Effects of a Singular Dose of Mangiferin–Quercetin Supplementation on Basketball Performance: A Double-Blind Crossover Study of High-Level Male Players
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Preliminary Measurements
2.3. Experimental Procedures
2.4. Field Basketball Exercise Simulation Test (BEST)
2.5. Supplement Administration
2.6. Other Measurements
2.7. Statistical Analysis
3. Results
4. Discussion
Strengths, Limitations, and Suggestions for Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Theodorou, A.S.; Rizou, H.P.; Zacharakis, E.; Ktistakis, I.; Bekris, E.; Panoutsakopoulos, V.; Strouzas, P.; Bourdas, D.I.; Kostopoulos, N. Pivot Step Jump: A New Test for Evaluating Jumping Ability in Young Basketball Players. J. Funct. Morphol. Kinesiol. 2022, 7, 116. [Google Scholar] [CrossRef] [PubMed]
- Paulauskas, R.; Kamarauskas, P.; Nekriošius, R.; Bigwood, N.M. Physical and Physiological Response to Different Modes of Repeated Sprint Exercises in Basketball Players. J. Hum. Kinet. 2020, 72, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, E.; Stojiljković, N.; Scanlan, A.T.; Dalbo, V.J.; Berkelmans, D.M.; Milanović, Z. The Activity Demands and Physiological Responses Encountered during Basketball Match-Play: A Systematic Review. Sport. Med. 2018, 48, 111–135. [Google Scholar] [CrossRef]
- Bourdas, D.I.; Zacharakis, E.D.; Travlos, A.K.; Souglis, A. Return to Basketball Play Following COVID-19 Lockdown. Sports 2021, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Petway, A.J.; Freitas, T.T.; Calleja-González, J.; Leal, D.M.; Alcaraz, P.E. Training load and match-play demands in basketball based on competition level: A systematic review. PLoS ONE 2020, 15, e0229212. [Google Scholar] [CrossRef]
- Bourdas, D.I.; Mitrousis, I.; Zacharakis, E.D.; Travlos, A.K. Home-audience advantage in basketball: Evidence from a natural experiment in Euro League games during the 2019–2021 COVID-19 era. J. Phys. Educ. Sport 2022, 22, 1761–1771. [Google Scholar] [CrossRef]
- Esen, O.; Rozwadowski, K.; Cepicka, L.; Gabrys, T.; Karayigit, R. Practical Nutrition Strategies to Support Basketball Performance during International Short-Term Tournaments: A Narrative Review. Nutrients 2022, 22, 4909. [Google Scholar] [CrossRef]
- Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Gericke, N.; Perez-Valera, M.; Curtelin, D.; Galvan-Alvarez, V.; Lopez-Rios, L.; Morales-Alamo, D.; Calbet, J.A.L. Mangifera indica L. leaf extract in combination with luteolin or quercetin enhances VO2peak and peak power output, and preserves skeletal muscle function during ischemia-reperfusion in humans. Front. Physiol. 2018, 9, 740. [Google Scholar] [CrossRef]
- Masibo, M.; Qian, H. Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef]
- Martinez-Canton, M.; Galvan-Alvarez, V.; Garcia-Gonzalez, E.; Gallego-Selles, A.; Gelabert-Rebato, M.; Garcia-Perez, G.; Santana, A.; Lopez-Rios, L.; Vega-Morales, T.; Martin-Rincon, M.; et al. A Mango Leaf Extract (Zynamite®) Combined with Quercetin Has Exercise-Mimetic Properties in Human Skeletal Muscle. Nutrients 2023, 15, 2848. [Google Scholar] [CrossRef]
- Kressler, J.; Millard-Stafford, M.; Warren, G.L. Quercetin and endurance exercise capacity: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 2011, 43, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Myburgh, K.H. Polyphenol supplementation: Benefits for exercise performance or oxidative stress? Sport. Med. 2014, 44, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Braakhuis, A.J.; Hopkins, W.G. Impact of Dietary Antioxidants on Sport Performance: A Review. Sport. Med. 2015, 45, 939–955. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Boumendjel, A.; Seteyen, A.L.S.; Boina, C.; Gasque, P.; Guiraud, P.; Sélambarom, J. Focus on the high therapeutic potentials of quercetin and its derivatives. Phytomed. Plus 2022, 2, 100220. [Google Scholar] [CrossRef] [PubMed]
- Bruton, J.D.; Place, N.; Yamada, T.; Silva, J.P.; Andrade, F.H.; Dahlstedt, A.J.; Zhang, S.J.; Katz, A.; Larsson, N.G.; Westerblad, H. Reactive oxygen species and fatigue-induced prolonged low-frequency force depression in skeletal muscle fibres of rats, mice and SOD2 overexpressing mice. J. Physiol. 2008, 586, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Sidhu, S.K.; Weavil, J.C.; Mangum, T.S.; Venturelli, M. Autonomic responses to exercise: Group III/IV muscle afferents and fatigue. Auton. Neurosci. Basic Clin. 2015, 188, 19–23. [Google Scholar] [CrossRef]
- Curtelin, D.; Morales-Alamo, D.; Torres-Peralta, R.; Rasmussen, P.; Martin-Rincon, M.; Perez-Valera, M.; Siebenmann, C.; Pérez-Suárez, I.; Cherouveim, E.; Sheel, A.W.; et al. Cerebral blood flow, frontal lobe oxygenation and intra-arterial blood pressure during sprint exercise in normoxia and severe acute hypoxia in humans. J. Cereb. Blood Flow Metab. 2018, 38, 136–150. [Google Scholar] [CrossRef]
- Gelabert-Rebato, M.; Wiebe, J.C.; Martin-Rincon, M.; Galvan-Alvarez, V.; Curtelin, D.; Perez-Valera, M.; Habib, J.J.; Pérez-López, A.; Vega, T.; Morales-Alamo, D.; et al. Enhancement of exercise performance by 48 hours, and 15-day supplementation with mangiferin and luteolin in men. Nutrients 2019, 11, 344. [Google Scholar] [CrossRef]
- López-Ríos, L.; Wiebe, J.C.; Vega-Morales, T.; Gericke, N. Central nervous system activities of extract Mangifera indica L. J. Ethnopharmacol. 2020, 260, 112996. [Google Scholar] [CrossRef]
- Martin-rincon, M.; Gelabert-rebato, M.; Galvan-alvarez, V.; Gallego-selles, A.; Martinez-canton, M.; Lopez-rios, L.; Wiebe, J.C.; Martin-rodriguez, S.; Arteaga-ortiz, R.; Dorado, C.; et al. Supplementation with a Mango Leaf Extract (Zynamite®) in Combination with Quercetin Attenuates Muscle Damage and Pain and Accelerates Recovery after Strenuous Damaging Exercise. Nutrients 2020, 12, 614. [Google Scholar] [CrossRef] [PubMed]
- Gelabert-Rebato, M.; Martin-Rincon, M.; Galvan-Alvarez, V.; Gallego-Selles, A.; Martinez-Canton, M.; Vega-Morales, T.; Wiebe, J.C.; Del Castillo, C.F.; Castilla-Hernandez, E.; Diaz-Tiberio, O.; et al. A single dose of the mango leaf extract zynamite® in combination with quercetin enhances peak power output during repeated sprint exercise in men and women. Nutrients 2019, 11, 2592. [Google Scholar] [CrossRef] [PubMed]
- McKay, A.K.A.; Stellingwerff, T.; Smith, E.S.; Martin, D.T.; Mujika, I.; Goosey-Tolfrey, V.L.; Sheppard, J.; Burke, L.M. Defining Training and Performance Caliber: A Participant Classification Framework. Int. J. Sports Physiol. Perform. 2022, 17, 317–331. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Health Behaviour in School-Aged Children Study: Physical Activity in Adolescents Key Facts and Figures; World Health Organization: Geneva, Switzerland, 2016; Volume 2. [Google Scholar]
- Bourdas, D.I.; Zacharakis, E.D. Impact of COVID-19 Lockdown on Physical Activity in a Sample of Greek Adults. Sports 2020, 8, 139. [Google Scholar] [CrossRef] [PubMed]
- Warburton, D.; Jamnik, V.; Bredin, S.; Gledhill, N. The 2018 Physical Activity Readiness Questionnaire for Everyone (PAR-Q+) and electronic Physical Activity Readiness Medical Examination (ePARmed-X+). Health Fit. J. Canada 2018, 11, 31–34. [Google Scholar] [CrossRef]
- Bourdas, D.I.; Zacharakis, E.D.; Travlos, A.K.; Souglis, A.; Georgali, T.I.; Gofas, D.C.; Ktistakis, I.E.; Deltsidou, A. Impact of lockdown on smoking and sleeping in the early COVID-19 presence: Datasets of Greek Adults sample. Data Br. 2021, 39, 107480. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki, Ethical Principles for Scientific Requirements and Research Protocols. Bull. World Health Organ. 2013, 79, 373. [Google Scholar]
- Nevill, A.M.; Metsios, G.S.; Jackson, A.S.; Wang, J.; Thornton, J.; Gallagher, D. Can we use the Jackson and Pollock equations to predict body density/fat of obese individuals in the 21st century? Int. J. Body Compos. Res. 2008, 6, 114–121. [Google Scholar]
- Jackson, A.S.; Pollock, M.L. Generalized equations for predicting body density of men. Br. J. Nutr. 1978, 40, 497–504. [Google Scholar] [CrossRef]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 10th ed.; Riebe, D., Ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2018; ISBN 9781496339065. [Google Scholar]
- Borg, G.A.V. Psychophysical bases of perceived exertion. Med. Sci. Sport Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- Scanlan, A.T.; Dascombe, B.J.; Reaburn, P.R.J. Development of the basketball exercise simulation test: A match-specific basketball fitness test. J. Hum. Sport Exerc. 2014, 9, 700–712. [Google Scholar] [CrossRef]
- Bourdas, D.I.; Souglis, A.; Zacharakis, E.D.; Geladas, N.D.; Travlos, A.K. Meta-Analysis of Carbohydrate Solution Intake during Prolonged Exercise in Adults: From the Last 45+ Years’ Perspective. Nutrients 2021, 13, 4223. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, A.; Dascombe, B.; Reaburn, P. A comparison of the activity demands of elite and sub-elite Australian men’s basketball competition. J. Sports Sci. 2011, 29, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Glaister, M.; Hauck, H.; Abraham, C.S.; Merry, K.L.; Beaver, D.; Woods, B.; McInnes, G. Familiarization, reliability, and comparability of a 40-m maximal shuttle run test. J. Sport. Sci. Med. 2009, 8, 77–82. [Google Scholar]
- Walther, L.H.; Zegers, F.; Nybo, M.; Mogensen, C.B.; Christensen, E.F.; Lassen, A.T.; Mikkelsen, S. Accuracy of a point-of-care blood lactate measurement device in a prehospital setting. J. Clin. Monit. Comput. 2022, 36, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- Nova Biomedical. Nova StatStrip Xpress Lactate Hospital Meter Instructions for Use Manual; Nova Biomedical Corporation: Waltham, MA, USA, 2011; pp. 1–68. [Google Scholar]
- Nosaka, K.; Sacco, P.; Mawatari, K. Effects of amino acid supplementation on muscle soreness and damage. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 620–635. [Google Scholar] [CrossRef] [PubMed]
- Abbey, E.L.; Rankin, J.W. Effect of quercetin supplementation on repeated-sprint performance, xanthine oxidase activity, and inflammation. Int. J. Sport Nutr. Exerc. Metab. 2011, 21, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Ying, W.; Han, S.K.; Miller, J.W.; Swanson, R.A. Acidosis potentiates oxidative neuronal death by multiple mechanisms. J. Neurochem. 1999, 73, 1549–1556. [Google Scholar] [CrossRef]
- Song, J.; Li, J.; Hou, F.; Wang, X.; Liu, B. Mangiferin inhibits endoplasmic reticulum stress-associated thioredoxin-interacting protein/NLRP3 inflammasome activation with regulation of AMPK in endothelial cells. Metabolism 2015, 64, 428–437. [Google Scholar] [CrossRef]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the cardiovascular system in health and in aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef]
- Gu, C.; Yang, M.; Zhou, Z.; Khan, A.; Cao, J.; Cheng, G. Purification and characterization of four benzophenone derivatives from Mangifera indica L. leaves and their antioxidant, immunosuppressive and α-glucosidase inhibitory activities. J. Funct. Foods 2019, 52, 709–714. [Google Scholar] [CrossRef]
- Nile, S.H.; Park, S.W. Total phenolics, antioxidant and xanthine oxidase inhibitory activity of three colored onions (Allium cepa L.). Front. Life Sci. 2013, 7, 224–228. [Google Scholar] [CrossRef]
- Merry, T.L.; Ristow, M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J. Physiol. 2016, 594, 5135–5147. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Liu, J.; Liu, H.Y.; Gao, L.H.; Feng, G.H.; Liu, X.; Li, L. Hypouricaemic action of mangiferin results from metabolite norathyriol via inhibiting xanthine oxidase activity. Pharm. Biol. 2016, 54, 1680–1686. [Google Scholar] [CrossRef]
- Özyürek, M.; Bektaşoǧlu, B.; Güçlü, K.; Apak, R. Measurement of xanthine oxidase inhibition activity of phenolics and flavonoids with a modified cupric reducing antioxidant capacity (CUPRAC) method. Anal. Chim. Acta 2009, 636, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Holland, J.A.; O’Donnell, R.W.; Chang, M.M.; Johnson, D.K.; Ziegler, L.M. Endothelial cell oxidant production: Effect of NADPH oxidase inhibitors. Endothel. J. Endothel. Cell Res. 2000, 7, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Morales-Alamo, D.; Calbet, J.A.L. Free radicals and sprint exercise in humans. Free Radic. Res. 2014, 48, 30–42. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Ponce-González, J.G.; Guadalupe-Grau, A.; Rodríguez-García, L.; Santana, A.; Cusso, R.; Guerrero, M.; Dorado, C.; Guerra, B.; Calbet, J.A.L. Critical role for free radicals on sprint exercise-induced CaMKII and AMPKα phosphorylation in human skeletal muscle. J. Appl. Physiol. 2013, 114, 566–577. [Google Scholar] [CrossRef]
- Cheng, A.J.; Bruton, J.D.; Lanner, J.T.; Westerblad, H. Antioxidant treatments do not improve force recovery after fatiguing stimulation of mouse skeletal muscle fibres. J. Physiol. 2015, 593, 457–472. [Google Scholar] [CrossRef]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar] [CrossRef]
- Reid, M.B.; Khawli, F.A.; Moody, M.R. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J. Appl. Physiol. 1993, 75, 1081–1087. [Google Scholar] [CrossRef] [PubMed]
- Shanely, R.A.; Knab, A.M.; Nieman, D.C.; Jin, F.; McAnulty, S.R.; Landram, M.J. Quercetin supplementation does not alter antioxidant status in humans. Free Radic. Res. 2010, 44, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Apontes, P.; Liu, Z.; Su, K.; Benard, O.; Youn, D.Y.; Li, X.; Li, W.; Mirza, R.H.; Bastie, C.C.; Jelicks, L.A.; et al. Mangiferin stimulates carbohydrate oxidation and protects against metabolic disorders induced by high-fat diets. Diabetes 2014, 63, 3626–3636. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Li, Y.; Song, J.; Hou, F.; Liu, B.; Li, A. Mangiferin protects mitochondrial function by preserving mitochondrial hexokinase-II in vessel endothelial cells. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 1829–1839. [Google Scholar] [CrossRef]
- Calbet, J.A.L.; Lundby, C.; Sander, M.; Robach, P.; Saltin, B.; Boushel, R. Effects of ATP-induced leg vasodilation on O2 peak and leg O2 extraction during maximal exercise in humans. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2006, 291, R447–R453. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.L.; Losa-Reyna, J.; Torres-Peralta, R.; Rasmussen, P.; Ponce-González, J.G.; Sheel, A.W.; de la Calle-Herrero, J.; Guadalupe-Grau, A.; Morales-Alamo, D.; Fuentes, T.; et al. Limitations to oxygen transport and utilization during sprint exercise in humans: Evidence for a functional reserve in muscle O2 diffusing capacity. J. Physiol. 2015, 593, 4649–4664. [Google Scholar] [CrossRef] [PubMed]
- Bourdas, D.I.; Geladas, N.D. Five repeated maximal efforts of apneas increase the time to exhaustion in subsequent high-intensity exercise. Respir. Physiol. Neurobiol. 2021, 292, 103703. [Google Scholar] [CrossRef]
- Cheetham, M.E.; Boobis, L.H.; Brooks, S.; Williams, C. Human muscle metabolism during sprint running. J. Appl. Physiol. 1986, 61, 54–60. [Google Scholar] [CrossRef]
- Morales-Alamo, D.; Ponce-González, J.G.; Guadalupe-Grau, A.; Rodríguez-García, L.; Santana, A.; Cusso, M.R.; Guerrero, M.; Guerra, B.; Dorado, C.; Calbet, J.A.L. Increased oxidative stress and anaerobic energy release, but blunted Thr172-AMPKα phosphorylation, in response to sprint exercise in severe acute hypoxia in humans. J. Appl. Physiol. 2012, 113, 917–928. [Google Scholar] [CrossRef]
- Light, A.R.; Hughen, R.W.; Zhang, J.; Rainier, J.; Liu, Z.; Lee, J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J. Neurophysiol. 2008, 100, 1184–1201. [Google Scholar] [CrossRef]
- Rauf, A.; Imranb, M.; Patel, S. Mangiferin: A phytochemical with panacea potential. Biomed. Pharmacother. 2017, 96, 1562–1564. [Google Scholar] [CrossRef] [PubMed]

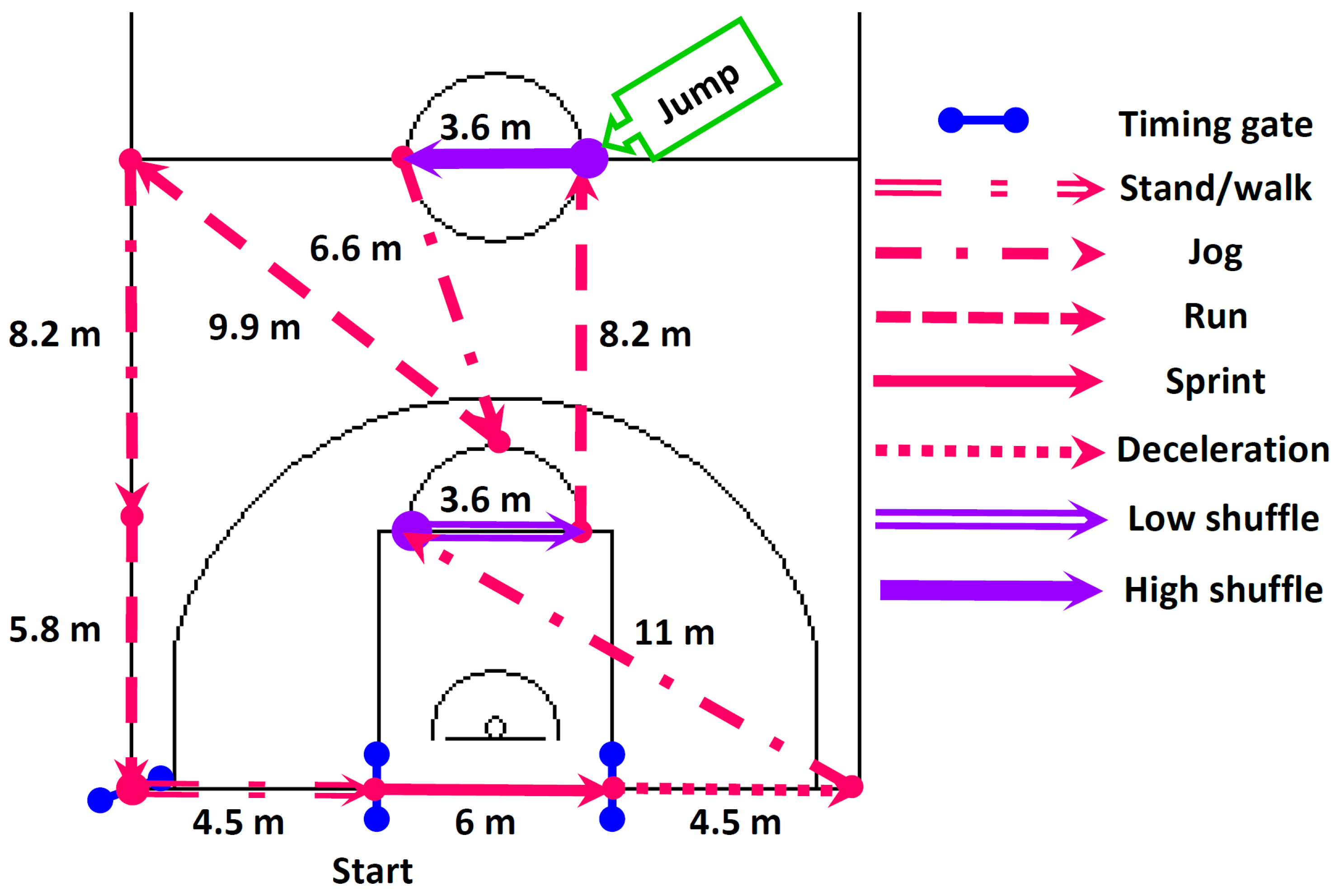
| Variables | EXP (Ne = 19) | CON (Nc = 19) |
|---|---|---|
| Height (cm) | 200.43 ± 5.40 [198–202.86] | 201.12 ± 4.71 [199–203.24] |
| Body mass (kg) | 96.2 ± 7.61 [92.78–99.62] | 97.67 ± 7.37 [94.36–100.98] |
| Body fat (%) | 10.95 ± 1.82 [10.13–11.77] | 11.11 ± 2.05 [10.19–12.03] |
| Age (year) | 23.21 ± 3.08 [21.83–24.59] | 24.05 ± 2.15 [23.08–25.02] |
| Experience practicing basketball (year) | 12.53 ± 3.03 [11.17–13.89] | 13.11 ± 2.47 [12–14.22] |
| Active in basketball competition (year) | 11.21 ± 3.08 [9.83–12.59] | 12.05 ± 2.15 [11.08–13.02] |
| Physical exercise training (h·week−1) | 3.97 ± 1.11 [3.47–4.47] | 3.79 ± 0.84 [3.41–4.17] |
| Basketball-related training (h·week−1) | 10.63 ± 1.16 [10.11–11.15] | 10.68 ± 1.2 [10.14–11.22] |
| O2max (mL·kg−1·min−1) | 59.32 ± 2.65 [58.13–60.51] | 59.05 ± 2.66 [57.85–60.25] |
| VT2 (%O2max) | 78.26 ± 3.12 [76.86–79.66] | 77.68 ± 2.98 [76.34–79.02] |
| HRmax (b·min−1) | 196.21 ± 2.07 [195.28–197.14] | 196.21 ± 1.72 [195.44–196.98] |
| CMJ height (cm) | 47.11 ± 4.64 [45.02–49.2] | 46.58 ± 4.21 [44.69–48.47] |
| EXP (Ne = 19) | CON (Nc = 19) | |||
|---|---|---|---|---|
| Dependent Variable | PRE | POST | PRE | POST |
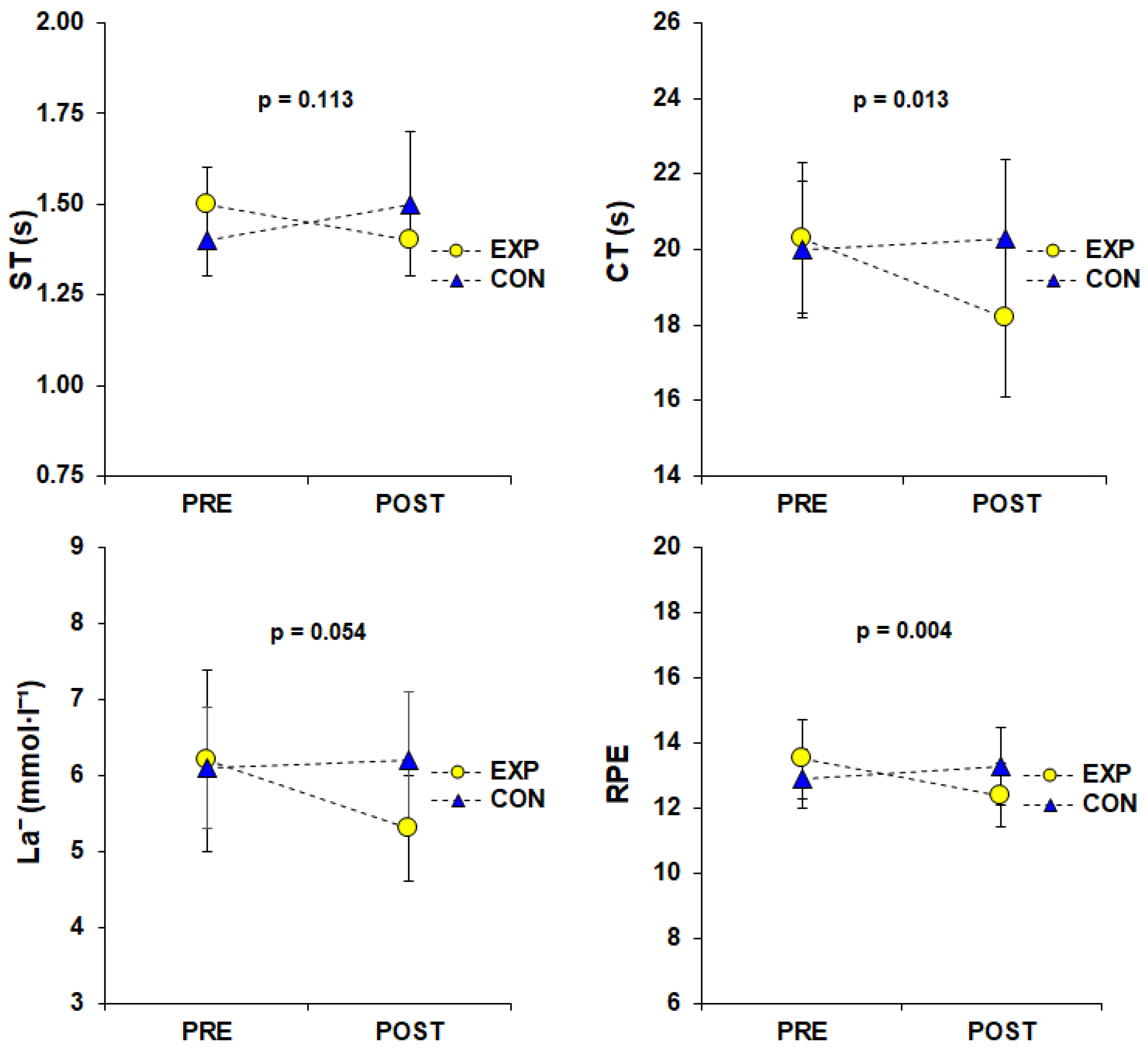
| ST (s) | 1.47 ± 0.11 | 1.39 ± 0.14 | 1.43 ± 0.12 | 1.45 ± 0.18 |
| [1.42–1.52] | [1.31–1.46] | [1.38–1.48] | [1.38–1.53] | |
| †‡ CT (s) | 20.33 ± 1.96 | 18.17 ± 2.08 | 20.02 ± 1.80 | 20.31 ± 2.10 |
| [19.45–21.20] | [17.20–19.14] | [19.14–20.89] | [19.33–21.23] | |
| HR (b·min−1) | 175.81 ± 5.80 | 176.46 ± 8.57 | 176.21 ± 6.29 | 174.15 ± 6.53 |
| [173.00–178.63] | [172.92–180.01] | [173.40–179.03] | [170.60–177.69] | |
| † La− (mmol·L−1) | 6.16 ± 1.17 | 5.32 ± 0.66 | 6.07 ± 0.80 | 6.19 ± 0.94 |
| [5.69–6.63] | [4.95–5.70] | [5.60–6.53] | [5.82–6.57] | |
| RPMS (Likert scale, 0–10) | 3.84 ± 0.90 | 4.11 ± 0.81 | 4.00 ± 0.58 | 4.42 ± 1.17 |
| [3.49–4.19] | [3.64–4.57] | [3.65–4.35] | [3.95–4.89] | |
| ‡ RPE (Borg scale, 6–20) | 13.47 ± 1.22 | 12.42 ± 1.02 | 12.95 ± 0.91 | 13.32 ± 1.16 |
| [12.97–13.97] | [11.91–12.93] | [12.45–13.45] | [12.81–13.82] | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourdas, D.I.; Travlos, A.K.; Souglis, A.; Stavropoulou, G.; Zacharakis, E.; Gofas, D.C.; Bakirtzoglou, P. Effects of a Singular Dose of Mangiferin–Quercetin Supplementation on Basketball Performance: A Double-Blind Crossover Study of High-Level Male Players. Nutrients 2024, 16, 170. https://doi.org/10.3390/nu16010170
Bourdas DI, Travlos AK, Souglis A, Stavropoulou G, Zacharakis E, Gofas DC, Bakirtzoglou P. Effects of a Singular Dose of Mangiferin–Quercetin Supplementation on Basketball Performance: A Double-Blind Crossover Study of High-Level Male Players. Nutrients. 2024; 16(1):170. https://doi.org/10.3390/nu16010170
Chicago/Turabian StyleBourdas, Dimitrios I., Antonios K. Travlos, Athanasios Souglis, Georgia Stavropoulou, Emmanouil Zacharakis, Dimitrios C. Gofas, and Panteleimon Bakirtzoglou. 2024. "Effects of a Singular Dose of Mangiferin–Quercetin Supplementation on Basketball Performance: A Double-Blind Crossover Study of High-Level Male Players" Nutrients 16, no. 1: 170. https://doi.org/10.3390/nu16010170





