38, 848–855. [Google Scholar] [CrossRef] [PubMed]
- Grønn, M.; Gørbitz, C.; Christensen, E.; Levorsen, A.; Ose, L.; Hagve, T.A.; Christophersen, B.O. Dietary n-6 fatty acids inhibit the incorporation of dietary n-3 fatty acids in thrombocyte and serum phospholipids in humans: A controlled dietetic study. Scand. J. Clin. Lab. Investig. 1991, 51, 255–263. [Google Scholar] [CrossRef]
- Huerta-Yépez, S.; Tirado-Rodriguez, A.B.; Hankinson, O. Role of diets rich in omega-3 and omega-6 in the development of cancer. Bol. Med. Hosp. Infant. Mex. 2016, 73, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Lotfi, K.; Salari-Moghaddam, A.; Yousefinia, M.; Larijani, B.; Esmaillzadeh, A. Dietary intakes of monounsaturated fatty acids and risk of mortality from all causes, cardiovascular disease and cancer: A systematic review and dose-response meta-analysis of prospective cohort studies. Ageing Res. Rev. 2021, 72, 101467. [Google Scholar] [CrossRef] [PubMed]
- Zong, G.; Li, Y.; Sampson, L.; Dougherty, L.W.; Willett, W.C.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Hu, F.B.; Sun, Q. Monounsaturated fats from plant and animal sources in relation to risk of coronary heart disease among US men and women. Am. J. Clin. Nutr. 2018, 107, 445–453. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Li, Y.; Willett, W.C.; Sun, Q.; Sampson, L.; Salas-Salvadó, J.; Martínez-González, M.A.; Stampfer, M.J.; Hu, F.B. Consumption of Olive Oil and Risk of Total and Cause-Specific Mortality among U.S. Adults. J. Am. Coll. Cardiol. 2022, 79, 101–112. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Hoffmann, G. Monounsaturated fatty acids, olive oil and health status: A systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014, 13, 154. [Google Scholar] [CrossRef]
- Micha, R.; Peñalvo, J.L.; Cudhea, F.; Imamura, F.; Rehm, C.D.; Mozaffarian, D. Association Between Dietary Factors and Mortality From Heart Disease, Stroke, and Type 2 Diabetes in the United States. JAMA 2017, 317, 912–924. [Google Scholar] [CrossRef]
- Bao, Y.; Han, J.; Hu, F.B.; Giovannucci, E.L.; Stampfer, M.J.; Willett, W.C.; Fuchs, C.S. Association of nut consumption with total and cause-specific mortality. N. Engl. J. Med. 2013, 369, 2001–2011. [Google Scholar] [CrossRef]
- Guasch-Ferré, M.; Zong, G.; Willett, W.C.; Zock, P.L.; Wanders, A.J.; Hu, F.B.; Sun, Q. Associations of Monounsaturated Fatty Acids from Plant and Animal Sources with Total and Cause-Specific Mortality in Two US Prospective Cohort Studies. Circ. Res. 2019, 124, 1266–1275. [Google Scholar] [CrossRef]
- Ros, E. Contrasting Effects on Mortality of Monounsaturated Fatty Acid Intake Depending on Vegetable or Animal Sources. Circ. Res. 2019, 124, 1154–1156. [Google Scholar] [CrossRef]
- Mohammadifard, N.; Mansourian, M.; Firouzi, S.; Taheri, M.; Haghighatdoost, F. Longitudinal association of dietary carbohydrate and the risk cardiovascular disease: A dose-response meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 6277–6292. [Google Scholar] [CrossRef]
- Liu, S. Intake of refined carbohydrates and whole grain foods in relation to risk of type 2 diabetes mellitus and coronary heart disease. J. Am. Coll. Nutr. 2002, 21, 298–306. [Google Scholar] [CrossRef]
- Ludwig, D.S. The glycemic index: Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA 2002, 287, 2414–2423. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Villegas, R.; Liu, S.; Gao, Y.T.; Yang, G.; Li, H.; Zheng, W.; Shu, X.O. Prospective study of dietary carbohydrates, glycemic index, glycemic load, and incidence of type 2 diabetes mellitus in middle-aged Chinese women. Arch. Intern. Med. 2007, 167, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.A.; Pan, A.; Malik, V.; Sun, Q. White rice consumption and risk of type 2 diabetes: Meta-analysis and systematic review. BMJ 2012, 344, e1454. [Google Scholar] [CrossRef] [PubMed]
- McKeown, N.M.; Meigs, J.B.; Liu, S.; Saltzman, E.; Wilson, P.W.; Jacques, P.F. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham Offspring Cohort. Diabetes Care 2004, 27, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Manson, J.E.; Buring, J.E.; Stampfer, M.J.; Willett, W.C.; Ridker, P.M. Relation between a diet with a high glycemic load and plasma concentrations of high-sensitivity C-reactive protein in middle-aged women. Am. J. Clin. Nutr. 2002, 75, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Mirrahimi, A.; de Souza, R.J.; Chiavaroli, L.; Sievenpiper, J.L.; Beyene, J.; Hanley, A.J.; Augustin, L.S.; Kendall, C.W.; Jenkins, D.J. Associations of glycemic index and load with coronary heart disease events: A systematic review and meta-analysis of prospective cohorts. J. Am. Heart Assoc. 2012, 1, e000752. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Song, Y.; Wang, Y.; Hui, R.; Zhang, W. Dietary glycemic index, glycemic load, and risk of coronary heart disease, stroke, and stroke mortality: A systematic review with meta-analysis. PLoS ONE 2012, 7, e52182. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.A.; Meilahn, E.; Kuller, L.H.; Kelsey, S.F.; Caggiula, A.W.; Wing, R.R. Menopause and risk factors for coronary heart disease. N. Engl. J. Med. 1989, 321, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Levitan, E.B.; Cook, N.R.; Stampfer, M.J.; Ridker, P.M.; Rexrode, K.M.; Buring, J.E.; Manson, J.E.; Liu, S. Dietary glycemic index, dietary glycemic load, blood lipids, and C-reactive protein. Metabolism 2008, 57, 437–443. [Google Scholar] [CrossRef] [PubMed]
- McGee, D.L.; Reed, D.M.; Yano, K.; Kagan, A.; Tillotson, J. Ten-year incidence of coronary heart disease in the Honolulu Heart Program: Relationship to nutrient intake. Am. J. Epidemiol. 1984, 119, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Posner, B.M.; Cobb, J.L.; Belanger, A.J.; Cupples, L.A.; D’Agostino, R.B.; Stokes, J., 3rd. Dietary lipid predictors of coronary heart disease in men. The Framingham Study. Arch. Intern. Med. 1991, 151, 1181–1187. [Google Scholar] [CrossRef]
- Fehily, A.M.; Yarnell, J.W.; Sweetnam, P.M.; Elwood, P.C. Diet and incident ischaemic heart disease: The Caerphilly Study. Br. J. Nutr. 1993, 69, 303–314. [Google Scholar] [CrossRef]
- Rohan, T.E.; Hiller, J.E.; McMichael, A.J. Dietary factors and survival from breast cancer. Nutr. Cancer 1993, 20, 167–177. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Madans, J.H.; Turnbull, B.; Cornoni-Huntley, J.; Dresser, C.; Everett, D.F.; Perrone, R.D. Diet, indicators of kidney disease, and later mortality among older persons in the NHANES I Epidemiologic Follow-Up Study. Am. J. Public. Health 1994, 84, 1299–1303. [Google Scholar] [CrossRef]
- Ascherio, A.; Rimm, E.B.; Stampfer, M.J.; Giovannucci, E.L.; Willett, W.C. Dietary intake of marine n-3 fatty acids, fish intake, and the risk of coronary disease among men. N. Engl. J. Med. 1995, 332, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Gillman, M.W.; Cupples, L.A.; Millen, B.E.; Ellison, R.C.; Wolf, P.A. Inverse association of dietary fat with development of ischemic stroke in men. JAMA 1997, 278, 2145–2150. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.; Colditz, G.A.; Rosner, B.A.; Hennekens, C.H.; Willett, W.C. Dietary fat intake and the risk of coronary heart disease in women. N. Engl. J. Med. 1997, 337, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Seino, F.; Date, C.; Nakayama, T.; Yoshiike, N.; Yokoyama, T.; Yamaguchi, M.; Tanaka, H. Dietary lipids and incidence of cerebral infarction in a Japanese rural community. J. Nutr. Sci. Vitaminol. 1997, 43, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.D.; Stampfer, M.J.; Colditz, G.A.; Rosner, B.; Hunter, D.J.; Willett, W.C. Dietary factors and the survival of women with breast carcinoma. Cancer 1999, 86, 826–835. [Google Scholar] [CrossRef]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Ascherio, A.; Colditz, G.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Am. J. Clin. Nutr. 1999, 70, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Stampfer, M.J.; Manson, J.E.; Rimm, E.; Colditz, G.A.; Speizer, F.E.; Hennekens, C.H.; Willett, W.C. Dietary protein and risk of ischemic heart disease in women. Am. J. Clin. Nutr. 1999, 70, 221–227. [Google Scholar] [CrossRef]
- Payette, H.; Coulombe, C.; Boutier, V.; Gray-Donald, K. Weight loss and mortality among free-living frail elders: A prospective study. J. Gerontol. A Biol. Sci. Med. Sci. 1999, 54, M440–M445. [Google Scholar] [CrossRef]
- Liu, S.; Willett, W.C.; Stampfer, M.J.; Hu, F.B.; Franz, M.; Sampson, L.; Hennekens, C.H.; Manson, J.E. A prospective study of dietary glycemic load, carbohydrate intake, and risk of coronary heart disease in US women. Am. J. Clin. Nutr. 2000, 71, 1455–1461. [Google Scholar] [CrossRef]
- Palli, D.; Russo, A.; Saieva, C.; Salvini, S.; Amorosi, A.; Decarli, A. Dietary and familial determinants of 10-year survival among patients with gastric carcinoma. Cancer 2000, 89, 1205–1213. [Google Scholar] [CrossRef]
- Iso, H.; Stampfer, M.J.; Manson, J.E.; Rexrode, K.; Hu, F.; Hennekens, C.H.; Colditz, G.A.; Speizer, F.E.; Willett, W.C. Prospective study of fat and protein intake and risk of intraparenchymal hemorrhage in women. Circulation 2001, 103, 856–863. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Merchant, A.; Rimm, E.B.; Rosner, B.A.; Stampfer, M.J.; Willett, W.C.; Ascherio, A. Dietary fat intake and risk of stroke in male US healthcare professionals: 14 year prospective cohort study. BMJ 2003, 327, 777–782. [Google Scholar] [CrossRef]
- Hu, F.B.; Cho, E.; Rexrode, K.M.; Albert, C.M.; Manson, J.E. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation 2003, 107, 1852–1857. [Google Scholar] [CrossRef] [PubMed]
- Iso, H.; Sato, S.; Kitamura, A.; Naito, Y.; Shimamoto, T.; Komachi, Y. Fat and protein intakes and risk of intraparenchymal hemorrhage among middle-aged Japanese. Am. J. Epidemiol. 2003, 157, 32–39. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, X.O.; Gao, Y.T.; Yang, G.; Li, Q.; Li, H.; Jin, F.; Zheng, W. Soy food consumption is associated with lower risk of coronary heart disease in Chinese women. J. Nutr. 2003, 133, 2874–2878. [Google Scholar] [CrossRef] [PubMed]
- Borugian, M.J.; Sheps, S.B.; Kim-Sing, C.; Van Patten, C.; Potter, J.D.; Dunn, B.; Gallagher, R.P.; Hislop, T.G. Insulin, macronutrient intake, and physical activity: Are potential indicators of insulin resistance associated with mortality from breast cancer? Cancer Epidemiol. Biomark. Prev. 2004, 13, 1163–1172. [Google Scholar] [CrossRef]
- Jakobsen, M.U.; Overvad, K.; Dyerberg, J.; Schroll, M.; Heitmann, B.L. Dietary fat and risk of coronary heart disease: Possible effect modification by gender and age. Am. J. Epidemiol. 2004, 160, 141–149. [Google Scholar] [CrossRef]
- Tanasescu, M.; Cho, E.; Manson, J.E.; Hu, F.B. Dietary fat and cholesterol and the risk of cardiovascular disease among women with type 2 diabetes. Am. J. Clin. Nutr. 2004, 79, 999–1005. [Google Scholar] [CrossRef]
- Kelemen, L.E.; Kushi, L.H.; Jacobs, D.R., Jr.; Cerhan, J.R. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. Am. J. Epidemiol. 2005, 161, 239–249. [Google Scholar] [CrossRef]
- Oh, K.; Hu, F.B.; Cho, E.; Rexrode, K.M.; Stampfer, M.J.; Manson, J.E.; Liu, S.; Willett, W.C. Carbohydrate intake, glycemic index, glycemic load, and dietary fiber in relation to risk of stroke in women. Am. J. Epidemiol. 2005, 161, 161–169. [Google Scholar] [CrossRef]
- Oh, K.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Willett, W.C. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the nurses’ health study. Am. J. Epidemiol. 2005, 161, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Trichopoulou, A.; Psaltopoulou, T.; Orfanos, P.; Trichopoulos, D. Diet and physical activity in relation to overall mortality amongst adult diabetics in a general population cohort. J. Intern. Med. 2006, 259, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Leosdottir, M.; Nilsson, P.M.; Nilsson, J.A.; Berglund, G. Cardiovascular event risk in relation to dietary fat intake in middle-aged individuals: Data from The Malmö Diet and Cancer Study. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Smit, E.; Garcia-Palmieri, M.R.; Figueroa, N.R.; McGee, D.L.; Messina, M.; Freudenheim, J.L.; Crespo, C.J. Protein and legume intake and prostate cancer mortality in Puerto Rican men. Nutr. Cancer 2007, 58, 146–152. [Google Scholar] [CrossRef]
- Boden-Albala, B.; Elkind, M.S.; White, H.; Szumski, A.; Paik, M.C.; Sacco, R.L. Dietary total fat intake and ischemic stroke risk: The Northern Manhattan Study. Neuroepidemiology 2009, 32, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Halbesma, N.; Bakker, S.J.; Jansen, D.F.; Stolk, R.P.; De Zeeuw, D.; De Jong, P.E.; Gansevoort, R.T. High protein intake associates with cardiovascular events but not with loss of renal function. J. Am. Soc. Nephrol. 2009, 20, 1797–1804. [Google Scholar] [CrossRef] [PubMed]
- Bates, C.J.; Mansoor, M.A.; Pentieva, K.D.; Hamer, M.; Mishra, G.D. Biochemical risk indices, including plasma homocysteine, that prospectively predict mortality in older British people: The National Diet and Nutrition Survey of People Aged 65 Years and Over. Br. J. Nutr. 2010, 104, 893–899. [Google Scholar] [CrossRef]
- Preis, S.R.; Stampfer, M.J.; Spiegelman, D.; Willett, W.C.; Rimm, E.B. Dietary protein and risk of ischemic heart disease in middle-aged men. Am. J. Clin. Nutr. 2010, 92, 1265–1272. [Google Scholar] [CrossRef]
- Yamagishi, K.; Iso, H.; Yatsuya, H.; Tanabe, N.; Date, C.; Kikuchi, S.; Yamamoto, A.; Inaba, Y.; Tamakoshi, A. Dietary intake of saturated fatty acids and mortality from cardiovascular disease in Japanese: The Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC) Study. Am. J. Clin. Nutr. 2010, 92, 759–765. [Google Scholar] [CrossRef]
- Atkinson, C.; Whitley, E.; Ness, A.; Baker, I. Associations between types of dietary fat and fish intake and risk of stroke in the Caerphilly Prospective Study (CaPS). Public Health 2011, 125, 345–348. [Google Scholar] [CrossRef]
- Houston, D.K.; Ding, J.; Lee, J.S.; Garcia, M.; Kanaya, A.M.; Tylavsky, F.A.; Newman, A.B.; Visser, M.; Kritchevsky, S.B. Dietary fat and cholesterol and risk of cardiovascular disease in older adults: The Health ABC Study. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Chiuve, S.E.; Rimm, E.B.; Sandhu, R.K.; Bernstein, A.M.; Rexrode, K.M.; Manson, J.E.; Willett, W.C.; Albert, C.M. Dietary fat quality and risk of sudden cardiac death in women. Am. J. Clin. Nutr. 2012, 96, 498–507. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Otto, M.C.; Mozaffarian, D.; Kromhout, D.; Bertoni, A.G.; Sibley, C.T.; Jacobs, D.R., Jr.; Nettleton, J.A. Dietary intake of saturated fat by food source and incident cardiovascular disease: The Multi-Ethnic Study of Atherosclerosis. Am. J. Clin. Nutr. 2012, 96, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C.; Virtamo, J.; Wolk, A. Dietary protein intake and risk of stroke in women. Atherosclerosis 2012, 224, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Nagata, C.; Nakamura, K.; Wada, K.; Oba, S.; Tsuji, M.; Tamai, Y.; Kawachi, T. Total fat intake is associated with decreased mortality in Japanese men but not in women. J. Nutr. 2012, 142, 1713–1719. [Google Scholar] [CrossRef]
- Wallström, P.; Sonestedt, E.; Hlebowicz, J.; Ericson, U.; Drake, I.; Persson, M.; Gullberg, B.; Hedblad, B.; Wirfält, E. Dietary fiber and saturated fat intake associations with cardiovascular disease differ by sex in the Malmö Diet and Cancer Cohort: A prospective study. PLoS ONE 2012, 7, e31637. [Google Scholar] [CrossRef]
- Yaemsiri, S.; Sen, S.; Tinker, L.; Rosamond, W.; Wassertheil-Smoller, S.; He, K. Trans fat, aspirin, and ischemic stroke in postmenopausal women. Ann. Neurol. 2012, 72, 704–715. [Google Scholar] [CrossRef]
- Argos, M.; Melkonian, S.; Parvez, F.; Rakibuz-Zaman, M.; Ahmed, A.; Chen, Y.; Ahsan, H. A population-based prospective study of energy-providing nutrients in relation to all-cause cancer mortality and cancers of digestive organs mortality. Int. J. Cancer 2013, 133, 2422–2428. [Google Scholar] [CrossRef]
- Similä, M.E.; Kontto, J.P.; Männistö, S.; Valsta, L.M.; Virtamo, J. Glycaemic index, carbohydrate substitution for fat and risk of CHD in men. Br. J. Nutr. 2013, 110, 1704–1711. [Google Scholar] [CrossRef]
- Yamagishi, K.; Iso, H.; Kokubo, Y.; Saito, I.; Yatsuya, H.; Ishihara, J.; Inoue, M.; Tsugane, S. Dietary intake of saturated fatty acids and incident stroke and coronary heart disease in Japanese communities: The JPHC Study. Eur. Heart J. 2013, 34, 1225–1232. [Google Scholar] [CrossRef]
- Yu, D.; Shu, X.O.; Li, H.; Xiang, Y.B.; Yang, G.; Gao, Y.T.; Zheng, W.; Zhang, X. Dietary carbohydrates, refined grains, glycemic load, and risk of coronary heart disease in Chinese adults. Am. J. Epidemiol. 2013, 178, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.L.; Weiss, T.; Duarte, C.K.; Gross, J.L.; de Azevedo, M.J.; Zelmanovitz, T. Dietary fat composition and cardiac events in patients with type 2 diabetes. Atherosclerosis 2014, 236, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Haring, B.; Gronroos, N.; Nettleton, J.A.; von Ballmoos, M.C.; Selvin, E.; Alonso, A. Dietary protein intake and coronary heart disease in a large community based cohort: Results from the Atherosclerosis Risk in Communities (ARIC) study [corrected]. PLoS ONE 2014, 9, e109552. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, N.; Miura, K.; Okuda, N.; Kadowaki, T.; Takashima, N.; Nagasawa, S.Y.; Nakamura, Y.; Matsumura, Y.; Hozawa, A.; Fujiyoshi, A.; et al. Long-chain n-3 polyunsaturated fatty acids intake and cardiovascular disease mortality risk in Japanese: A 24-year follow-up of NIPPON DATA80. Atherosclerosis 2014, 232, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Rebello, S.A.; Koh, H.; Chen, C.; Naidoo, N.; Odegaard, A.O.; Koh, W.P.; Butler, L.M.; Yuan, J.M.; van Dam, R.M. Amount, type, and sources of carbohydrates in relation to ischemic heart disease mortality in a Chinese population: A prospective cohort study. Am. J. Clin. Nutr. 2014, 100, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, J.K.; Mursu, J.; Tuomainen, T.P.; Voutilainen, S. Dietary fatty acids and risk of coronary heart disease in men: The Kuopio Ischemic Heart Disease Risk Factor Study. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 2679–2687. [Google Scholar] [CrossRef]
- Campmans-Kuijpers, M.J.; Sluijs, I.; Nöthlings, U.; Freisling, H.; Overvad, K.; Weiderpass, E.; Fagherazzi, G.; Kühn, T.; Katzke, V.A.; Mattiello, A.; et al. Isocaloric substitution of carbohydrates with protein: The association with weight change and mortality among patients with type 2 diabetes. Cardiovasc. Diabetol. 2015, 14, 39. [Google Scholar] [CrossRef]
- Chiuve, S.E.; Sandhu, R.K.; Moorthy, M.V.; Glynn, R.J.; Albert, C.M. Dietary Fat Intake Is Differentially Associated with Risk of Paroxysmal Compared with Sustained Atrial Fibrillation in Women. J. Nutr. 2015, 145, 2092–2101. [Google Scholar] [CrossRef]
- Li, Y.; Hruby, A.; Bernstein, A.M.; Ley, S.H.; Wang, D.D.; Chiuve, S.E.; Sampson, L.; Rexrode, K.M.; Rimm, E.B.; Willett, W.C.; et al. Saturated Fats Compared with Unsaturated Fats and Sources of Carbohydrates in Relation to Risk of Coronary Heart Disease: A Prospective Cohort Study. J. Am. Coll. Cardiol. 2015, 66, 1538–1548. [Google Scholar] [CrossRef]
- Nagata, C.; Wada, K.; Tamura, T.; Kawachi, T.; Konishi, K.; Tsuji, M.; Nakamura, K. Dietary intakes of glutamic acid and glycine are associated with stroke mortality in Japanese adults. J. Nutr. 2015, 145, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Puaschitz, N.G.; Strand, E.; Norekvål, T.M.; Dierkes, J.; Dahl, L.; Svingen, G.F.; Assmus, J.; Schartum-Hansen, H.; Øyen, J.; Pedersen, E.K.; et al. Dietary intake of saturated fat is not associated with risk of coronary events or mortality in patients with established coronary artery disease. J. Nutr. 2015, 145, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, Y.; Sun, Q.; Pan, A.; Manson, J.E.; Rexrode, K.M.; Willett, W.C.; Rimm, E.B.; Hu, F.B. Dairy fat and risk of cardiovascular disease in 3 cohorts of US adults. Am. J. Clin. Nutr. 2016, 104, 1209–1217. [Google Scholar] [CrossRef] [PubMed]
- Courand, P.Y.; Lesiuk, C.; Milon, H.; Defforges, A.; Fouque, D.; Harbaoui, B.; Lantelme, P. Association Between Protein Intake and Mortality in Hypertensive Patients Without Chronic Kidney Disease in the OLD-HTA Cohort. Hypertension 2016, 67, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Alonso, P.; Salas-Salvadó, J.; Ruiz-Canela, M.; Corella, D.; Estruch, R.; Fitó, M.; Arós, F.; Gómez-Gracia, E.; Fiol, M.; Lapetra, J.; et al. High dietary protein intake is associated with an increased body weight and total death risk. Clin. Nutr. 2016, 35, 496–506. [Google Scholar] [CrossRef]
- Owen, A.J.; Magliano, D.J.; O’Dea, K.; Barr, E.L.; Shaw, J.E. Polyunsaturated fatty acid intake and risk of cardiovascular mortality in a low fish-consuming population: A prospective cohort analysis. Eur. J. Nutr. 2016, 55, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Praagman, J.; Beulens, J.W.; Alssema, M.; Zock, P.L.; Wanders, A.J.; Sluijs, I.; van der Schouw, Y.T. The association between dietary saturated fatty acids and ischemic heart disease depends on the type and source of fatty acid in the European Prospective Investigation into Cancer and Nutrition-Netherlands cohort. Am. J. Clin. Nutr. 2016, 103, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of Animal and Plant Protein Intake with All-Cause and Cause-Specific Mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef]
- Xu, H.; Rossi, M.; Campbell, K.L.; Sencion, G.L.; Ärnlöv, J.; Cederholm, T.; Sjögren, P.; Risérus, U.; Lindholm, B.; Carrero, J.J. Excess protein intake relative to fiber and cardiovascular events in elderly men with chronic kidney disease. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 597–602. [Google Scholar] [CrossRef]
- Zong, G.; Li, Y.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Willett, W.C.; Hu, F.B.; Sun, Q. Intake of individual saturated fatty acids and risk of coronary heart disease in US men and women: Two prospective longitudinal cohort studies. BMJ 2016, 355, i5796. [Google Scholar] [CrossRef]
- Holmes, M.D.; Wang, J.; Hankinson, S.E.; Tamimi, R.M.; Chen, W.Y. Protein Intake and Breast Cancer Survival in the Nurses’ Health Study. J. Clin. Oncol. 2017, 35, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Rhee, J.J.; Kim, E.; Buring, J.E.; Kurth, T. Fish Consumption, Omega-3 Fatty Acids, and Risk of Cardiovascular Disease. Am. J. Prev. Med. 2017, 52, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Zaslavsky, O.; Zelber-Sagi, S.; Hebert, J.R.; Steck, S.E.; Shivappa, N.; Tabung, F.K.; Wirth, M.D.; Bu, Y.; Shikany, J.M.; Orchard, T.; et al. Biomarker-calibrated nutrient intake and healthy diet index associations with mortality risks among older and frail women from the Women’s Health Initiative. Am. J. Clin. Nutr. 2017, 105, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- AlEssa, H.B.; Cohen, R.; Malik, V.S.; Adebamowo, S.N.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Hu, F.B. Carbohydrate quality and quantity and risk of coronary heart disease among US women and men. Am. J. Clin. Nutr. 2018, 107, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Arthur, A.E.; Goss, A.M.; Demark-Wahnefried, W.; Mondul, A.M.; Fontaine, K.R.; Chen, Y.T.; Carroll, W.R.; Spencer, S.A.; Rogers, L.Q.; Rozek, L.S.; et al. Higher carbohydrate intake is associated with increased risk of all-cause and disease-specific mortality in head and neck cancer patients: Results from a prospective cohort study. Int. J. Cancer 2018, 143, 1105–1113. [Google Scholar] [CrossRef]
- Ricci, C.; Baumgartner, J.; Zec, M.; Kruger, H.S.; Smuts, C.M. Type of dietary fat intakes in relation to all-cause and cause-specific mortality in US adults: An iso-energetic substitution analysis from the American National Health and Nutrition Examination Survey linked to the US mortality registry. Br. J. Nutr. 2018, 119, 456–463. [Google Scholar] [CrossRef]
- Song, M.; Wu, K.; Meyerhardt, J.A.; Yilmaz, O.; Wang, M.; Ogino, S.; Fuchs, C.S.; Giovannucci, E.L.; Chan, A.T. Low-Carbohydrate Diet Score and Macronutrient Intake in Relation to Survival After Colorectal Cancer Diagnosis. JNCI Cancer Spectr. 2018, 2, pky077. [Google Scholar] [CrossRef]
- Tharrey, M.; Mariotti, F.; Mashchak, A.; Barbillon, P.; Delattre, M.; Fraser, G.E. Patterns of plant and animal protein intake are strongly associated with cardiovascular mortality: The Adventist Health Study-2 cohort. Int. J. Epidemiol. 2018, 47, 1603–1612. [Google Scholar] [CrossRef]
- Chan, R.; Leung, J.; Woo, J. High Protein Intake Is Associated with Lower Risk of All-Cause Mortality in Community-Dwelling Chinese Older Men and Women. J. Nutr. Health Aging 2019, 23, 987–996. [Google Scholar] [CrossRef]
- Jiao, J.; Liu, G.; Shin, H.J.; Hu, F.B.; Rimm, E.B.; Rexrode, K.M.; Manson, J.E.; Zong, G.; Sun, Q. Dietary fats and mortality among patients with type 2 diabetes: Analysis in two population based cohort studies. BMJ 2019, 366, l4009. [Google Scholar] [CrossRef]
- Kurihara, A.; Okamura, T.; Sugiyama, D.; Higashiyama, A.; Watanabe, M.; Okuda, N.; Kadota, A.; Miyagawa, N.; Fujiyoshi, A.; Yoshita, K.; et al. Vegetable Protein Intake was Inversely Associated with Cardiovascular Mortality in a 15-Year Follow-Up Study of the General Japanese Population. J. Atheroscler. Thromb. 2019, 26, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Mazidi, M.; Katsiki, N.; Mikhailidis, D.P.; Sattar, N.; Banach, M. Lower carbohydrate diets and all-cause and cause-specific mortality: A population-based cohort study and pooling of prospective studies. Eur. Heart J. 2019, 40, 2870–2879. [Google Scholar] [CrossRef] [PubMed]
- Okada, C.; Imano, H.; Yamagishi, K.; Cui, R.; Umesawa, M.; Maruyama, K.; Muraki, I.; Hayama-Terada, M.; Shimizu, Y.; Sankai, T.; et al. Dietary Intake of Energy and Nutrients from Breakfast and Risk of Stroke in The Japanese Population: The Circulatory Risk in Communities Study (CIRCS). J. Atheroscler. Thromb. 2019, 26, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Ho, F.K.; Gray, S.R.; Welsh, P.; Petermann-Rocha, F.; Foster, H.; Waddell, H.; Anderson, J.; Lyall, D.; Sattar, N.; Gill, J.M.R.; et al. Associations of fat and carbohydrate intake with cardiovascular disease and mortality: Prospective cohort study of UK Biobank participants. BMJ 2020, 368, m688. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Liao, L.M.; Weinstein, S.J.; Sinha, R.; Graubard, B.I.; Albanes, D. Association Between Plant and Animal Protein Intake and Overall and Cause-Specific Mortality. JAMA Intern. Med. 2020, 180, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Langsetmo, L.; Harrison, S.; Jonnalagadda, S.; Pereira, S.L.; Shikany, J.M.; Farsijani, S.; Lane, N.E.; Cauley, J.A.; Stone, K.; Cawthon, P.M. Low Protein Intake Irrespective of Source is Associated with Higher Mortality among Older Community-dwelling Men. J. Nutr. Health Aging 2020, 24, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Lelli, D.; Antonelli Incalzi, R.; Ferrucci, L.; Bandinelli, S.; Pedone, C. Association between PUFA intake and serum concentration and mortality in older adults: A cohort study. Clin. Nutr. 2020, 39, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Liu, C.S.; Li, C.I.; Lin, C.H.; Lin, W.Y.; Wang, M.C.; Yang, S.Y.; Li, T.C. Dietary Macronutrient Intakes and Mortality among Patients with Type 2 Diabetes. Nutrients 2020, 12, 1665. [Google Scholar] [CrossRef]
- Mao, L.; Zhang, Y.; Wang, W.; Zhuang, P.; Wu, F.; Jiao, J. Plant-sourced and animal-sourced monounsaturated fatty acid intakes in relation to mortality: A prospective nationwide cohort study. Eur. J. Nutr. 2020, 59, 1989–1998. [Google Scholar] [CrossRef]
- Mendonça, N.; Kingston, A.; Granic, A.; Hill, T.R.; Mathers, J.C.; Jagger, C. Contribution of protein intake and its interaction with physical activity to transitions between disability states and to death in very old adults: The Newcastle 85+ Study. Eur. J. Nutr. 2020, 59, 1909–1918. [Google Scholar] [CrossRef]
- Mirmiran, P.; Houshialsadat, Z.; Bahadoran, Z.; Khalili-Moghadam, S.; Sheikholeslami, F.; Azizi, F. Association of dietary fatty acids and the incidence risk of cardiovascular disease in adults: The Tehran Lipid and Glucose Prospective Study. BMC Public Health 2020, 20, 1743. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, I.; Miura, K.; Miyagawa, N.; Kondo, K.; Kadota, A.; Okuda, N.; Fujiyoshi, A.; Chihara, I.; Nakamura, Y.; Hozawa, A.; et al. Relationship between carbohydrate and dietary fibre intake and the risk of cardiovascular disease mortality in Japanese: 24-year follow-up of NIPPON DATA80. Eur. J. Clin. Nutr. 2020, 74, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Shan, Z.; Guo, Y.; Hu, F.B.; Liu, L.; Qi, Q. Association of Low-Carbohydrate and Low-Fat Diets with Mortality among US Adults. JAMA Intern. Med. 2020, 180, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, M.; Krogh, V.; Grioni, S.; Farinaro, E. Mediterranean diet and all-cause mortality: A cohort of Italian men. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1673–1678. [Google Scholar] [CrossRef] [PubMed]
- Akter, S.; Mizoue, T.; Nanri, A.; Goto, A.; Noda, M.; Sawada, N.; Yamaji, T.; Iwasaki, M.; Inoue, M.; Tsugane, S. Low carbohydrate diet and all cause and cause-specific mortality. Clin. Nutr. 2021, 40, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Hyun, D.S.; Koh, S.B.; Lee, J.W. Differential relationship between dietary fat and cholesterol on total mortality in Korean population cohorts. J. Intern. Med. 2021, 290, 866–877. [Google Scholar] [CrossRef]
- Sadeghi, M.; Simani, M.; Mohammadifard, N.; Talaei, M.; Roohafza, H.; Hassannejad, R.; Sarrafzadegan, N. Longitudinal association of dietary fat intake with cardiovascular events in a prospective cohort study in Eastern Mediterranean region. Int. J. Food Sci. Nutr. 2021, 72, 1095–1104. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, B.; Snetselaar, L.G.; Wallace, R.B.; Shadyab, A.H.; Kroenke, C.H.; Haring, B.; Howard, B.V.; Shikany, J.M.; Valdiviezo, C.; et al. Association of Major Dietary Protein Sources with All-Cause and Cause-Specific Mortality: Prospective Cohort Study. J. Am. Heart Assoc. 2021, 10, e015553. [Google Scholar] [CrossRef]
- Yao, X.; Xu, X.; Wang, S.; Xia, D. Associations of Dietary Fat Intake with Mortality From All Causes, Cardiovascular Disease, and Cancer: A Prospective Study. Front. Nutr. 2021, 8, 701430. [Google Scholar] [CrossRef]
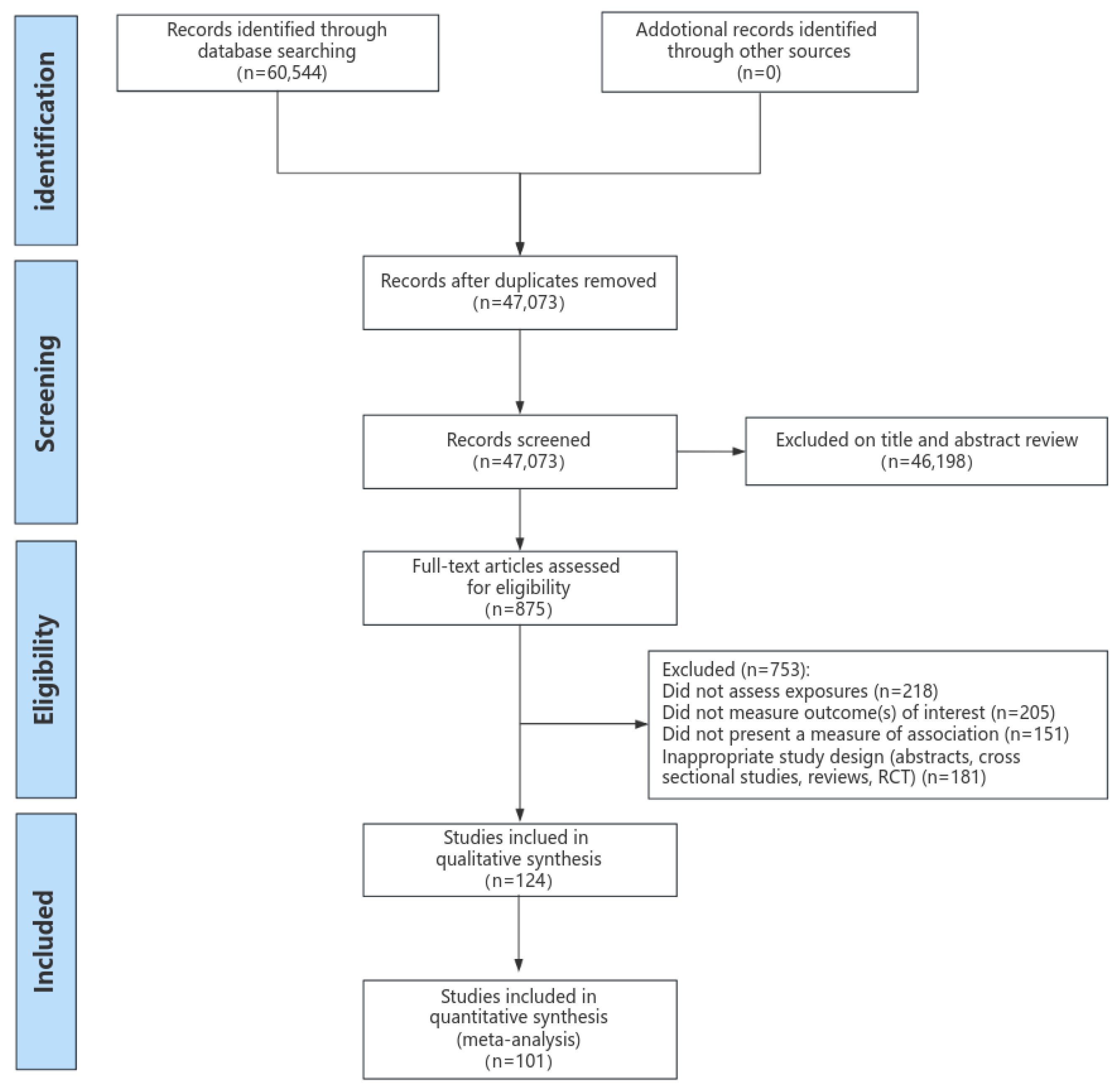



| Characteristic | All-Cause Mortality and Cause-Specific Mortality | CVD Morbidity | ||||
|---|---|---|---|---|---|---|
| Protein | Fats | Carbohydrate | Protein | Fats | Carbohydrate | |
| Number of reports | 40 | 46 | 19 | 10 | 44 | 9 |
| Study location * | USA (17), Canada (3), Australia (1), Italy (3), Japan (4), Sweden (2), Netherlands (2), UK (2), Bangladesh (1), France (1), Spain (1), Finland (1), China (1) | USA (20), Australia (2), UK (4), Finland (1), Italy (4), Canada (1), Japan (5), Sweden (1), Greece (2), China (3), Korea (2), Bangladesh (1), Norway (1), Spain (1) | USA (4), Sweden (4), Japan (1) | USA (4), Sweden (4), Japan (1) | USA (25), Japan (6), Sweden (5) Finland (2), Iran (2), Greece (2), UK (1), Israel (1), Denmark (1), Finland (1), Norway (1), Spain (1), Holland (1) | USA (6), Sweden (2), Finland (1), China (1), Japan (1) |
| Sample size (range) | 36,035 (278 to 416,104) | 39,484 (278 to 521,120) | 34,895 (278 to 135,335) | 4424 (13 to 135,335) | 32,476 (227 to 135,335) | 58,191 (3248 to 58,191) |
| Baseline age (range) | 20–74 | 20–80 | 20–86 y | 40–83 y | 34–79 y | 38–75 y |
| Duration (range) | 2.2–26 y | 4.8–30 y | 2.2–26 y | 2.5–24.6 | 4.6–30 y | 5.4–30 y |
| Dietary assessment method | FFQ (28), 24 h recall (10), food record (2) | FFQ (32), 24 h recall (12), food record (2) | FFQ (13), 24 h recall (6) | FFQ (7), 24 h recall (1), record (2) | FFQ (34), 24 h recall (5), record (5) | FFQ (7), 24 h recall (1), record (1) |
| Outcome assessment method | Confirmed or conducted by investigators (40) | Confirmed or conducted by investigators (46) | Confirmed or conducted by investigators (19) | Conducted by investigators (7), self-reported (3) | Conducted by investigators (25), self-reported (19) | Conducted by investigators (4), self-reported (5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Zheng, Z.; Zhuang, L.; Wang, H.; Li, A.; Chen, L.; Liu, L. Dietary Macronutrient Intake and Cardiovascular Disease Risk and Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Nutrients 2024, 16, 152. https://doi.org/10.3390/nu16010152
Ma Y, Zheng Z, Zhuang L, Wang H, Li A, Chen L, Liu L. Dietary Macronutrient Intake and Cardiovascular Disease Risk and Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Nutrients. 2024; 16(1):152. https://doi.org/10.3390/nu16010152
Chicago/Turabian StyleMa, Yibin, Zekun Zheng, Litao Zhuang, Huiting Wang, Anni Li, Liangkai Chen, and Liegang Liu. 2024. "Dietary Macronutrient Intake and Cardiovascular Disease Risk and Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies" Nutrients 16, no. 1: 152. https://doi.org/10.3390/nu16010152
APA StyleMa, Y., Zheng, Z., Zhuang, L., Wang, H., Li, A., Chen, L., & Liu, L. (2024). Dietary Macronutrient Intake and Cardiovascular Disease Risk and Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. Nutrients, 16(1), 152. https://doi.org/10.3390/nu16010152





