Compositional Aspects of Beverages Designed to Promote Hydration Before, During, and After Exercise: Concepts Revisited
Abstract
:1. Introduction
2. Materials and Methods
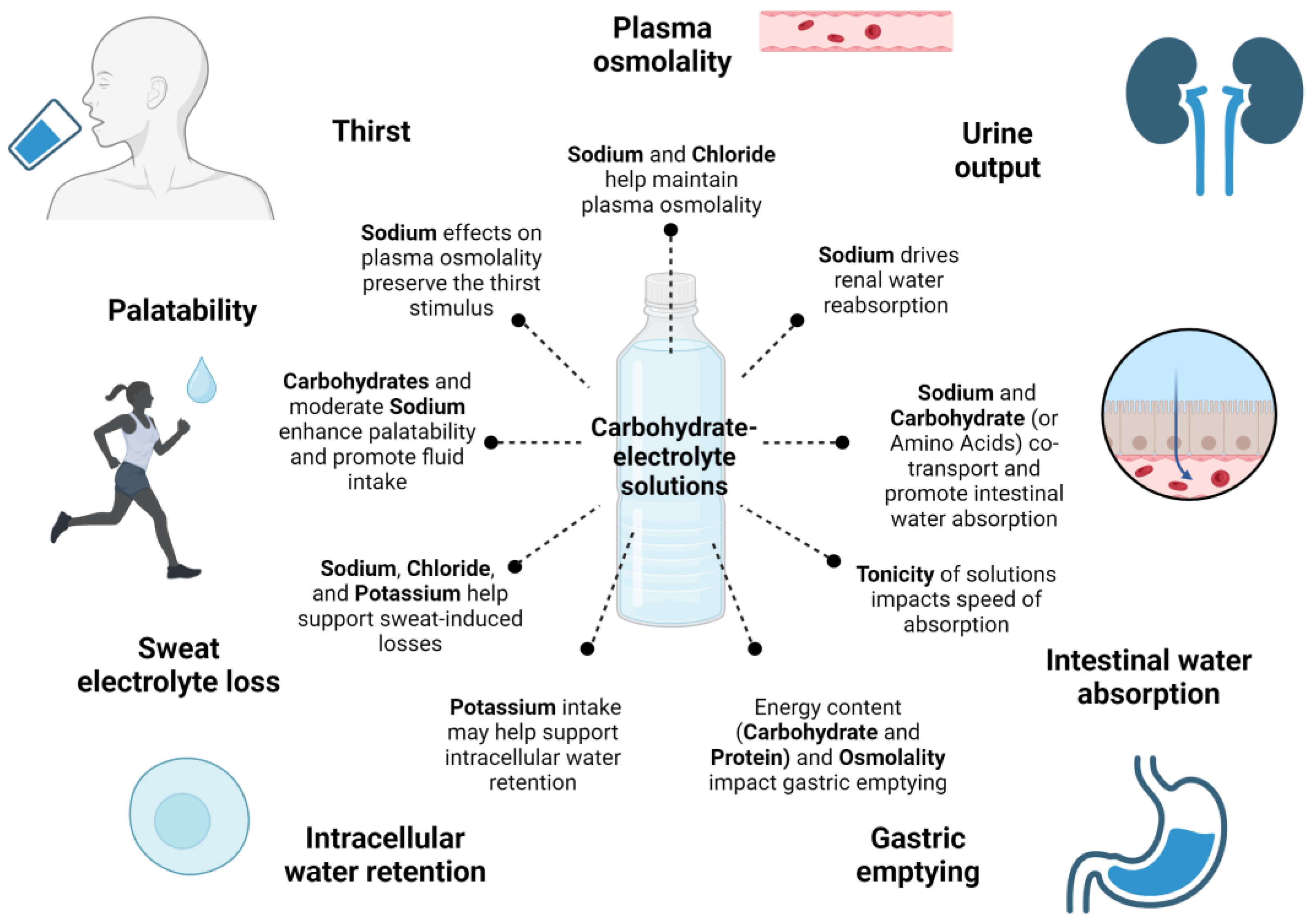
3. Compositional Aspects of Carbohydrate–Electrolyte Solutions
3.1. Electrolytes
3.2. Carbohydrates
3.3. Osmolality
3.4. Other Potential Ingredients
4. Impact of Exercise Timing on Carbohydrate–Electrolyte Solutions
4.1. Before Exercise
4.2. During Exercise
4.3. After Exercise
5. Outlook to Hypotonic Beverages in Sports
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, L.E.; Johnson, E.C. Water Intake, Water Balance, and the Elusive Daily Water Requirement. Nutrients 2018, 10, 1928. [Google Scholar] [CrossRef]
- McDermott, B.P.; Anderson, S.A.; Armstrong, L.E.; Casa, D.J.; Cheuvront, S.N.; Cooper, L.; Kenney, W.L.; O’Connor, F.G.; Roberts, W.O. National Athletic Trainers’ Association Position Statement: Fluid Replacement for the Physically Active. J. Athl. Train. 2017, 52, 877–895. [Google Scholar] [CrossRef] [PubMed]
- Horswill, C.; Fransen, J. Body Water: Balance, Turnover, Regulation, and Evaluation. In Fluid Balance, Hydration, and Athletic Performance; CRC Press: Boca Raton, FL, USA, 2016; pp. 3–22. [Google Scholar]
- Barley, O.R.; Chapman, D.W.; Abbiss, C.R. Reviewing the current methods of assessing hydration in athletes. J. Int. Soc. Sports Nutr. 2020, 17, 52. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar] [CrossRef] [PubMed]
- Cheuvront, S.N.; Kenefick, R.W. Dehydration: Physiology, assessment, and performance effects. Compr. Physiol. 2014, 4, 257–285. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N.; Cheuvront, S.N.; Kenefick, R.W. Hypohydration and Human Performance: Impact of Environment and Physiological Mechanisms. Sports Med. 2015, 45 (Suppl. S1), S51–S60. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Maresh, C.M.; Armstrong, L.E.; Gabaree, C.L.; Bergeron, M.F.; Kenefick, R.W.; Castellani, J.W.; Ahlquist, L.E.; Ward, A. Effects of hydration state on plasma testosterone, cortisol and catecholamine concentrations before and during mild exercise at elevated temperature. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 294–300. [Google Scholar] [CrossRef] [PubMed]
- López-Torres, O.; Rodríguez-Longobardo, C.; Escribano-Tabernero, R.; Fernández-Elías, V.E. Hydration, Hyperthermia, Glycogen, and Recovery: Crucial Factors in Exercise Performance—A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 4442. [Google Scholar] [CrossRef] [PubMed]
- Sawka, M.N.; Burke, L.M.; Eichner, E.R.; Maughan, R.J.; Montain, S.J.; Stachenfeld, N.S. American College of Sports Medicine position stand. Exercise and fluid replacement. Med. Sci. Sports Exerc. 2007, 39, 377–390. [Google Scholar] [CrossRef]
- McCubbin, A.J.; Allanson, B.A.; Caldwell Odgers, J.N.; Cort, M.M.; Costa, R.J.S.; Cox, G.R.; Crawshay, S.T.; Desbrow, B.; Freney, E.G.; Gaskell, S.K.; et al. Sports Dietitians Australia Position Statement: Nutrition for Exercise in Hot Environments. Int. J. Sport Nutr. Exerc. Metab. 2020, 30, 83–98. [Google Scholar] [CrossRef]
- Racinais, S.; Hosokawa, Y.; Akama, T.; Bermon, S.; Bigard, X.; Casa, D.J.; Grundstein, A.; Jay, O.; Massey, A.; Migliorini, S.; et al. IOC consensus statement on recommendations and regulations for sport events in the heat. Br. J. Sports Med. 2023, 57, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Lara, B.; Gallo-Salazar, C.; Puente, C.; Areces, F.; Salinero, J.J.; Del Coso, J. Interindividual variability in sweat electrolyte concentration in marathoners. J. Int. Soc. Sports Nutr. 2016, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, Y.; Pandolf, K.B.; Goldman, R.F. Predicting sweat loss response to exercise, environment and clothing. Eur. J. Appl. Physiol. Occup. Physiol. 1982, 48, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Buono, M.J.; Ball, K.D.; Kolkhorst, F.W. Sodium ion concentration vs. sweat rate relationship in humans. J. Appl. Physiol. 2007, 103, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Montain, S.J.; Cheuvront, S.N.; Sawka, M.N. Exercise associated hyponatraemia: Quantitative analysis to understand the aetiology. Br. J. Sports Med. 2006, 40, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Montain, S.J.; Sawka, M.N.; Wenger, C.B. Hyponatremia Associated with Exercise: Risk Factors and Pathogenesis. Exerc. Sport Sci. Rev. 2001, 29, 113–117. [Google Scholar] [CrossRef]
- Vist, G.E.; Maughan, R.J. The effect of osmolality and carbohydrate content on the rate of gastric emptying of liquids in man. J. Physiol. 1995, 486 Pt 2, 523–531. [Google Scholar] [CrossRef]
- Gisolfi, C.V.; Summers, R.W.; Schedl, H.P.; Bleiler, T.L. Intestinal water absorption from select carbohydrate solutions in humans. J. Appl. Physiol. 1992, 73, 2142–2150. [Google Scholar] [CrossRef]
- Okabe, T.; Terashima, H.; Sakamoto, A. Determinants of liquid gastric emptying: Comparisons between milk and isocalorically adjusted clear fluids. BJA Br. J. Anaesth. 2014, 114, 77–82. [Google Scholar] [CrossRef]
- James, L.J.; Clayton, D.; Evans, G.H. Effect of milk protein addition to a carbohydrate-electrolyte rehydration solution ingested after exercise in the heat. Br. J. Nutr. 2011, 105, 393–399. [Google Scholar] [CrossRef]
- Evans, G.H.; Shirreffs, S.M.; Maughan, R.J. Postexercise rehydration in man: The effects of osmolality and carbohydrate content of ingested drinks. Nutrition 2009, 25, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Murray, R. Sports Drinks: Basic Science and Practical Aspects; CRC Press: Boca Raton, FL, USA, 2000. [Google Scholar]
- Shirreffs, S.M. The optimal sports drink. Schweiz. Z. Sportmed. Sport. 2003, 51, 25–30. [Google Scholar]
- Evans, G.H.; James, L.J.; Shirreffs, S.M.; Maughan, R.J. Optimizing the restoration and maintenance of fluid balance after exercise-induced dehydration. J. Appl. Physiol. 2017, 122, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; Jeukendrup, A.E. Optimal composition of fluid-replacement beverages. Compr. Physiol. 2014, 4, 575–620. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Watson, P.; Cordery, P.A.; Walsh, N.P.; Oliver, S.J.; Dolci, A.; Rodriguez-Sanchez, N.; Galloway, S.D. A randomized trial to assess the potential of different beverages to affect hydration status: Development of a beverage hydration index. Am. J. Clin. Nutr. 2016, 103, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Sollanek, K.J.; Tsurumoto, M.; Vidyasagar, S.; Kenefick, R.W.; Cheuvront, S.N. Neither body mass nor sex influences beverage hydration index outcomes during randomized trial when comparing 3 commercial beverages. Am. J. Clin. Nutr. 2018, 107, 544–549. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.J.; Lee, J.Y.; Jeon, M.; Oh, S.E.; Park, J.H.; Yoon, J. The effects of fluid absorption and plasma volume changes in athletes following consumption of various beverages. BMC Sports Sci. Med. Rehabil. 2022, 14, 207. [Google Scholar] [CrossRef]
- Clarke, M.M.; Stanhewicz, A.E.; Wolf, S.T.; Cheuvront, S.N.; Kenefick, R.W.; Kenney, W.L. A randomized trial to assess beverage hydration index in healthy older adults. Am. J. Clin. Nutr. 2019, 109, 1640–1647. [Google Scholar] [CrossRef]
- Millard-Stafford, M.; Snow, T.K.; Jones, M.L.; Suh, H. The Beverage Hydration Index: Influence of Electrolytes, Carbohydrate and Protein. Nutrients 2021, 13, 2933. [Google Scholar] [CrossRef]
- UK Sports and Energy Drinks Market Report 2019. Available online: https://store.mintel.com/report/uk-sports-and-energy-drinks-market-report-2 (accessed on 1 February 2023).
- Shirreffs, S.M. Hydration in sport and exercise: Water, sports drinks and other drinks. Nutr. Bull. 2009, 34, 374–379. [Google Scholar] [CrossRef]
- Berry, C.; Murray, B.; Kenney, W. Scientific basis for a milk permeate-based sports drink—A critical review. Int. Dairy J. 2021, 127, 105296. [Google Scholar] [CrossRef]
- SCF. Report of the Scientific Committee on Food on Composition and Specification of Food Intended to Meet the Expenditure of Intense Muscular Effort, Especially for Sportsmen; European Commission: Brussels, Belgium, 2001. [Google Scholar]
- Atia, A.N.; Buchman, A.L. Oral rehydration solutions in non-cholera diarrhea: A review. Am. J. Gastroenterol. 2009, 104, 2596–2604; quiz 2605. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.W.; Burns, S.F.; Lee, J.K.W. Efficacy of Ingesting an Oral Rehydration Solution after Exercise on Fluid Balance and Endurance Performance. Nutrients 2020, 12, 3826. [Google Scholar] [CrossRef] [PubMed]
- Schleh, M.W.; Dumke, C.L. Comparison of Sports Drink Versus Oral Rehydration Solution During Exercise in the Heat. Wilderness Environ. Med. 2018, 29, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Peden, D.L.; Funnell, M.P.; Reynolds, K.M.; Kenefick, R.W.; Cheuvront, S.N.; Mears, S.A.; James, L.J. Post-exercise rehydration: Comparing the efficacy of three commercial oral rehydration solutions. Front. Sports Act. Living 2023, 5, 1158167. [Google Scholar] [CrossRef] [PubMed]
- Kitson, O.; Rutherfurd-Markwick, K.; Foskett, A.; Lee, J.K.W.; Diako, C.; Wong, M.; Ali, A. Sensory Perception of an Oral Rehydration Solution during Exercise in the Heat. Nutrients 2021, 13, 3313. [Google Scholar] [CrossRef]
- Lau, W.Y.; Kato, H.; Nosaka, K. Effect of oral rehydration solution versus spring water intake during exercise in the heat on muscle cramp susceptibility of young men. J. Int. Soc. Sports Nutr. 2021, 18, 22. [Google Scholar] [CrossRef]
- World Health Organization. Reduced Osmolarity: Oral Rehydration Salts (ORS) Formulation: A Report from a Meeting of Experts Jointly Organised by UNICEF and WHO: UNICEF House: New York, USA; WHO: Geneva, Switzerland, 2002. [Google Scholar]
- Guarino, A.; Albano, F.; Guandalini, S. Oral rehydration: Toward a real solution. J. Pediatr. Gastroenterol. Nutr. 2001, 33 (Suppl. S2), S2–S12. [Google Scholar] [CrossRef]
- Subcommittee on Acute Gastroenteritis; Provisional Committee on Quality Improvement. Practice Parameter: The Management of Acute Gastroenteritis in Young Children. Pediatrics 1996, 97, 424–435. [Google Scholar] [CrossRef]
- Water with sugar and salt. Lancet 1978, 2, 300–301.
- Booth, I.; Cunha Ferreira, R.; Desjeux, J.F.; Farthing, M.; Guandalini, S.; Hoekstra, J.H. Recommendations for composition of oral rehydration solutions for the children of Europe. Report of an ESPGAN Working Group. J. Pediatr. Gastroenterol. Nutr. 1992, 14, 113–115. [Google Scholar]
- Guarino, A.; Ashkenazi, S.; Gendrel, D.; Lo Vecchio, A.; Shamir, R.; Szajewska, H. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: Update 2014. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 132–152. [Google Scholar] [CrossRef] [PubMed]
- Lun, V.; Erdman, K.A.; Fung, T.S.; Reimer, R.A. Dietary supplementation practices in Canadian high-performance athletes. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Chlibkova, D.; Nikolaidis, P.T.; Rosemann, T.; Knechtle, B.; Bednar, J. Reported Hydration Beliefs and Behaviors without Effect on Plasma Sodium in Endurance Athletes. Front. Physiol. 2017, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.A.; Naughton, R.J.; Harper, L.D. Investigating the Nutritional and Recovery Habits of Tennis Players. Nutrients 2018, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- McCubbin, A.J.; Cox, G.R.; Costa, R.J.S. Sodium Intake Beliefs, Information Sources, and Intended Practices of Endurance Athletes Before and During Exercise. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 371–381. [Google Scholar] [CrossRef]
- Maughan, R.J. Fluid and electrolyte loss and replacement in exercise. J. Sports Sci. 1991, 9, 117–142. [Google Scholar] [CrossRef]
- Baker, L.B.; De Chavez, P.J.D.; Ungaro, C.T.; Sopena, B.C.; Nuccio, R.P.; Reimel, A.J.; Barnes, K.A. Exercise intensity effects on total sweat electrolyte losses and regional vs. whole-body sweat [Na(+)], [Cl(−)], and [K(+)]. Eur. J. Appl. Physiol. 2019, 119, 361–375. [Google Scholar] [CrossRef]
- Holmes, N.; Bates, G.; Zhao, Y.; Sherriff, J.; Miller, V. The effect of exercise intensity on sweat rate and sweat sodium and potassium losses in trained endurance athletes. Ann. Sports Med. Res. 2016, 3, 1–4. [Google Scholar]
- Crane, R.K. Intestinal Absorption of Sugars. Physiol. Rev. 1960, 40, 789–825. [Google Scholar] [CrossRef]
- Skou, J.C.; Esmann, M. The Na,K-ATPase. J. Bioenerg. Biomembr. 1992, 24, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Watson, F.; Austin, P. Physiology of human fluid balance. Anaesth. Intensive Care Med. 2021, 22, 644–651. [Google Scholar] [CrossRef]
- Thornton, S.N. Thirst and hydration: Physiology and consequences of dysfunction. Physiol. Behav. 2010, 100, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Wemple, R.D.; Morocco, T.S.; Mack, G.W. Influence of sodium replacement on fluid ingestion following exercise-induced dehydration. Int. J. Sport Nutr. 1997, 7, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Passe, D.H.; Stofan, J.R.; Rowe, C.L.; Horswill, C.A.; Murray, R. Exercise condition affects hedonic responses to sodium in a sport drink. Appetite 2009, 52, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Takamata, A.; Mack, G.W.; Gillen, C.M.; Nadel, E.R. Sodium appetite, thirst, and body fluid regulation in humans during rehydration without sodium replacement. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1994, 266, R1493–R1502. [Google Scholar] [CrossRef] [PubMed]
- Murray, D.; Miller, K.C.; Edwards, J.E. Does a Reduction in Serum Sodium Concentration or Serum Potassium Concentration Increase the Prevalence of Exercise-Associated Muscle Cramps? J. Sport Rehabil. 2016, 25, 301–304. [Google Scholar] [CrossRef]
- Rehrer, N.J. Fluid and Electrolyte Balance in Ultra-Endurance Sport. Sports Med. 2001, 31, 701–715. [Google Scholar] [CrossRef]
- Nielsen, B.; Sjøgaard, G.; Ugelvig, J.; Knudsen, B.; Dohlmann, B. Fluid balance in exercise dehydration and rehydration with different glucose-electrolyte drinks. Eur. J. Appl. Physiol. Occup. Physiol. 1986, 55, 318–325. [Google Scholar] [CrossRef]
- Maughan, R.J.; Owen, J.H.; Shirreffs, S.M.; Leiper, J.B. Post-exercise rehydration in man: Effects of electrolyte addition to ingested fluids. Eur. J. Appl. Physiol. Occup. Physiol. 1994, 69, 209–215. [Google Scholar] [CrossRef]
- Pérez-Idárraga, A.; Aragón-Vargas, L.F. Postexercise rehydration: Potassium-rich drinks versus water and a sports drink. Appl. Physiol. Nutr. Metab. 2014, 39, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Shirreffs, S.M.; Maughan, R.J. Volume repletion after exercise-induced volume depletion in humans: Replacement of water and sodium losses. Am. J. Physiol. 1998, 274, F868–F875. [Google Scholar] [CrossRef]
- Lau, W.Y.; Kato, H.; Nosaka, K. Water intake after dehydration makes muscles more susceptible to cramp but electrolytes reverse that effect. BMJ Open Sport Exerc. Med. 2019, 5, e000478. [Google Scholar] [CrossRef] [PubMed]
- Earp, J.E.; Stearns, R.L.; Stranieri, A.; Agostinucci, J.; Lepley, A.S.; Matson, T.; Ward-Ritacco, C.L. Electrolyte beverage consumption alters electrically induced cramping threshold. Muscle Nerve 2019, 60, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Baker, L.B.; Ungaro, C.T.; Sopeña, B.C.; Nuccio, R.P.; Reimel, A.J.; Carter, J.M.; Stofan, J.R.; Barnes, K.A. Body map of regional vs. whole body sweating rate and sweat electrolyte concentrations in men and women during moderate exercise-heat stress. J. Appl. Physiol. 2018, 124, 1304–1318. [Google Scholar] [CrossRef]
- Osterberg, K.L.; Pallardy, S.E.; Johnson, R.J.; Horswill, C.A. Carbohydrate exerts a mild influence on fluid retention following exercise-induced dehydration. J. Appl. Physiol. 2010, 108, 245–250. [Google Scholar] [CrossRef]
- Roebber, J.K.; Roper, S.D.; Chaudhari, N. The Role of the Anion in Salt (NaCl) Detection by Mouse Taste Buds. J. Neurosci. 2019, 39, 6224–6232. [Google Scholar] [CrossRef]
- Siegler, J.C.; Carr, A.J.; Jardine, W.T.; Convit, L.; Cross, R.; Chapman, D.; Burke, L.M.; Ross, M. The Hyperhydration Potential. of Sodium Bicarbonate and Sodium Citrate. Int. J. Sport Nutr. Exerc. Metab. 2022, 32, 74–81. [Google Scholar] [CrossRef]
- Brouns, F.; Kovacs, E.; Senden, J. The effect of different rehydration drinks on post-exercise electrolyte excretion in trained athletes. Int. J. Sports Med. 1998, 19, 56–60. [Google Scholar] [CrossRef]
- Reuter, S.E.; Schultz, H.B.; Ward, M.B.; Grant, C.L.; Paech, G.M.; Banks, S.; Evans, A.M. The effect of high-dose, short-term caffeine intake on the renal clearance of calcium, sodium and creatinine in healthy adults. Br. J. Clin. Pharmacol. 2021, 87, 4461–4466. [Google Scholar] [CrossRef]
- Garrison, S.R.; Korownyk, C.S.; Kolber, M.R.; Allan, G.M.; Musini, V.M.; Sekhon, R.K.; Dugré, N. Magnesium for skeletal muscle cramps. Cochrane Database Syst. Rev. 2020, 9. [Google Scholar] [CrossRef]
- Jeukendrup, A.E. Carbohydrate intake during exercise and performance. Nutrition 2004, 20, 669–677. [Google Scholar] [CrossRef]
- Koepsell, H. Glucose transporters in the small intestine in health and disease. Pflug. Arch. 2020, 472, 1207–1248. [Google Scholar] [CrossRef]
- Rowlands, D.S.; Houltham, S.; Musa-Veloso, K.; Brown, F.; Paulionis, L.; Bailey, D. Fructose-Glucose Composite Carbohydrates and Endurance Performance: Critical Review and Future Perspectives. Sports Med. 2015, 45, 1561–1576. [Google Scholar] [CrossRef]
- Rumessen, J.J.; Gudmand-Høyer, E. Absorption capacity of fructose in healthy adults. Comparison with sucrose and its constituent monosaccharides. Gut 1986, 27, 1161–1168. [Google Scholar] [CrossRef]
- Liu, W.; Jin, Y.; Wilde, P.J.; Hou, Y.; Wang, Y.; Han, J. Mechanisms, physiology, and recent research progress of gastric emptying. Crit. Rev. Food Sci. Nutr. 2021, 61, 2742–2755. [Google Scholar] [CrossRef]
- Clayton, D.J.; Evans, G.H.; James, L.J. Effect of drink carbohydrate content on postexercise gastric emptying, rehydration, and the calculation of net fluid balance. Int. J. Sport Nutr. Exerc. Metab. 2014, 24, 79–89. [Google Scholar] [CrossRef]
- Shi, X.; Horn, M.K.; Osterberg, K.L.; Stofan, J.R.; Zachwieja, J.J.; Horswill, C.A.; Passe, D.H.; Murray, R. Gastrointestinal discomfort during intermittent high-intensity exercise: Effect of carbohydrate-electrolyte beverage. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 673–683. [Google Scholar] [CrossRef]
- Hunt, J.N. The site of receptors slowing gastric emptying in response to starch in test meals. J. Physiol. 1960, 154, 270–276. [Google Scholar] [CrossRef]
- Costill, D.L.; Kammer, W.F.; Fisher, A. Fluid Ingestion During Distance Running. Arch. Environ. Health Int. J. 1970, 21, 520–525. [Google Scholar] [CrossRef]
- Mitchell, J.B.; Costill, D.L.; Houmard, J.A.; Fink, W.J.; Robergs, R.A.; Davis, J.A. Gastric emptying: Influence of prolonged exercise and carbohydrate concentration. Med. Sci. Sports Exerc. 1989, 21, 269–274. [Google Scholar] [CrossRef]
- Houmard, J.A.; Egan, P.C.; Johns, R.A.; Neufer, P.D.; Chenier, T.C.; Israel, R.G. Gastric emptying during 1 h of cycling and running at 75% VO2max. Med. Sci. Sports Exerc. 1991, 23, 320–325. [Google Scholar] [CrossRef]
- Murray, R.; Bartoli, W.; Stofan, J.; Horn, M.; Eddy, D. A comparison of the gastric emptying characteristics of selected sports drinks. Int. J. Sport Nutr. 1999, 9, 263–274. [Google Scholar] [CrossRef]
- Murray, R.; Bartoli, W.P.; Eddy, D.E.; Horn, M.K. Gastric emptying and plasma deuterium accumulation following ingestion of water and two carbohydrate-electrolyte beverages. Int. J. Sport Nutr. 1997, 7, 144–153. [Google Scholar] [CrossRef]
- Murray, R.; Eddy, D.E.; Bartoli, W.P.; Paul, G.L. Gastric emptying of water and isocaloric carbohydrate solutions consumed at rest. Med. Sci. Sports Exerc. 1994, 26, 725–732. [Google Scholar] [CrossRef]
- Vist, G.E.; Maughan, R.J. Gastric emptying of ingested solutions in man: Effect of beverage glucose concentration. Med. Sci. Sports Exerc. 1994, 26, 1269–1273. [Google Scholar] [CrossRef]
- Neufer, P.D.; Costill, D.L.; Fink, W.J.; Kirwan, J.P.; Fielding, R.A.; Flynn, M.G. Effects of exercise and carbohydrate composition on gastric emptying. Med. Sci. Sports Exerc. 1986, 18, 658–662. [Google Scholar] [CrossRef]
- Coyle, E.F.; Costill, D.L.; Fink, W.J.; Hoopes, D.G. Gastric emptying rates for selected athletic drinks. Res. Q. 1978, 49, 119–124. [Google Scholar] [CrossRef]
- Costill, D.L.; Saltin, B. Factors limiting gastric emptying during rest and exercise. J. Appl. Physiol. 1974, 37, 679–683. [Google Scholar] [CrossRef]
- Cole, K.J.; Grandjean, P.W.; Sobszak, R.J.; Mitchell, J.B. Effect of carbohydrate composition on fluid balance, gastric emptying, and exercise performance. Int. J. Sport Nutr. 1993, 3, 408–417. [Google Scholar] [CrossRef]
- Owen, M.D.; Kregel, K.C.; Wall, P.T.; Gisolfi, C.V. Effects of ingesting carbohydrate beverages during exercise in the heat. Med. Sci. Sports Exerc. 1986, 18, 568–575. [Google Scholar] [CrossRef]
- Zachwieja, J.J.; Costill, D.L.; Beard, G.C.; Robergs, R.A.; Pascoe, D.D.; Anderson, D.E. The effects of a carbonated carbohydrate drink on gastric emptying, gastrointestinal distress, and exercise performance. Int. J. Sport Nutr. 1992, 2, 239–250. [Google Scholar] [CrossRef]
- Shi, X.; Osterberg, K.L.; Petrie, H.; Stofan, J.R.; Murray, R. Effect of Different Osmolalities, CHO Types, and [CHO] on Gastric Emptying in Humans. Med. Sci. Sports Exerc. 2017, 49, 1015–1021. [Google Scholar] [CrossRef]
- Davis, J.M.; Lamb, D.R.; Burgess, W.A.; Bartoli, W.P. Accumulation of deuterium oxide in body fluids after ingestion of D2O-labeled beverages. J. Appl. Physiol. 1987, 63, 2060–2066. [Google Scholar] [CrossRef]
- Leiper, J.B. Fate of ingested fluids: Factors affecting gastric emptying and intestinal absorption of beverages in humans. Nutr. Rev. 2015, 73 (Suppl. S2), 57–72. [Google Scholar] [CrossRef]
- Millard-Stafford, M.L.; Sparling, P.B.; Rosskopf, L.B.; Snow, T.K. Should Carbohydrate Concentration of a Sports Drink Be Less Than 8% During Exercise in the Heat? Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 117–130. [Google Scholar] [CrossRef]
- Jeukendrup, A.E.; Currell, K.; Clarke, J.; Cole, J.; Blannin, A.K. Effect of beverage glucose and sodium content on fluid delivery. Nutr. Metab. 2009, 6, 9. [Google Scholar] [CrossRef]
- Davis, J.M.; Burgess, W.A.; Slentz, C.A.; Bartoli, W.P. Fluid availability of sports drinks differing in carbohydrate type and concentration. Am. J. Clin. Nutr. 1990, 51, 1054–1057. [Google Scholar] [CrossRef]
- Koulmann, N.; Melin, B.; Jimenez, C.; Charpenet, A.; Savourey, G.; Bittel, J. Effects of different carbohydrate-electrolyte beverages on the appearance of ingested deuterium in body fluids during moderate exercise by humans in the heat. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 525–531. [Google Scholar] [CrossRef]
- Gisolfi, C.V.; Summers, R.W.; Schedl, H.P.; Bleiler, T.L.; Oppliger, R.A. Human intestinal water absorption: Direct vs. indirect measurements. Am. J. Physiol. 1990, 258, G216–G222. [Google Scholar] [CrossRef]
- Shiose, K.; Takahashi, H.; Yamada, Y. Muscle Glycogen Assessment and Relationship with Body Hydration Status: A Narrative Review. Nutrients 2023, 15, 155. [Google Scholar] [CrossRef]
- Fernández-Elías, V.E.; Ortega, J.F.; Nelson, R.K.; Mora-Rodriguez, R. Relationship between muscle water and glycogen recovery after prolonged exercise in the heat in humans. Eur. J. Appl. Physiol. 2015, 115, 1919–1926. [Google Scholar] [CrossRef]
- Maughan, R.J.; Shirreffs, S.M.; Leiper, J.B. Errors in the estimation of hydration status from changes in body mass. J. Sports Sci. 2007, 25, 797–804. [Google Scholar] [CrossRef]
- King, R.F.G.J.; Jones, B.; O’Hara, J.P. The availability of water associated with glycogen during dehydration: A reservoir or raindrop? Eur. J. Appl. Physiol. 2018, 118, 283–290. [Google Scholar] [CrossRef]
- Brands, M.W. Role of Insulin-Mediated Antinatriuresis in Sodium Homeostasis and Hypertension. Hypertension 2018, 72, 1255–1262. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Brand-Miller, J.C.; Foster-Powell, K.; Buyken, A.E.; Goletzke, J. International tables of glycemic index and glycemic load values 2021: A systematic review. Am. J. Clin. Nutr. 2021, 114, 1625–1632. [Google Scholar] [CrossRef]
- Sole, C.; Noakes, T. Faster gastric emptying for glucose-polymer and fructose solutions than for glucose in humans. Eur. J. Appl. Physiol. Occup. Physiol. 1989, 58, 605–612. [Google Scholar] [CrossRef]
- Ryan, A.J.; Bleiler, T.L.; Carter, J.E.; Gisolfi, C.V. Gastric emptying during prolonged cycling exercise in the heat. Med. Sci. Sports Exerc. 1989, 21, 51–58. [Google Scholar] [CrossRef]
- Sutehall, S.; Galloway, S.D.R.; Bosch, A.; Pitsiladis, Y. Addition of an Alginate Hydrogel to a Carbohydrate Beverage Enhances Gastric Emptying. Med. Sci. Sports Exerc. 2020, 52, 1785–1792. [Google Scholar] [CrossRef]
- Gisolfi, C.V.; Lambert, G.P.; Summers, R.W. Intestinal fluid absorption during exercise: Role of sport drink osmolality and [Na+]. Med. Sci. Sports Exerc. 2001, 33, 907–915. [Google Scholar] [CrossRef]
- Hunt, J.; Elliott, E.; Fairclough, P.; Clark, M.; Farthing, M. Water and solute absorption from hypotonic glucose-electrolyte solutions in human jejunum. Gut 1992, 33, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Wapnir, R.A.; Lifshitz, F. Osmolality and solute concentration--their relationship with oral hydration solution effectiveness: An experimental assessment. Pediatr. Res. 1985, 19, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Passe, D.H. Water and Solute Absorption from Carbohydrate-Electrolyte Solutions in the Human Proximal Small Intestine: A Review and Statistical Analysis. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Phillips, S.M. Protein requirements and supplementation in strength sports. Nutrition 2004, 20, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.; Rosenbloom, C. Fundamentals of glycogen metabolism for coaches and athletes. Nutr. Rev. 2018, 76, 243–259. [Google Scholar] [CrossRef] [PubMed]
- Seifert, J.; Harmon, J.; DeClercq, P. Protein added to a sports drink improves fluid retention. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 420–429. [Google Scholar] [CrossRef] [PubMed]
- James, L.J.; Gingell, R.; Evans, G.H. Whey protein addition to a carbohydrate-electrolyte rehydration solution ingested after exercise in the heat. J. Athl. Train. 2012, 47, 61–66. [Google Scholar] [CrossRef] [PubMed]
- James, L.J.; Evans, G.H.; Madin, J.; Scott, D.; Stepney, M.; Harris, R.; Stone, R.; Clayton, D.J. Effect of varying the concentrations of carbohydrate and milk protein in rehydration solutions ingested after exercise in the heat. Br. J. Nutr. 2013, 110, 1285–1291. [Google Scholar] [CrossRef]
- James, L.J.; Mattin, L.; Aldiss, P.; Adebishi, R.; Hobson, R.M. Effect of whey protein isolate on rehydration after exercise. Amino Acids 2014, 46, 1217–1224. [Google Scholar] [CrossRef]
- Hobson, R.; James, L. The addition of whey protein to a carbohydrate–electrolyte drink does not influence post-exercise rehydration. J. Sports Sci. 2015, 33, 77–84. [Google Scholar] [CrossRef]
- Desbrow, B.; Jansen, S.; Barrett, A.; Leveritt, M.D.; Irwin, C. Comparing the rehydration potential of different milk-based drinks to a carbohydrate-electrolyte beverage. Appl. Physiol. Nutr. Metab. 2014, 39, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- Russo, I.; Della Gatta, P.A.; Garnham, A.; Porter, J.; Burke, L.M.; Costa, R.J.S. Assessing Overall Exercise Recovery Processes Using Carbohydrate and Carbohydrate-Protein Containing Recovery Beverages. Front. Physiol. 2021, 12, 628863. [Google Scholar] [CrossRef] [PubMed]
- Seery, S.; Jakeman, P. A metered intake of milk following exercise and thermal dehydration restores whole-body net fluid balance better than a carbohydrate-electrolyte solution or water in healthy young men. Br. J. Nutr. 2016, 116, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Shirreffs, S.M.; Watson, P.; Maughan, R.J. Milk as an effective post-exercise rehydration drink. Br. J. Nutr. 2007, 98, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.; Love, T.D.; Maughan, R.J.; Shirreffs, S.M. A comparison of the effects of milk and a carbohydrate-electrolyte drink on the restoration of fluid balance and exercise capacity in a hot, humid environment. Eur. J. Appl. Physiol. 2008, 104, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Ye, A.; Cui, J.; Dalgleish, D.; Singh, H. Formation of a structured clot during the gastric digestion of milk: Impact on the rate of protein hydrolysis. Food Hydrocoll. 2016, 52, 478–486. [Google Scholar] [CrossRef]
- Boulier, A.; Denis, S.; Henry, G.; Guérin, S.; Alric, M.; Meunier, N.; Blot, A.; Pereira, B.; Malpuech-Brugere, C.; Remond, D.; et al. Casein structures differently affect postprandial amino acid delivery through their intra-gastric clotting properties. Food Chem. 2023, 415, 135779. [Google Scholar] [CrossRef]
- James, L.J.; Stevenson, E.J.; Rumbold, P.L.S.; Hulston, C.J. Cow’s milk as a post-exercise recovery drink: Implications for performance and health. Eur. J. Sport Sci. 2019, 19, 40–48. [Google Scholar] [CrossRef]
- Millen, B.E.; Abrams, S.; Adams-Campbell, L.; Anderson, C.A.M.; Brenna, J.T.; Campbell, W.W.; Clinton, S.; Hu, F.; Nelson, M.; Neuhouser, M.L.; et al. The 2015 Dietary Guidelines Advisory Committee Scientific Report: Development and Major Conclusions. Adv. Nutr. 2016, 7, 438–444. [Google Scholar] [CrossRef]
- Storhaug, C.L.; Fosse, S.K.; Fadnes, L.T. Country, regional, and global estimates for lactose malabsorption in adults: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2017, 2, 738–746. [Google Scholar] [CrossRef]
- Goto, M.; Okazaki, K.; Kamijo, Y.; Ikegawa, S.; Masuki, S.; Miyagawa, K.; Nose, H. Protein and carbohydrate supplementation during 5-day aerobic training enhanced plasma volume expansion and thermoregulatory adaptation in young men. J. Appl. Physiol. 2010, 109, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, K.; Goto, M.; Nose, H. Protein and carbohydrate supplementation increases aerobic and thermoregulatory capacities. J. Physiol. 2009, 587, 5585–5590. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S.; Fairweather, S.J. Amino Acid Transport Across the Mammalian 2009.180562.Intestine. Compr. Physiol. 2018, 9, 343–373. [Google Scholar] [CrossRef] [PubMed]
- Luiking, Y.C.; Engelen, M.P.; Deutz, N.E. Regulation of nitric oxide production in health and disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mourad, F.H.; Turvill, J.L.; Farthing, M.J.G. Role of nitric oxide in intestinal water and electrolyte transport. Gut 1999, 44, 143. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.S.; Clarke, J.; Green, J.G.; Shi, X. l-Arginine but not l-glutamine likely increases exogenous carbohydrate oxidation during endurance exercise. Eur. J. Appl. Physiol. 2012, 112, 2443–2453. [Google Scholar] [CrossRef] [PubMed]
- Bardhan, P.K. Improving the ORS: Does glutamine have a role? J. Health Popul. Nutr. 2007, 25, 263–266. [Google Scholar] [PubMed]
- Wapnir, R.A.; Zdanowicz, M.M.; Teichberg, S.; Lifshitz, F. Oral hydration solutions in experimental osmotic diarrhea: Enhancement by alanine and other amino acids and oligopeptides. Am. J. Clin. Nutr. 1988, 48, 84–90. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Ratamess, N.A.; Kang, J.; Rashti, S.L.; Kelly, N.; Gonzalez, A.M.; Stec, M.; Anderson, S.; Bailey, B.L.; Yamamoto, L.M.; et al. Examination of the efficacy of acute L-alanyl-L-glutamine ingestion during hydration stress in endurance exercise. J. Int. Soc. Sports Nutr. 2010, 7, 8. [Google Scholar] [CrossRef]
- Hoffman, J.; Ratamess, N.; Kang, J.; Rashti, S.; Kelly, N.; Gonzalez, A.; Stec, M.; Anderson, S.; Bailey, B.; Yamamoto, L.; et al. Acute L-alanyl-L-glutamine ingestion during short duration, high intensity exercise and a mild hydration stress. Kinesiology 2011, 43, 125–136. [Google Scholar]
- Hoffman, J.R.; Williams, D.R.; Emerson, N.S.; Hoffman, M.W.; Wells, A.J.; McVeigh, D.M.; McCormack, W.P.; Mangine, G.T.; Gonzalez, A.M.; Fragala, M.S. L-alanyl-L-glutamine ingestion maintains performance during a competitive basketball game. J. Int. Soc. Sports Nutr. 2012, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.J.S.; Henningsen, K.; Gaskell, S.K.; Alcock, R.; Mika, A.; Rauch, C.; Cheuvront, S.N.; Blazy, P.; Kenefick, R. Amino Acid-Based Beverage Interventions Ameliorate Exercise-Induced Gastrointestinal Syndrome in Response to Exertional-Heat Stress: The Heat Exertion Amino Acid Technology (HEAAT) Study. Int. J. Sport Nutr. Exerc. Metab. 2023, 33, 230–242. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the substantiation of health claims related to caffeine and increase in physical performance during short-term high-intensity exercise (ID 737, 1486, 1489), increase in endurance performance (ID 737, 1486), increase in endurance capacity (ID 1488) and reduction in the rated perceived exertion/effort during exercise (ID 1488, 1490) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2053. [Google Scholar] [CrossRef]
- Maughan, R.J.; Burke, L.M.; Dvorak, J.; Larson-Meyer, D.E.; Peeling, P.; Phillips, S.M.; Rawson, E.S.; Walsh, N.P.; Garthe, I.; Geyer, H.; et al. IOC Consensus Statement: Dietary Supplements and the High-Performance Athlete. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 104–125. [Google Scholar] [CrossRef] [PubMed]
- Passmore, A.P.; Kondowe, G.B.; Johnston, G.D. Renal and cardiovascular effects of caffeine: A dose-response study. Clin. Sci. 1987, 72, 749–756. [Google Scholar] [CrossRef]
- Robertson, D.; Frölich, J.C.; Carr, R.K.; Watson, J.T.; Hollifield, J.W.; Shand, D.G.; Oates, J.A. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. N. Engl. J. Med. 1978, 298, 181–186. [Google Scholar] [CrossRef]
- Neuhäuser, B.; Beine, S.; Verwied, S.C.; Lührmann, P.M. Coffee consumption and total body water homeostasis as measured by fluid balance and bioelectrical impedance analysis. Ann. Nutr. Metab. 1997, 41, 29–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Coca, A.; Casa, D.J.; Antonio, J.; Green, J.M.; Bishop, P.A. Caffeine and diuresis during rest and exercise: A meta-analysis. J. Sci. Med. Sport 2015, 18, 569–574. [Google Scholar] [CrossRef]
- Silva, A.M.; Júdice, P.B.; Matias, C.N.; Santos, D.A.; Magalhães, J.P.; St-Onge, M.P.; Gonçalves, E.M.; Armada-da-Silva, P.; Sardinha, L.B. Total body water and its compartments are not affected by ingesting a moderate dose of caffeine in healthy young adult males. Appl. Physiol. Nutr. Metab. 2013, 38, 626–632. [Google Scholar] [CrossRef]
- Grandjean, A.C.; Reimers, K.J.; Bannick, K.E.; Haven, M.C. The effect of caffeinated, non-caffeinated, caloric and non-caloric beverages on hydration. J. Am. Coll. Nutr. 2000, 19, 591–600. [Google Scholar] [CrossRef]
- Conway, K.J.; Orr, R.; Stannard, S.R. Effect of a divided caffeine dose on endurance cycling performance, postexercise urinary caffeine concentration, and plasma paraxanthine. J. Appl. Physiol. 2003, 94, 1557–1562. [Google Scholar] [CrossRef] [PubMed]
- Wemple, R.D.; Lamb, D.R.; McKeever, K.H. Caffeine vs caffeine-free sports drinks: Effects on urine production at rest and during prolonged exercise. Int. J. Sports Med. 1997, 18, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Guimarães, V.; Figueiredo, N.; Queiroz, M.; Gentil, P.; Mota, J.F.; Pimentel, G.D. Acute caffeine mouth rinse does not change the hydration status following a 10 km run in recreationally trained runners. BioMed Res. Int. 2020, 2020, 6598753. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.M.R.; Stegen, J.H.C.H.; Brouns, F. Effect of caffeinated drinks on substrate metabolism, caffeine excretion, and performance. J. Appl. Physiol. 1998, 85, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Shirreffs, S.M.; Maughan, R.J. Restoration of fluid balance after exercise-induced dehydration: Effects of alcohol consumption. J. Appl. Physiol. 1997, 83, 1152–1158. [Google Scholar] [CrossRef]
- Hobson, R.M.; Maughan, R.J. Hydration status and the diuretic action of a small dose of alcohol. Alcohol Alcohol. 2010, 45, 366–373. [Google Scholar] [CrossRef]
- Turner, C. How much alcohol is in a ‘standard drink’? An. analysis of 125 studies. Br. J. Addict. 1990, 85, 1171–1175. [Google Scholar] [CrossRef]
- Desbrow, B.; Murray, D.; Leveritt, M. Beer as a sports drink? Manipulating beer’s ingredients to replace lost fluid. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 593–600. [Google Scholar] [CrossRef]
- Flores-Salamanca, R.; Aragón-Vargas, L.F. Postexercise rehydration with beer impairs fluid retention, reaction time, and balance. Appl. Physiol. Nutr. Metab. 2014, 39, 1175–1181. [Google Scholar] [CrossRef]
- Desbrow, B.; Cecchin, D.; Jones, A.; Grant, G.; Irwin, C.; Leveritt, M. Manipulations to the Alcohol and Sodium Content of Beer for Postexercise Rehydration. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 262–270. [Google Scholar] [CrossRef]
- Wijnen, A.H.; Steennis, J.; Catoire, M.; Wardenaar, F.C.; Mensink, M. Post-Exercise Rehydration: Effect of Consumption of Beer with Varying Alcohol Content on Fluid Balance after Mild Dehydration. Front. Nutr. 2016, 3, 45. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, L.; Tassignon, B.; Rommers, N.; Mertens, E.; Mullie, P.; Clarys, P. Pre-exercise hypohydration prevalence in soccer players: A quantitative systematic review. Eur. J. Sport Sci. 2020, 20, 744–755. [Google Scholar] [CrossRef] [PubMed]
- Deshayes, T.A.; Jeker, D.; Goulet, E.D.B. Impact of Pre-exercise Hypohydration on Aerobic Exercise Performance, Peak Oxygen Consumption and Oxygen Consumption at Lactate Threshold: A Systematic Review with Meta-analysis. Sports Med. 2020, 50, 581–596. [Google Scholar] [CrossRef] [PubMed]
- Horswill, C.A. Applied Physiology of Amateur Wrestling. Sports Med. 1992, 14, 114–143. [Google Scholar] [CrossRef] [PubMed]
- Valiente, J.S.; Utter, A.C.; Quindry, J.C.; Nieman, D.C. Effects of commercially formulated water on the hydration status of dehydrated collegiate wrestlers. J. Strength Cond. Res. 2009, 23, 2210–2216. [Google Scholar] [CrossRef] [PubMed]
- McKenna, Z.J.; Gillum, T.L. Effects of Exercise Induced Dehydration and Glycerol Rehydration on Anaerobic Power in Male Collegiate Wrestlers. J. Strength Cond. Res. 2017, 31, 2965–2968. [Google Scholar] [CrossRef]
- Timpmann, S.; Burk, A.; Medijainen, L.; Tamm, M.; Kreegipuu, K.; Vähi, M.; Unt, E.; Ööpik, V. Dietary sodium citrate supplementation enhances rehydration and recovery from rapid body mass loss in trained wrestlers. Appl. Physiol. Nutr. Metab. 2012, 37, 1028–1037. [Google Scholar] [CrossRef]
- Burke, L.M.; Slater, G.J.; Matthews, J.J.; Langan-Evans, C.; Horswill, C.A. ACSM Expert Consensus Statement on Weight Loss in Weight-Category Sports. Curr. Sports Med. Rep. 2021, 20, 199–217. [Google Scholar] [CrossRef]
- Barley, O.R.; Chapman, D.W.; Abbiss, C.R. The Current State of Weight-Cutting in Combat Sports. Sports 2019, 7, 123. [Google Scholar] [CrossRef]
- Nelson, J.L.; Robergs, R.A. Exploring the Potential Ergogenic Effects of Glycerol Hyperhydration. Sports Med. 2007, 37, 981–1000. [Google Scholar] [CrossRef]
- Freund, B.J.; Montain, S.J.; Young, A.J.; Sawka, M.N.; DeLuca, J.P.; Pandolf, K.B.; Valeri, C.R. Glycerol hyperhydration: Hormonal, renal, and vascular fluid responses. J. Appl. Physiol. 1995, 79, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Lyons, T.P.; Riedesel, M.L.; Meuli, L.E.; Chick, T.W. Effects of glycerol-induced hyperhydration prior to exercise in the heat on sweating and core temperature. Med. Sci. Sports Exerc. 1990, 22, 477–483. [Google Scholar] [CrossRef]
- Van Rosendal, S.P.; Osborne, M.A.; Fassett, R.G.; Coombes, J.S. Physiological and performance effects of glycerol hyperhydration and rehydration. Nutr. Rev. 2009, 67, 690–705. [Google Scholar] [CrossRef] [PubMed]
- van Rosendal, S.P.; Osborne, M.A.; Fassett, R.G.; Coombes, J.S. Guidelines for glycerol use in hyperhydration and rehydration associated with exercise. Sports Med. 2010, 40, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.; Braun, H.; de Marees, M.; Geyer, H.; Thevis, M.; Mester, J.; Schaenzer, W. Glycerol administration before endurance exercise: Metabolism, urinary glycerol excretion and effects on doping-relevant blood parameters. Drug Test. Anal. 2014, 6, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Jolicoeur Desroches, A.; Naulleau, C.; Deshayes, T.A.; Parent-Roberge, H.; Pancrate, T.; Goulet, E.D.B. Effect of Glycerol-Induced Hyperhydration on a 5-kilometer Running Time-Trial Performance in the Heat in Recreationally Active Individuals. Nutrients 2023, 15, 599. [Google Scholar] [CrossRef] [PubMed]
- Koehler, K.; Thevis, M.; Schaenzer, W. Meta-analysis: Effects of glycerol administration on plasma volume, haemoglobin, and haematocrit. Drug Test. Anal. 2013, 5, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.; Eddy, D.E.; Paul, G.L.; Seifert, J.G.; Halaby, G.A. Physiological responses to glycerol ingestion during exercise. J. Appl. Physiol. 1991, 71, 144–149. [Google Scholar] [CrossRef]
- Coutts, A.; Reaburn, P.; Mummery, K.; Holmes, M. The Effect of Glycerol Hyperhydration on Olympic Distance Triathlon Performance in High Ambient Temperatures. Int. J. Sport Nutr. Exerc. Metab. 2002, 12, 105–119. [Google Scholar] [CrossRef]
- Easton, C.; Turner, S.; Pitsiladis, Y.P. Creatine and Glycerol Hyperhydration in Trained Subjects before Exercise in the Heat. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 70–91. [Google Scholar] [CrossRef]
- Goulet, E.D.B.; Aubertin-Leheudre, M.; Plante, G.E.; Dionne, I.J. A Meta-Analysis of the Effects of Glycerol-Induced Hyperhydration on Fluid Retention and Endurance Performance. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Coles, M.G.; Luetkemeier, M.J. Sodium-facilitated hypervolemia, endurance performance, and thermoregulation. Int. J. Sports Med. 2005, 26, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Sims, S.T.; Rehrer, N.J.; Bell, M.L.; Cotter, J.D. Preexercise sodium loading aids fluid balance and endurance for women exercising in the heat. J. Appl. Physiol. 2007, 103, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Sims, S.T.; van Vliet, L.; Cotter, J.D.; Rehrer, N.J. Sodium loading aids fluid balance and reduces physiological strain of trained men exercising in the heat. Med. Sci. Sports Exerc. 2007, 39, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Gigou, P.Y.; Dion, T.; Asselin, A.; Berrigan, F.; Goulet, E.D. Pre-exercise hyperhydration-induced bodyweight gain does not alter prolonged treadmill running time-trial performance in warm ambient conditions. Nutrients 2012, 4, 949–966. [Google Scholar] [CrossRef] [PubMed]
- Savoie, F.A.; Dion, T.; Asselin, A.; Goulet, E.D. Sodium-induced hyperhydration decreases urine output and improves fluid balance compared with glycerol- and water-induced hyperhydration. Appl. Physiol. Nutr. Metab. 2015, 40, 51–58. [Google Scholar] [CrossRef]
- Savoie, F.A.; Asselin, A.; Goulet, E.D. Comparison of Sodium Chloride Tablets-Induced, Sodium Chloride Solution-Induced, and Glycerol-Induced Hyperhydration on Fluid Balance Responses in Healthy Men. J. Strength Cond. Res. 2016, 30, 2880–2891. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Sugihara, A.; Watanabe, K.; Niwa, T.; Katagiri, A.; Moriyama, S.; Miyanagi, I.; Kojima, J.; Nishiyasu, T. Carbohydrate hastens hypervolemia achieved through ingestion of aqueous sodium solution in resting euhydrated humans. Eur. J. Appl. Physiol. 2021, 121, 3527–3537. [Google Scholar] [CrossRef]
- Sugihara, A.; Fujii, N.; Tsuji, B.; Watanabe, K.; Niwa, T.; Nishiyasu, T. Hypervolemia induced by fluid ingestion at rest: Effect of sodium concentration. Eur. J. Appl. Physiol. 2014, 114, 2139–2145. [Google Scholar] [CrossRef]
- Johannsen, N.M.; Sullivan, Z.M.; Warnke, N.R.; Smiley-Oyen, A.L.; King, D.S.; Sharp, R.L. Effect of preexercise soup ingestion on water intake and fluid balance during exercise in the heat. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 287–296. [Google Scholar] [CrossRef]
- Castro-Sepulveda, M.; Johannsen, N.; Astudillo, S.; Jorquera, C.; Álvarez, C.; Zbinden-Foncea, H.; Ramírez-Campillo, R. Effects of Beer, Non-Alcoholic Beer and Water Consumption before Exercise on Fluid and Electrolyte Homeostasis in Athletes. Nutrients 2016, 8, 345. [Google Scholar] [CrossRef] [PubMed]
- García-Berger, D.; Mackay, K.; Monsalves-Alvarez, M.; Jorquera, C.; Ramirez-Campillo, R.; Zbinden-Foncea, H.; Castro-Sepulveda, M. Effects of skim milk and isotonic drink consumption before exercise on fluid homeostasis and time-trial performance in cyclists: A randomized cross-over study. J. Int. Soc. Sports Nutr. 2020, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Laitano, O.; Trangmar, S.J.; Marins, D.d.M.; Menezes, E.S.; Reis, G.d.S. Improved exercise capacity in the heat followed by coconut water consumption. Mot. Rev. Educ. Física 2014, 20, 107–111. [Google Scholar] [CrossRef]
- Kerksick, C.M.; Arent, S.; Schoenfeld, B.J.; Stout, J.R.; Campbell, B.; Wilborn, C.D.; Taylor, L.; Kalman, D.; Smith-Ryan, A.E.; Kreider, R.B.; et al. International society of sports nutrition position stand: Nutrient timing. J. Int. Soc. Sports Nutr. 2017, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, D.S.; Kopetschny, B.H.; Badenhorst, C.E. The Hydrating Effects of Hypertonic, Isotonic and Hypotonic Sports Drinks and Waters on Central Hydration During Continuous Exercise: A Systematic Meta-Analysis and Perspective. Sports Med. 2022, 52, 349–375. [Google Scholar] [CrossRef]
- Maughan, R.J.; Leiper, J.B. Sodium intake and post-exercise rehydration in man. Eur. J. Appl. Physiol. Occup. Physiol. 1995, 71, 311–319. [Google Scholar] [CrossRef]
- Maughan, R.; Shirreffs, S.; Merson, S.; Horswill, C. Fluid and electrolyte balance in elite male football (soccer) players training in a cool environment. J. Sports Sci. 2005, 23, 73–79. [Google Scholar] [CrossRef]
- Maughan, R.J.; Watson, P.; Evans, G.H.; Broad, N.; Shirreffs, S.M. Water balance and salt losses in competitive football. Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 583–594. [Google Scholar] [CrossRef]
- Maughan, R.J.; Merson, S.J.; Broad, N.P.; Shirreffs, S.M. Fluid and electrolyte intake and loss in elite soccer players during training. Int. J. Sport Nutr. Exerc. Metab. 2004, 14, 333–346. [Google Scholar] [CrossRef]
- Kurdak, S.S.; Shirreffs, S.M.; Maughan, R.J.; Ozgünen, K.T.; Zeren, C.; Korkmaz, S.; Yazici, Z.; Ersöz, G.; Binnet, M.S.; Dvorak, J. Hydration and sweating responses to hot-weather football competition. Scand. J. Med. Sci. Sports 2010, 20 (Suppl. S3), 133–139. [Google Scholar] [CrossRef]
- Barnes, K.A.; Anderson, M.L.; Stofan, J.R.; Dalrymple, K.J.; Reimel, A.J.; Roberts, T.J.; Randell, R.K.; Ungaro, C.T.; Baker, L.B. Normative data for sweating rate, sweat sodium concentration, and sweat sodium loss in athletes: An update and analysis by sport. J. Sports Sci. 2019, 37, 2356–2366. [Google Scholar] [CrossRef] [PubMed]
- Barr, S.I.; Costill, D.L.; Fink, W.J. Fluid replacement during prolonged exercise: Effects of water, saline, or no fluid. Med. Sci. Sports Exerc. 1991, 23, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Del Coso, J.; González-Millán, C.; Salinero, J.J.; Abián-Vicén, J.; Areces, F.; Lledó, M.; Lara, B.; Gallo-Salazar, C.; Ruiz-Vicente, D. Effects of oral salt supplementation on physical performance during a half-ironman: A randomized controlled trial. Scand. J. Med. Sci. Sports 2016, 26, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Speedy, D.B.; Thompson, J.M.D.; Rodgers, I.; Collins, M.; Sharwood, K. Oral Salt Supplementation During Ultradistance Exercise. Clin. J. Sport Med. 2002, 12, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B.; Noakes, T.D.; Dennis, S.C. Sodium replacement and fluid shifts during prolonged exercise in humans. Eur. J. Appl. Physiol. 2001, 84, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Wijering, L.A.J.; Cotter, J.D.; Rehrer, N.J. A randomized, cross-over trial assessing effects of beverage sodium concentration on plasma sodium concentration and plasma volume during prolonged exercise in the heat. Eur. J. Appl. Physiol. 2023, 123, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, C.A.; Kavouras, S.A.; Arnaoutis, G.; Gioxari, A.; Kollia, M.; Botoula, E.; Sidossis, L.S. Sodium replacement and plasma sodium drop during exercise in the heat when fluid intake matches fluid loss. J. Athl. Train. 2009, 44, 117–123. [Google Scholar] [CrossRef]
- Moss, K.N. Some effects of high air temperatures and muscular exertion upon colliers. Proc. R. Soc. Lond. Ser. B Contain. Pap. A Biol. Character 1923, 95, 181–200. [Google Scholar] [CrossRef]
- Schwellnus, M.P.; Drew, N.; Collins, M. Increased running speed and previous cramps rather than dehydration or serum sodium changes predict exercise-associated muscle cramping: A prospective cohort study in 210 Ironman triathletes. Br. J. Sports Med. 2011, 45, 650–656. [Google Scholar] [CrossRef]
- Maughan, R.J.; Shirreffs, S.M. Muscle Cramping During Exercise: Causes, Solutions, and Questions Remaining. Sports Med. 2019, 49, 115–124. [Google Scholar] [CrossRef]
- Ohno, M.; Lavender, A.P.; Sawai, A. Heat-induced Body Fluid Loss Causes Muscle Cramp during Maximal Voluntary Contraction for the Knee Flexors. Int. J. Sport Health Sci. 2018, 16, 191–199. [Google Scholar] [CrossRef]
- Jung, A.P.; Bishop, P.A.; Al-Nawwas, A.; Dale, R.B. Influence of Hydration and Electrolyte Supplementation on Incidence and Time to Onset of Exercise-Associated Muscle Cramps. J. Athl. Train. 2005, 40, 71–75. [Google Scholar] [PubMed]
- Miller, K.C.; Knight, K.L. Initial electrical stimulation frequency and cramp threshold frequency and force. J. Athl. Train. 2012, 47, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Ørtenblad, N.; Westerblad, H.; Nielsen, J. Muscle glycogen stores and fatigue. J. Physiol. 2013, 591, 4405–4413. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Costill, D.L.; Fink, W.J.; King, D.S.; Hargreaves, M.; Kovaleski, J.E. Effect of carbohydrate feeding frequencies and dosage on muscle glycogen use during exercise. Med. Sci. Sports Exerc. 1985, 17, 472–476. [Google Scholar] [CrossRef]
- Smith, J.W.; Pascoe, D.D.; Passe, D.H.; Ruby, B.C.; Stewart, L.K.; Baker, L.B.; Zachwieja, J.J. Curvilinear Dose–Response Relationship of Carbohydrate (0–120 g·h−1) and Performance. Med. Sci. Sports Exerc. 2013, 45, 336–341. [Google Scholar] [CrossRef]
- Smith, J.W.; Zachwieja, J.J.; Péronnet, F.; Passe, D.H.; Massicotte, D.; Lavoie, C.; Pascoe, D.D. Fuel selection and cycling endurance performance with ingestion of [13C]glucose: Evidence for a carbohydrate dose response. J. Appl. Physiol. 2010, 108, 1520–1529. [Google Scholar] [CrossRef]
- Maughan, R.J.; Leiper, J.B.; Shirreffs, S.M. Restoration of fluid balance after exercise-induced dehydration: Effects of food and fluid intake. Eur. J. Appl. Physiol. Occup. Physiol. 1996, 73, 317–325. [Google Scholar] [CrossRef]
- Nose, H.; Mack, G.W.; Shi, X.R.; Nadel, E.R. Involvement of sodium retention hormones during rehydration in humans. J. Appl. Physiol. 1988, 65, 332–336. [Google Scholar] [CrossRef]
- Nose, H.; Mack, G.W.; Shi, X.R.; Nadel, E.R. Role of osmolality and plasma volume during rehydration in humans. J. Appl. Physiol. 1988, 65, 325–331. [Google Scholar] [CrossRef]
- González-Alonso, J.; Heaps, C.L.; Coyle, E.F. Rehydration after exercise with common beverages and water. Int. J. Sports Med. 1992, 13, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Shirreffs, S.M.; Taylor, A.J.; Leiper, J.B.; Maughan, R.J. Post-exercise rehydration in man: Effects of volume consumed and drink sodium content. Med. Sci. Sports Exerc. 1996, 28, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Merson, S.J.; Maughan, R.J.; Shirreffs, S.M. Rehydration with drinks differing in sodium concentration and recovery from moderate exercise-induced hypohydration in man. Eur. J. Appl. Physiol. 2008, 103, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Kamijo, Y.; Ikegawa, S.; Okada, Y.; Masuki, S.; Okazaki, K.; Uchida, K.; Sakurai, M.; Nose, H. Enhanced renal Na+ reabsorption by carbohydrate in beverages during restitution from thermal and exercise-induced dehydration in men. Am. J. Physiology. Regul. Integr. Comp. Physiol. 2012, 303, R824–R833. [Google Scholar] [CrossRef] [PubMed]
- Amano, T.; Sugiyama, Y.; Okumura, J.; Fujii, N.; Kenny, G.P.; Nishiyasu, T.; Inoue, Y.; Kondo, N.; Sasagawa, K.; Enoki, Y.; et al. Effects of isomaltulose ingestion on postexercise hydration state and heat loss responses in young men. Exp. Physiol. 2019, 104, 1494–1504. [Google Scholar] [CrossRef]
- Amano, T.; Watanabe, D.; Otsuka, J.; Okamoto, Y.; Takada, S.; Fujii, N.; Kenny, G.P.; Enoki, Y.; Maejima, D. Comparison of hydration efficacy of carbohydrate-electrolytes beverages consisting of isomaltulose and sucrose in healthy young adults: A randomized crossover trial. Physiol. Behav. 2022, 249, 113770. [Google Scholar] [CrossRef]
- Ly, N.Q.; Hamstra-Wright, K.L.; Horswill, C.A. Post-Exercise Rehydration in Athletes: Effects of Sodium and Carbohydrate in Commercial Hydration Beverages. Nutrients 2023, 15, 4759. [Google Scholar] [CrossRef]
| Institution/Organization | Before Exercise | During Exercise | After Exercise |
|---|---|---|---|
| General | |||
| American College of Sports Medicine (ACSM) (2007) [10] | Consume 5–10 mL fluid/kg body weight 2–4 h before exercise to allow sufficient time for voiding and achieve pale yellow color urine. | Consume sufficient fluid to replace sweat loss and limit BML to <2%. A plan suitable for most athletes may consist of consuming 0.4–0.8 L/h fluid during exercise. | Consume ≈ 1.5 L/kg BML at a modest rate to minimize urine loss. |
| National Athletic Trainers’ Association (NATA) (2017) [2] | Individualize fluid intake plans to achieve euhydration or <2% hyperhydration (the latter only under medical oversight, and before endurance events where fluid supply is limited). | Consume enough fluid to approximate personal sweat loss and limit BML to <2%. | Consume fluid up to 150% of estimated fluid loss in <4 h. |
| Hot environments | |||
| Sports Dietitians Australia (SDA) (2020) [11] | Fluid intake strategies should be adapted to fluid balance and anticipated substrate and fluid requirements. Pre-event hyperhydration may be useful in situations of limited fluid intake opportunities. | Individualized fluid intake plans should be adjusted to real-time assessment and be based on prior fluid balance, thirst, gastrointestinal tolerance, and previous experience. | To rapidly reverse moderate–severe fluid deficit, volumes up to 150% of BML should be consumed during the hours following exercise. |
| International Olympic Committee (IOC) (2022) [12] | Fluid intake plans should ascertain sufficient fluid intake before training and competition in the heat to achieve BML < 1–2%, urine specific gravity < 1.020, and/or plasma osmolality < 290 mmol/kg. | Fluid intake plans should aim to minimize loss without increasing body weight during the event. Plans should be practiced at training under conditions similar to those of competition. | Rehydrate after exercise in the heat consuming fluids to restore fluid balance slightly over BML (i.e., 100–120%). |
| Carbohydrate–Electrolyte Solutions | Osmolality (mOsm/Kg) | Carbohydrate (Glucose) (g/L) | Sodium (mmol/L) | Potassium (mmol/L) | Chloride (mmol/L) |
|---|---|---|---|---|---|
| ORS (WHO, 1975) [45] | 311 | 20 | 90 | 20 | 80 |
| ORS (ESPGHAN, 1992) [46] * | 200–250 | 13.3–20 | 50–60 | 20 | 60 |
| ORS (WHO, 2002) [42] | 245 | 13.5 | 75 | 20 | 65 |
| Commonly Marketed ORS [36] | 220–270 | ≤25 | 45–50 | 20–25 | 35–45 |
| Commonly Marketed Sports Drinks [26] | >280–380 | 60–80 | 10–35 | 3–5 | 10–12 |
| Before Exercise | During Exercise | After Exercise | |
|---|---|---|---|
| Rationale | Athletes might pursue commencing training and competition in a hyperhydrated state in situations of low fluid availability or when euhydration is insufficient to compensate for challenges in fluid balance [2]. | Athletes typically want to prevent dehydration losses ≥2%, restore sweat electrolyte losses, or prevent EAMC during exercise [2]. | Athletes might want to accelerate the restoration of fluid and electrolyte losses and reestablish net fluid balance before a further bout of exercise [2]. |
| Evidence | Sodium loading has been shown to be more effective in increasing pre-exercise plasma volume than hyperhydration with water or glycerol [191]. | Hypotonic formulations have been shown to absorb faster than isotonic or hypertonic formulas during exercise [200]. The amounts of sodium contained in isotonic or hypertonic sports drinks (10–25 mmol/L) may be insufficient to match sweat-induced losses in most situations [201]. Fluid and/or electrolyte imbalances might be associated with EAMC [215]. | Sodium is the most important compositional aspect controlling urine production and retention of plasma volume when rehydrating after exercise [201]. Other macronutrients such as carbohydrates and proteins might promote water retention by delaying gastric emptying [21,82]. Carbohydrates might involve additional mechanisms (i.e., insulin response) [230]. |
| Gaps in CES Research | Sodium levels at which CES produce a mild hypervolemic response and aid in fluid retention (i.e., ≥45 mmol/L) should be further researched. Palatability aspects of these formulations should also be considered. Most research to date has focused on maximizing glycogen stores, and further research on well-designed CES compositions to improve fluid balance before exercise is warranted. | Studies comparing isotonic/hypertonic sports drinks and hypotonic CES containing moderate to high amounts of sodium (i.e., ≥45 mmol/L) and low quantities of carbohydrate (i.e., 2–6%) and are scarce, and might shed light upon the role of these hypotonic solutions in aiding intestinal water absorption, restoring sweat electrolyte losses, and potentially preventing or attenuating EAMC during exercise. Raising sodium content of common isotonic/hypertonic sports drinks might help restore electrolyte balance during exercise, which merits further research. These studies might also benefit from exploring high-intensity exercise protocols to provide real-world evidence. | Further research on CES containing moderate to high amounts of sodium (i.e., ≥45 mmol/L) to help reduce urine output and increase plasma volume during post-exercise rehydration without compromising gastric motility is needed. Studies exploring slow- and fast-digestible carbohydrates might help elucidate mechanisms involved in water retention properties of carbohydrate-containing beverages. |
| Reference | Study Design and Sample | Beverages | Exercise Protocol | Fluid Intake | Main Results |
|---|---|---|---|---|---|
| Schleh et al., 2018 [38] | Randomized double-blind cross-over trial n = 10 aerobically fit men | ORS: 235 mOsm/L; 60.9 mmol/L Na+; 3.4% carbohydrate; 20 mmol/L K+. Sports drink: 355 mOsm/L; 18.4 mmol/L Na+; 5.9% carbohydrate; 3.2 mmol/L K+. | Walking (50% VO2max, 90 min, 39 °C) | 150% BML volume (calculated in the first 45 min) provided during exercise | Similar changes in percentage of dehydration, urine specific gravity, urine volume, and plasma volume were observed between treatments. |
| Fan et al., 2020 [37] | Randomized double-blind cross-over trial n = 9 physically active men | ORS: 216 mOsm/L; 60 mmol/L Na+; 3.3% carbohydrate; 18.2 mmol/L K+. Sports drink: 382 mOsm/L: 31 mmol/L Na+; 6.2% carbohydrate; 5.3 mmol/L K+. Water. | Cycling (65% VO2max, 75 min, 30.4 °C) | 150% BML volume provided during exercise and 2 h post-exercise | Cumulative urine output over 5 h of recovery was lower and percentage fluid retention was higher with ORS than with sports drink and water. Serum sodium levels were maintained better with ORS than with sports drinks and water at 3 h of recovery. Net fluid balance, changes in plasma volume, skin temperature, and palatability ratings were similar across treatments. |
| Ly et al., 2023 [232] | Randomized double-blind cross-over trial n = 26 physically fit men | ORS: 270 mOsm/Kg; 45 mmol/L Na+; 2.5% carbohydrate; 20 mmol/L K+. Sports drink: 330–380 mOsm/Kg; 18 mmol/L Na+; 6% carbohydrate; 3 mmol/L K+. Water placebo. | Interval training performed until 2.6% BML (intermittent and variable intensity, 90 min, 22–29 °C) | 100% BML volume provided 45 min post-exercise | Fluid retention at 3.5 h post-exercise was similar between ORS and sports drink, but urine volume was significantly reduced with the ORS at 30–60 min into recovery, which was associated with improved sodium balance. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Castillo, Í.M.; Williams, J.A.; López-Chicharro, J.; Mihic, N.; Rueda, R.; Bouzamondo, H.; Horswill, C.A. Compositional Aspects of Beverages Designed to Promote Hydration Before, During, and After Exercise: Concepts Revisited. Nutrients 2024, 16, 17. https://doi.org/10.3390/nu16010017
Pérez-Castillo ÍM, Williams JA, López-Chicharro J, Mihic N, Rueda R, Bouzamondo H, Horswill CA. Compositional Aspects of Beverages Designed to Promote Hydration Before, During, and After Exercise: Concepts Revisited. Nutrients. 2024; 16(1):17. https://doi.org/10.3390/nu16010017
Chicago/Turabian StylePérez-Castillo, Íñigo M., Jennifer A. Williams, José López-Chicharro, Niko Mihic, Ricardo Rueda, Hakim Bouzamondo, and Craig A. Horswill. 2024. "Compositional Aspects of Beverages Designed to Promote Hydration Before, During, and After Exercise: Concepts Revisited" Nutrients 16, no. 1: 17. https://doi.org/10.3390/nu16010017
APA StylePérez-Castillo, Í. M., Williams, J. A., López-Chicharro, J., Mihic, N., Rueda, R., Bouzamondo, H., & Horswill, C. A. (2024). Compositional Aspects of Beverages Designed to Promote Hydration Before, During, and After Exercise: Concepts Revisited. Nutrients, 16(1), 17. https://doi.org/10.3390/nu16010017





