The Importance of Nutrition in Menopause and Perimenopause—A Review
Abstract
:1. Introduction
2. Balanced Nutrition Recommendation
2.1. Nutritional Status during Perimenopause
- Body fat percentage ≥ 30%;
- Abdominal circumference ≥ 88 cm (abdominal, visceral obesity);
- Waist–hip ratio ≥ 0.8 (abdominal, visceral obesity);
- FFMI ≤ 15 kg/m2 (fat-free mass index);
- ASMI ≤ 5.25 kg/m2 (appendicular skeletal muscle index);
- For BMI ≤ 23 kg/m2: ≤17 kgf;
- For BMI 23.1–26 kg/m2: ≤17.3 kgf;
- For BMI ≤ 26.1-29 kg/m2: ≤18 kgf;
- For BMI > 29.1 kg/m2: ≤21 kgf;
- Short physical performance battery (SPPB) SPPB ≤ 8.
2.2. Maintaining and Achieving a Healthy Nutritional Status
2.3. Fluid Intake in Menopause
3. Dietary Intervention of Chronic Diseases in Menopause
3.1. Lipids Metabolism Disorders in Menopause
- (a)
- Body weight control in menopause is recommended with energy intake corresponding to body composition measurements;
- (b)
- Salt consumption should be as close as possible to 5 g/day, preferring green and dried vegetable spices for seasoning;
- (c)
3.2. Carbohydrate Metabolism Disorders in Menopause
- In the absence of estrogen, the insulin secretion of the pancreatic beta cells decreases;
- Decreased insulin sensitivity in the muscles results in a decrease in glucose uptake;
- As a result of deteriorating insulin sensitivity in the liver, gluconeogenesis and lipogenesis increase, triglyceride accumulation increases, VLDL production increases, and insulin clearance decreases;
- As a result of the reduced insulin effect on adipose tissue, lipolysis increases, the size of fat cells increases, and inflammatory mediators accumulate;
- The resulting metabolic changes lead to the development of metabolic syndrome.
3.3. Bone Metabolism Changes in Menopause
3.4. Cancer Prevention during Perimenopause and Menopause
3.5. The Role of Micronutrients in Menopause
3.6. Soy and Phytoestrogens and Menopause
4. The Role of Microbiom in Menopause
5. Sleep and Menopause
6. Conclusions
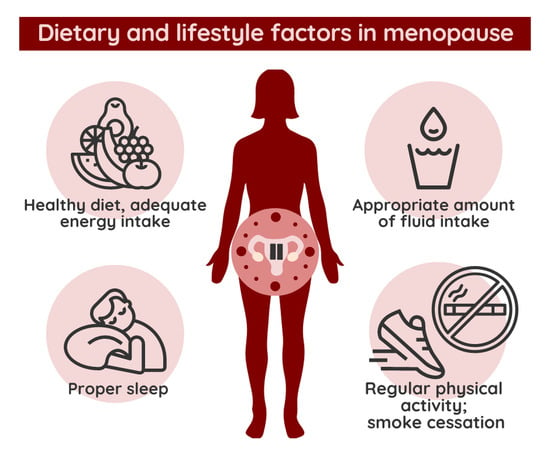
- Achieving/maintaining a healthy nutritional status (BMI = 18.5 − 24.9 kg/m2, normal range of fat mass, and skeletal muscle mass) is the goal even during the perimenopause period;
- In case of overweight or obesity, an energy intake less than the current energy requirement (reduced by 500–700 kcal/day) and a protein intake of 1–1.2 g/day are recommended while following the recommendations of a balanced diet. Energy intake below BMR is not recommended in the long term;
- During perimenopause, the process of dietetic care for women should be based on the Nutrition Care Process Model (NCPM);
- The use of body composition analysis tools is ideal for assessing nutritional status;
- Following the guidelines of a balanced diet reduces symptoms and preserves health. Ensuring energy, nutrient, and fluid requirements appropriate to age, nutritional status, physical activity, and diseases are required as follows:
- Establishing a physiological eating schedule is necessary;
- Simple, fast-acting sugars should be avoided;
- Protein intake should be 0.8–1–1.2 g/kg/day, half of which should come from plant sources;
- Adequate intake of calcium, vitamin D, vitamin C, and B vitamins is important;
- Adequate intake of n-3 LCPUFA and omega-3 fatty acids is necessary;
- Sugary and alcoholic beverages should be avoided;
- Fruits and vegetables provide vitamins, minerals, fiber, and other plant nutrients, such as antioxidants, to help protect the heart. The recommended daily intake of vegetables and fruits is 5 portions (500 g/day: 300–400 g of vegetables and 200–100 g of fruit), i.e., 3–4 portions of vegetables and 1–2 portions of fruit;
- Eating legumes (beans, peas, lentils, chickpeas, or soy) at least once a week is recommended;
- Regular consumption of low-fat protein sources (e.g., poultry, low-fat dairy products) helps to cover calcium needs;
- Moderate consumption of red and processed meats is recommended;
- Consumption of no more than 350–500 g boiled/steamed/fried (500–700 g of raw meat) red meat (e.g., beef and pork) per week is recommended. Intake of processed meat products should be only occasional, in small quantities. Incorporating at least one meat-free day per week can be useful. Meat can be replaced with fish, eggs, dairy products, and the right combination of legumes, grains, and nuts;
- Moderate consumption of fats and sweets is important. Consumption of vegetable fats and occasional consumption of high-fat foods is recommended. Sunflower oil for frying, and olive, rapeseed, linseed, soybean oil, etc., as salad dressing are recommended;
- Using as little amount of sugar and salt as possible to flavor food and drinks is important. A portion of salt can be replaced with fresh or dried herbs;
- At least two servings per week (100–120 g/occasion) of deep-sea fish with fatty meat (e.g., consumption of salmon, mackerel, tuna, herring, and sardines) or freshwater fish (e.g., trout and silver carp) is recommended;
- Consumption of 30 g of unsalted nuts, other oily seeds, or seeds per day can be beneficial. When it comes to frequency, it is important to take body weight into account;
- Incorporation of foods and ingredients with a higher fiber content daily is recommended: whole grain bread, fiber-rich breakfast cereals without added sugar, and brown rice. Oats, whole grains, whole wheat bread, and legumes such as lentils, chickpeas, and beans are excellent sources of fiber. The daily amount of dietary fiber should be 30–45 g, preferably mainly whole grains. One-third of the amount of grain consumed should be whole grains;
- The amount of saturated fat should not exceed 10% of the total energy intake. Replacement of saturated fats with monounsaturated and polyunsaturated fatty acids, or carbohydrates from whole grains is recommended. The amount of TFA should be reduced to the smallest possible amount so that the consumption of processed products is limited and the natural TFA intake is kept below <1E%;
- Eighty per cent of salt intake comes from processed foods, and only 20% is consumed in the form of added salt. It is recommended to reduce the amount and frequency of processed food consumption. Salt consumption should be as close as possible to 5 g/day, preferring fresh and dried vegetable spices for seasoning;
- Consuming dairy products corresponding to the calcium content of half a liter of milk per day is recommended. It is necessary to introduce lifestyle changes during this period to reduce the risk of fracture associated with osteoporosis. Changes should include maintaining/achieving a healthy nutritional status and balanced nutrition focusing on adequate intake of vitamin D and calcium, regular exercise, as well as quitting smoking and stopping alcohol drinking. Dietary supplementation of calcium and vitamin D should be considered based on the season and daily intake, as well as on the presence of osteoporosis and cardiovascular risk factors;
- A smoking-free lifestyle is recommended;
- Regular physical activity is essential.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Hill, K. The demography of menopause. Maturitas 1996, 23, 113–127. [Google Scholar] [CrossRef] [PubMed]
- Foryst-Ludwig, A.; Kintscher, U. Metabolic impact of estrogen signalling through ERalpha and ERbeta. J. Steroid. Biochem. Mol. Biol. 2010, 122, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Poehlman, E.T. Menopause, energy expenditure, and body composition. Acta Obstet. Gynecol. Scand. 2002, 81, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Barrea, L.; Pugliese, G.; Laudisio, D.; Colao, A.; Savastano, S.; Muscogiuri, G. Mediterranean diet as medical prescription in menopausal women with obesity: A practical guide for nutritionists. Crit. Rev. Food Sci. Nutr. 2021, 61, 1201–1211. [Google Scholar] [CrossRef] [PubMed]
- Lovejoy, J.C.; Champagne, C.M.; de Jonge, L.; Xie, H.; Smith, S.R. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int. J. Obes. 2008, 32, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; An, Y.A.; Scherer, P.E. The ominous triad of adipose tissue dysfunction: Inflammation, fibrosis, and impaired angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulos, N.; Katritsis, D.; Raggi, P. Visceral adipose tissue as a source of inflammation and promoter of atherosclerosis. Atherosclerosis 2014, 233, 104–112. [Google Scholar] [CrossRef]
- Harvie, M. Breast cancer. In Manula of Dietetic Practic; Gandy, J., Ed.; Wiley Blackwell: Oxford, UK, 2019; pp. 842–846. [Google Scholar]
- Martin, S.; Schneider, B.; Heinemann, L.; Lodwig, V.; Kurth, H.J.; Kolb, H.; Scherbaum, W.A. Self-monitoring of blood glucose in type 2 diabetes and long-term outcome: An epidemiological cohort study. Diabetologia 2006, 49, 271–278. [Google Scholar] [CrossRef]
- Bussell, G. Menopause. In Manual of Dietetic Practice; Gandy, J., Ed.; Wiley Blackwell: Oxford, UK, 2019; pp. 85–87. [Google Scholar]
- Huang, A.J.; Subak, L.L.; Wing, R.; West, D.S.; Hernandez, A.L.; Macer, J.; Grady, D.; Program to Reduce Incontinence by Diet and Exercise Investigators. An intensive behavioral weight loss intervention and hot flushes in women. Arch. Intern. Med. 2010, 170, 1161–1167. [Google Scholar] [CrossRef]
- Herforth, A.; Arimond, M.; Álvarez-Sánchez, C.; Coates, J.; Christianson, K.; Muehlhoff, E. A Global Review of Food-Based Dietary Guidelines. Adv. Nutr. 2019, 10, 590–605. [Google Scholar] [CrossRef]
- Daley, A.; Jolly, K.; Madigan, C.; Griffin, R.; Roalfe, A.; Lewis, A.; Nickless, A.; Aveyard, P. Public Health Research. In A Brief Behavioural Intervention to Promote Regular Self-Weighing to Prevent Weight Regain after Weight Loss: A RCT; NIHR Journals Library: Southampton, UK, 2019. [Google Scholar] [CrossRef]
- Camara, M.; Giner, R.M.; Gonzalez-Fandos, E.; Lopez-Garcia, E.; Manes, J.; Portillo, M.P.; Rafecas, M.; Dominguez, L.; Martinez, J.A. Food-Based Dietary Guidelines around the World: A Comparative Analysis to Update AESAN Scientific Committee Dietary Recommendations. Nutrients 2021, 13, 3131. [Google Scholar] [CrossRef]
- Springmann, M.; Spajic, L.; Clark, M.A.; Poore, J.; Herforth, A.; Webb, P.; Rayner, M.; Scarborough, P. The healthiness and sustainability of national and global food based dietary guidelines: Modelling study. BMJ 2020, 370, m2322. [Google Scholar] [CrossRef]
- Corrêa Rezende, J.L.; de Medeiros Frazão Duarte, M.C.; Melo, G.; Dos Santos, L.C.; Toral, N. Food-based dietary guidelines for children and adolescents. Front. Public Health 2022, 10, 1033580. [Google Scholar] [CrossRef]
- Food-Based Dietary Guidelines. Available online: https://www.fao.org/nutrition/education/food-based-dietary-guidelines (accessed on 2 February 2023).
- The Hungarian Dietetic Association. The OKOSTÁNYÉR® New Dietary Guideline for the Healthy Adult Population. Available online: https://www.okostanyer.hu/wp-content/uploads/2021/11/2021_OKOSTANYER_ANGOL_felnott_A4.pdf (accessed on 12 February 2021).
- European Food Safety Authority. Dietary Reference Values for nutrients Summary report. EFSA Support. Publ. 2017, 14, 98. [Google Scholar]
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 9 June 2021).
- Strohm, D.B.H.; Leschik-Bonnet, E.; Heseker, H.; Arens-Aze-vêdo, U.; Bechthold, A.; Knorpp, L.; Kroke, A.; for the German Nutrition Society (DGE). Salt intake in Germany, health consequences, and resulting recommendations for action. A scientific statement from the German Nutrition Society (DGE). Ernahr. Umsch. 2016, 63, 62–70. [Google Scholar]
- Heller, B.; Reiter, F.P.; Leicht, H.B.; Fiessler, C.; Bergheim, I.; Heuschmann, P.U.; Geier, A.; Rau, M. Salt-Intake-Related Behavior Varies between Sexes and Is Strongly Associated with Daily Salt Consumption in Obese Patients at High Risk for MASLD. Nutrients 2023, 15, 3942. [Google Scholar] [CrossRef]
- Di Rosa, C.; Di Francesco, L.; Spiezia, C.; Khazrai, Y.M. Effects of Animal and Vegetable Proteins on Gut Microbiota in Subjects with Overweight or Obesity. Nutrients 2023, 15, 2675. [Google Scholar] [CrossRef]
- Quattrini, S.; Pampaloni, B.; Gronchi, G.; Giusti, F.; Brandi, M.L. The Mediterranean Diet in Osteoporosis Prevention: An Insight in a Peri- and Post-Menopausal Population. Nutrients 2021, 13, 531. [Google Scholar] [CrossRef]
- Silva, T.R.; Oppermann, K.; Reis, F.M.; Spritzer, P.M. Nutrition in Menopausal Women: A Narrative Review. Nutrients 2021, 13, 2149. [Google Scholar] [CrossRef]
- Ojo, O. Nutrition and Chronic Conditions. Nutrients 2019, 11, 459. [Google Scholar] [CrossRef]
- Rees, M.; Abernethy, K.; Bachmann, G.; Bretz, S.; Ceausu, I.; Durmusoglu, F.; Erkkola, R.; Fistonic, I.; Gambacciani, M.; Geukes, M.; et al. The essential menopause curriculum for healthcare professionals: A European Menopause and Andropause Society (EMAS) position statement. Maturitas 2022, 158, 70–77. [Google Scholar] [CrossRef]
- van Eekelen, E.; Geelen, A.; Alssema, M.; Lamb, H.J.; de Roos, A.; Rosendaal, F.R.; de Mutsert, R. Sweet Snacks Are Positively and Fruits and Vegetables Are Negatively Associated with Visceral or Liver Fat Content in Middle-Aged Men and Women. J. Nutr. 2019, 149, 304–313. [Google Scholar] [CrossRef]
- Bechthold, A.; Boeing, H.; Tetens, I.; Schwingshackl, L.; Nöthlings, U. Perspective: Food-Based Dietary Guidelines in Europe-Scientific Concepts, Current Status, and Perspectives. Adv. Nutr. 2018, 9, 544–560. [Google Scholar] [CrossRef]
- Yumuk, V.; Tsigos, C.; Fried, M.; Schindler, K.; Busetto, L.; Micic, D.; Toplak, H.; Obesity Management Task Force of the European Association for the Study of Obesity. European Guidelines for Obesity Management in Adults. Obes. Facts 2015, 8, 402–424. [Google Scholar] [CrossRef]
- Burgess, N.S. Effect of a very-low-calorie diet on body composition and resting metabolic rate in obese men and women. J. Am. Diet. Assoc. 1991, 91, 430–434. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Kodoth, V.; Scaccia, S.; Aggarwal, B. Adverse Changes in Body Composition During the Menopausal Transition and Relation to Cardiovascular Risk: A Contemporary Review. Women’s Health Rep. 2022, 3, 573–581. [Google Scholar] [CrossRef]
- Musa, I.R.; Omar, S.M.; Adam, I. Mid-upper arm circumference as a substitute for body mass index in the assessment of nutritional status among adults in eastern Sudan. BMC Public Health 2022, 22, 2056. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Gomez, J.M.; Heitmann, B.L.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part I: Review of principles and methods. Clin. Nutr. 2004, 23, 1226–1243. [Google Scholar] [CrossRef]
- Kyle, U.G.; Bosaeus, I.; De Lorenzo, A.D.; Deurenberg, P.; Elia, M.; Manuel Gomez, J.; Lilienthal Heitmann, B.; Kent-Smith, L.; Melchior, J.C.; Pirlich, M.; et al. Bioelectrical impedance analysis—Part II: Utilization in clinical practice. Clin. Nutr. 2004, 23, 1430–1453. [Google Scholar] [CrossRef]
- Baranauskas, M.; Kupčiūnaitė, I.; Stukas, R. Dietary Intake of Protein and Essential Amino Acids for Sustainable Muscle Development in Elite Male Athletes. Nutrients 2023, 15, 4003. [Google Scholar] [CrossRef]
- Sharma, A.M.; Kushner, R.F. A proposed clinical staging system for obesity. Int. J. Obes. 2009, 33, 289–295. [Google Scholar] [CrossRef]
- BAPEN. BAPEN The ‘MUST’ Explanatory Booklet. Available online: https://www.bapen.org.uk/screening-and-must/must/must-toolkit/the-must-explanatory-booklet (accessed on 13 August 2023).
- Cederholm, T.; Jensen, G.L.; Correia, M.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. J. Cachexia Sarcopenia Muscle 2019, 10, 207–217. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Biolo, G.; Cederholm, T.; Muscaritoli, M. Muscle contractile and metabolic dysfunction is a common feature of sarcopenia of aging and chronic diseases: From sarcopenic obesity to cachexia. Clin. Nutr. 2014, 33, 737–748. [Google Scholar] [CrossRef]
- Fricker, J.; Rozen, R.; Melchior, J.C.; Apfelbaum, M. Energy-metabolism adaptation in obese adults on a very-low-calorie diet. Am. J. Clin. Nutr. 1991, 53, 826–830. [Google Scholar] [CrossRef]
- Hassapidou, M.; Vlassopoulos, A.; Kalliostra, M.; Govers, E.; Mulrooney, H.; Ells, L.; Salas, X.R.; Muscogiuri, G.; Darleska, T.H.; Busetto, L.; et al. European Association for the Study of Obesity Position Statement on Medical Nutrition Therapy for the Management of Overweight and Obesity in Adults Developed in Collaboration with the European Federation of the Associations of Dietitians. Obes. Facts 2023, 16, 11–28. [Google Scholar] [CrossRef]
- Semlitsch, T.; Stigler, F.L.; Jeitler, K.; Horvath, K.; Siebenhofer, A. Management of overweight and obesity in primary care-A systematic overview of international evidence-based guidelines. Obes. Rev. 2019, 20, 1218–1230. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef]
- Thomas, D.R. Loss of skeletal muscle mass in aging: Examining the relationship of starvation, sarcopenia and cachexia. Clin. Nutr. 2007, 26, 389–399. [Google Scholar] [CrossRef]
- Rust, C.; Prior, R.M.; Stec, M. Implementation of a clinical practice guideline in a primary care setting for the prevention and management of obesity in adults. Nurs. Forum. 2020, 55, 485–490. [Google Scholar] [CrossRef]
- Wharton, S.; Lau, D.C.W.; Vallis, M.; Sharma, A.M.; Biertho, L.; Campbell-Scherer, D.; Adamo, K.; Alberga, A.; Bell, R.; Boule, N.; et al. Obesity in adults: A clinical practice guideline. CMAJ 2020, 192, E875–E891. [Google Scholar] [CrossRef]
- Dinh, Q.N.; Drummond, G.R.; Sobey, C.G. Estrogen: Reducing the pressure by arginine vasopressin. Cardiovasc. Res. 2021, 117, 2143–2144. [Google Scholar] [CrossRef]
- Stachenfeld, N.S. Hormonal changes during menopause and the impact on fluid regulation. Reprod. Sci. 2014, 21, 555–561. [Google Scholar] [CrossRef]
- Stachenfeld, N.S.; DiPietro, L.; Palter, S.F.; Nadel, E.R. Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. Am. J. Physiol. 1998, 274, R187–R195. [Google Scholar] [CrossRef]
- Corona, G.; Giuliani, C.; Parenti, G.; Colombo, G.L.; Sforza, A.; Maggi, M.; Forti, G.; Peri, A. The Economic Burden of Hyponatremia: Systematic Review and Meta-Analysis. Am. J. Med. 2016, 129, 823–835.e824. [Google Scholar] [CrossRef]
- Fernando, S.; Sivagnanam, F.; Rathish, D. A compulsive act of excess water intake leading to hyponatraemia and rhabdomyolysis: A case report. Int. J. Emerg. Med. 2019, 12, 34. [Google Scholar] [CrossRef]
- Mallett, L.J.; Premkumar, V.; Brown, L.J.; May, J.; Rollo, M.E.; Schumacher, T.L. Total water intake by kilogram of body weight: Analysis of the Australian 2011 to 2013 National Nutrition and Physical Activity Survey. Nutr. Diet. 2021, 78, 496–505. [Google Scholar] [CrossRef]
- Raj, A.; Chakole, S.; Agrawal, S.; Gupta, A.; Khekade, H.; Prasad, R.; Lohakare, T.; Wanjari, M. The Impact of Menopause on Cardiovascular Aging: A Comprehensive Review of Androgen Influences. Cureus 2023, 15, e43569. [Google Scholar] [CrossRef]
- Mishra, S.R.; Chung, H.F.; Waller, M.; Mishra, G.D. Duration of estrogen exposure during reproductive years, age at menarche and age at menopause, and risk of cardiovascular disease events, all-cause and cardiovascular mortality: A systematic review and meta-analysis. BJOG 2021, 128, 809–821. [Google Scholar] [CrossRef]
- Anagnostis, P.; Stevenson, J.C.; Crook, D.; Johnston, D.G.; Godsland, I.F. Effects of menopause, gender and age on lipids and high-density lipoprotein cholesterol subfractions. Maturitas 2015, 81, 62–68. [Google Scholar] [CrossRef]
- Ryczkowska, K.; Adach, W.; Janikowski, K.; Banach, M.; Bielecka-Dabrowa, A. Menopause and women’s cardiovascular health: Is it really an obvious relationship? Arch. Med. Sci. 2023, 19, 458–466. [Google Scholar] [CrossRef]
- Novella, S.; Perez-Cremades, D.; Mompeon, A.; Hermenegildo, C. Mechanisms underlying the influence of oestrogen on cardiovascular physiology in women. J. Physiol. 2019, 597, 4873–4886. [Google Scholar] [CrossRef]
- Gardner, C.D.; Vadiveloo, M.K.; Petersen, K.S.; Anderson, C.A.M.; Springfield, S.; Van Horn, L.; Khera, A.; Lamendola, C.; Mayo, S.M.; Joseph, J.J.; et al. Popular Dietary Patterns: Alignment With American Heart Association 2021 Dietary Guidance: A Scientific Statement From the American Heart Association. Circulation 2023, 147, 1715–1730. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Back, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Zwart, J.J.; Richters, J.M.; Ory, F.; de Vries, J.I.; Bloemenkamp, K.W.; van Roosmalen, J. Uterine rupture in The Netherlands: A nationwide population-based cohort study. BJOG 2009, 116, 1069–1080. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Paschou, S.A.; Anagnostis, P.; Pavlou, D.I.; Vryonidou, A.; Goulis, D.G.; Lambrinoudaki, I. Diabetes in Menopause: Risks and Management. Curr. Vasc. Pharmacol. 2019, 17, 556–563. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Manson, J.E.; Stevenson, J.C.; Fonseca, V.A. Menopausal Hormone Therapy and Type 2 Diabetes Prevention: Evidence, Mechanisms, and Clinical Implications. Endocr. Rev. 2017, 38, 173–188. [Google Scholar] [CrossRef]
- Wang, M.; Gan, W.; Kartsonaki, C.; Guo, Y.; Lv, J.; Chen, Z.; Li, L.; Yang, L.; Yu, M. Menopausal status, age at natural menopause and risk of diabetes in China: A 10-year prospective study of 300,000 women. Nutr. Metab. 2022, 19, 7. [Google Scholar] [CrossRef]
- Wainberg, M.; Mahajan, A.; Kundaje, A.; McCarthy, M.I.; Ingelsson, E.; Sinnott-Armstrong, N.; Rivas, M.A. Homogeneity in the association of body mass index with type 2 diabetes across the UK Biobank: A Mendelian randomization study. PLoS Med. 2019, 16, e1002982. [Google Scholar] [CrossRef]
- Slopien, R.; Wender-Ozegowska, E.; Rogowicz-Frontczak, A.; Meczekalski, B.; Zozulinska-Ziolkiewicz, D.; Jaremek, J.D.; Cano, A.; Chedraui, P.; Goulis, D.G.; Lopes, P.; et al. Menopause and diabetes: EMAS clinical guide. Maturitas 2018, 117, 6–10. [Google Scholar] [CrossRef]
- Briggs Early, K.; Stanley, K. Position of the Academy of Nutrition and Dietetics: The Role of Medical Nutrition Therapy and Registered Dietitian Nutritionists in the Prevention and Treatment of Prediabetes and Type 2 Diabetes. J. Acad. Nutr. Diet. 2018, 118, 343–353. [Google Scholar] [CrossRef]
- Paschou, S.A.; Marina, L.V.; Spartalis, E.; Anagnostis, P.; Alexandrou, A.; Goulis, D.G.; Lambrinoudaki, I. Therapeutic strategies for type 2 diabetes mellitus in women after menopause. Maturitas 2019, 126, 69–72. [Google Scholar] [CrossRef]
- Cooney, C.; Daly, E.; McDonagh, M.; Ryan, L. Evaluation of Measured Resting Metabolic Rate for Dietary Prescription in Ageing Adults with Overweight and Adiposity-Based Chronic Disease. Nutrients 2021, 13, 1229. [Google Scholar] [CrossRef]
- Guideline: Sugars Intake for Adults and Children; WHO: Geneva, Switzerland, 2015.
- Cobiac, L.J.; Scarborough, P.; Kaur, A.; Rayner, M. The Eatwell Guide: Modelling the Health Implications of Incorporating New Sugar and Fibre Guidelines. PLoS ONE 2016, 11, e0167859. [Google Scholar] [CrossRef]
- Abdelsalam, S.E.; Ismaail, M.A.R.; Sultan, E.A.; Elsherif, O.E.; Salama, H.M.; Hassan, S.I. The outcome of medical nutrition therapy on glycemic control among type 2 diabetic patients. Pan Afr. Med. J. 2023, 45, 85. [Google Scholar] [CrossRef]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Lepping, K.; Adams-Campbell, L.L.; Hicks, J.; Mills, M.; Dash, C. Dietary fiber intake and metabolic syndrome in postmenopausal African American women with obesity. PLoS ONE 2022, 17, e0273911. [Google Scholar] [CrossRef]
- Singh, V.; Park, Y.J.; Lee, G.; Unno, T.; Shin, J.H. Dietary regulations for microbiota dysbiosis among post-menopausal women with type 2 diabetes. Crit. Rev. Food Sci. Nutr. 2023, 63, 9961–9976. [Google Scholar] [CrossRef]
- Zengul, A.G.; Demark-Wahnefried, W.; Barnes, S.; Morrow, C.D.; Bertrand, B.; Berryhill, T.F.; Fruge, A.D. Associations between Dietary Fiber, the Fecal Microbiota and Estrogen Metabolism in Postmenopausal Women with Breast Cancer. Nutr. Cancer 2021, 73, 1108–1117. [Google Scholar] [CrossRef]
- WHO. Joint FDA/WHO Expert Committee on Food Additives. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/ (accessed on 23 August 2018).
- Management of osteoporosis in postmenopausal women: The 2021 position statement of The North American Menopause Society. Menopause 2021, 28, 973–997. [CrossRef]
- Várbíró, S.; Takács, I.; Tűű, L.; Nas, K.; Sziva, R.E.; Hetthéssy, J.R.; Török, M. Effects of Vitamin D on Fertility, Pregnancy and Polycystic Ovary Syndrome-A Review. Nutrients 2022, 14, 1649. [Google Scholar] [CrossRef]
- Agostini, D.; Gervasi, M.; Ferrini, F.; Bartolacci, A.; Stranieri, A.; Piccoli, G.; Barbieri, E.; Sestili, P.; Patti, A.; Stocchi, V.; et al. An Integrated Approach to Skeletal Muscle Health in Aging. Nutrients 2023, 15, 1802. [Google Scholar] [CrossRef]
- Takacs, I.; Dank, M.; Majnik, J.; Nagy, G.; Szabo, A.; Szabo, B.; Szekanecz, Z.; Sziller, I.; Toldy, E.; Tisler, A.; et al. Hungarian consensus recommendation on the role of vitamin D in disease prevention and treatment. Orv. Hetil. 2022, 163, 575–584. [Google Scholar] [CrossRef]
- Hodges, J.K.; Cao, S.; Cladis, D.P.; Weaver, C.M. Lactose Intolerance and Bone Health: The Challenge of Ensuring Adequate Calcium Intake. Nutrients 2019, 11, 718. [Google Scholar] [CrossRef]
- de Villiers, T.J.; Goldstein, S.R. Update on bone health: The International Menopause Society White Paper 2021. Climacteric 2021, 24, 498–504. [Google Scholar] [CrossRef]
- Nutrition Working, G.; O’Connor, D.L.; Blake, J.; Bell, R.; Bowen, A.; Callum, J.; Fenton, S.; Gray-Donald, K.; Rossiter, M.; Adamo, K.; et al. Canadian Consensus on Female Nutrition: Adolescence, Reproduction, Menopause, and Beyond. J. Obstet. Gynaecol. Can. 2016, 38, 508–554.e518. [Google Scholar] [CrossRef]
- Porter, K.; Hoey, L.; Hughes, C.F.; Ward, M.; McNulty, H. Causes, Consequences and Public Health Implications of Low B-Vitamin Status in Ageing. Nutrients 2016, 8, 725. [Google Scholar] [CrossRef]
- Homocysteine Studies, C. Homocysteine and risk of ischemic heart disease and stroke: A meta-analysis. JAMA 2002, 288, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Wang, R.; Ang, L.W.; Yuan, J.M.; Koh, W.P. Dietary B vitamin intake and risk of hip fracture: The Singapore Chinese Health Study. Osteoporos. Int. 2013, 24, 2049–2059. [Google Scholar] [CrossRef] [PubMed]
- Enneman, A.W.; Swart, K.M.; Zillikens, M.C.; van Dijk, S.C.; van Wijngaarden, J.P.; Brouwer-Brolsma, E.M.; Dhonukshe-Rutten, R.A.; Hofman, A.; Rivadeneira, F.; van der Cammen, T.J.; et al. The association between plasma homocysteine levels and bone quality and bone mineral density parameters in older persons. Bone 2014, 63, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Gjesdal, C.G.; Vollset, S.E.; Ueland, P.M.; Refsum, H.; Drevon, C.A.; Gjessing, H.K.; Tell, G.S. Plasma total homocysteine level and bone mineral density: The Hordaland Homocysteine Study. Arch. Intern. Med. 2006, 166, 88–94. [Google Scholar] [CrossRef]
- McLean, R.R.; Jacques, P.F.; Selhub, J.; Fredman, L.; Tucker, K.L.; Samelson, E.J.; Kiel, D.P.; Cupples, L.A.; Hannan, M.T. Plasma B vitamins, homocysteine, and their relation with bone loss and hip fracture in elderly men and women. J. Clin. Endocrinol. Metab. 2008, 93, 2206–2212. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Hannan, M.T.; Qiao, N.; Jacques, P.F.; Selhub, J.; Cupples, L.A.; Kiel, D.P. Low plasma vitamin B12 is associated with lower BMD: The Framingham Osteoporosis Study. J. Bone Miner Res. 2005, 20, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, X.; Zhang, Q.; Cao, H.; Wang, J.; Liu, B. Homocysteine level and risk of fracture: A meta-analysis and systematic review. Bone 2012, 51, 376–382. [Google Scholar] [CrossRef]
- Yazdanpanah, N.; Zillikens, M.C.; Rivadeneira, F.; de Jong, R.; Lindemans, J.; Uitterlinden, A.G.; Pols, H.A.; van Meurs, J.B. Effect of dietary B vitamins on BMD and risk of fracture in elderly men and women: The Rotterdam study. Bone 2007, 41, 987–994. [Google Scholar] [CrossRef]
- Brown, B.; Huang, M.H.; Karlamangla, A.; Seeman, T.; Kado, D. Do the effects of APOE-epsilon4 on cognitive function and decline depend upon vitamin status? MacArthur Studies of Successful Aging. J. Nutr. Health Aging 2011, 15, 196–201. [Google Scholar] [CrossRef]
- Horvat, P.; Gardiner, J.; Kubinova, R.; Pajak, A.; Tamosiunas, A.; Schottker, B.; Pikhart, H.; Peasey, A.; Jansen, E.; Bobak, M. Serum folate, vitamin B-12 and cognitive function in middle and older age: The HAPIEE study. Exp. Gerontol. 2016, 76, 33–38. [Google Scholar] [CrossRef]
- Kado, D.M.; Karlamangla, A.S.; Huang, M.H.; Troen, A.; Rowe, J.W.; Selhub, J.; Seeman, T.E. Homocysteine versus the vitamins folate, B6, and B12 as predictors of cognitive function and decline in older high-functioning adults: MacArthur Studies of Successful Aging. Am. J. Med. 2005, 118, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, D.; Peter, I.; Scott, T.M.; Parnell, L.D.; Lai, C.Q.; Crott, J.W.; Ordovas, J.M.; Selhub, J.; Griffith, J.; Rosenberg, I.H.; et al. Status of vitamins B-12 and B-6 but not of folate, homocysteine, and the methylenetetrahydrofolate reductase C677T polymorphism are associated with impaired cognition and depression in adults. J. Nutr. 2012, 142, 1554–1560. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S. The role of B vitamins in preventing and treating cognitive impairment and decline. Adv. Nutr. 2012, 3, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.S.; Selhub, J.; Jacques, P.F. Vitamin B-12 and folate status in relation to decline in scores on the mini-mental state examination in the framingham heart study. J. Am. Geriatr. Soc. 2012, 60, 1457–1464. [Google Scholar] [CrossRef] [PubMed]
- Alrubaye, H.S.; Kohl, K.D. Abundance and Compositions of B-Vitamin-Producing Microbes in the Mammalian Gut Vary Based on Feeding Strategies. mSystems 2021, 6, e0031321. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.S.; Amarasena, S.; Mayengbam, S. B Vitamins and Their Roles in Gut Health. Microorganisms 2022, 10, 1168. [Google Scholar] [CrossRef] [PubMed]
- Taku, K.; Melby, M.K.; Kronenberg, F.; Kurzer, M.S.; Messina, M. Extracted or synthesized soybean isoflavones reduce menopausal hot flash frequency and severity: Systematic review and meta-analysis of randomized controlled trials. Menopause 2012, 19, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.J.; Rowland, I. Soy products in the management of breast cancer. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 586–591. [Google Scholar] [CrossRef]
- Steinberg, F.M.; Murray, M.J.; Lewis, R.D.; Cramer, M.A.; Amato, P.; Young, R.L.; Barnes, S.; Konzelmann, K.L.; Fischer, J.G.; Ellis, K.J.; et al. Clinical outcomes of a 2-y soy isoflavone supplementation in menopausal women. Am. J. Clin. Nutr. 2011, 93, 356–367. [Google Scholar] [CrossRef]
- van Duursen, M.B.; Nijmeijer, S.M.; de Morree, E.S.; de Jong, P.C.; van den Berg, M. Genistein induces breast cancer-associated aromatase and stimulates estrogen-dependent tumor cell growth in in vitro breast cancer model. Toxicology 2011, 289, 67–73. [Google Scholar] [CrossRef]
- Guha, N.; Kwan, M.L.; Quesenberry, C.P., Jr.; Weltzien, E.K.; Castillo, A.L.; Caan, B.J. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: The Life After Cancer Epidemiology study. Breast Cancer Res. Treat. 2009, 118, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.O.; Zheng, Y.; Cai, H.; Gu, K.; Chen, Z.; Zheng, W.; Lu, W. Soy food intake and breast cancer survival. JAMA 2009, 302, 2437–2443. [Google Scholar] [CrossRef] [PubMed]
- Deli, T.; Orosz, M.; Jakab, A. Hormone Replacement Therapy in Cancer Survivors—Review of the Literature. Pathol. Oncol. Res. 2020, 26, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xu, J.; Lefever, D.E.; Glenn, T.C.; Nagy, T.; Guo, T.L. Genistein prevention of hyperglycemia and improvement of glucose tolerance in adult non-obese diabetic mice are associated with alterations of gut microbiome and immune homeostasis. Toxicol. Appl. Pharmacol. 2017, 332, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Messina, N.; Mohn, W.W.; Finlay, B.B.; Curtis, N. Association between the intestinal microbiota and allergic sensitization, eczema, and asthma: A systematic review. J. Allergy Clin. Immunol. 2019, 143, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Plottel, C.S.; Blaser, M.J. Microbiome and malignancy. Cell Host Microbe 2011, 10, 324–335. [Google Scholar] [CrossRef]
- Vieira, A.T.; Castelo, P.M.; Ribeiro, D.A.; Ferreira, C.M. Influence of Oral and Gut Microbiota in the Health of Menopausal Women. Front. Microbiol. 2017, 8, 1884. [Google Scholar] [CrossRef]
- Szulińska, M.; Łoniewski, I.; van Hemert, S.; Sobieska, M.; Bogdański, P. Dose-Dependent Effects of Multispecies Probiotic Supplementation on the Lipopolysaccharide (LPS) Level and Cardiometabolic Profile in Obese Postmenopausal Women: A 12-Week Randomized Clinical Trial. Nutrients 2018, 10, 773. [Google Scholar] [CrossRef]
- Barrea, L.; Verde, L.; Auriemma, R.S.; Vetrani, C.; Cataldi, M.; Frias-Toral, E.; Pugliese, G.; Camajani, E.; Savastano, S.; Colao, A.; et al. Probiotics and Prebiotics: Any Role in Menopause-Related Diseases? Curr. Nutr. Rep. 2023, 12, 83–97. [Google Scholar] [CrossRef]
- Baker, F.C.; de Zambotti, M.; Colrain, I.M.; Bei, B. Sleep problems during the menopausal transition: Prevalence, impact, and management challenges. Nat. Sci. Sleep 2018, 10, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Hasheminezhad, R.; Hosseinian-Far, A.; Rasoulpoor, S.; Assefi, M.; Nankali, S.; Nankali, A.; Mohammadi, M. Global prevalence of sleep disorders during menopause: A meta-analysis. Sleep Breath 2023, 27, 1883–1897. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.; Kontopantelis, E.; Kuligowski, G.; Gray, M.; Muhyaldeen, A.; Gale, C.P.; Peat, G.M.; Cleator, J.; Chew-Graham, C.; Loke, Y.K.; et al. Self-Reported Sleep Duration and Quality and Cardiovascular Disease and Mortality: A Dose-Response Meta-Analysis. J. Am. Heart Assoc. 2018, 7, e008552. [Google Scholar] [CrossRef] [PubMed]
- Sejbuk, M.; Mironczuk-Chodakowska, I.; Witkowska, A.M. Sleep Quality: A Narrative Review on Nutrition, Stimulants, and Physical Activity as Important Factors. Nutrients 2022, 14, 1912. [Google Scholar] [CrossRef] [PubMed]
- Lloyd-Jones, D.M.; Allen, N.B.; Anderson, C.A.M.; Black, T.; Brewer, L.C.; Foraker, R.E.; Grandner, M.A.; Lavretsky, H.; Perak, A.M.; Sharma, G.; et al. Life’s Essential 8: Updating and Enhancing the American Heart Association’s Construct of Cardiovascular Health: A Presidential Advisory From the American Heart Association. Circulation 2022, 146, e18–e43. [Google Scholar] [CrossRef] [PubMed]
- Szakács, Z. The relationship between sleep and digestion. Cent. Eur. J. Gastroenterol. Hepatol. 2020, 6, 106–112. [Google Scholar] [CrossRef]
- Khanijow, V.; Prakash, P.; Emsellem, H.A.; Borum, M.L.; Doman, D.B. Sleep Dysfunction and Gastrointestinal Diseases. Gastroenterol. Hepatol. 2015, 11, 817–825. [Google Scholar]
- Nagai, M.; Hoshide, S.; Kario, K. Sleep duration as a risk factor for cardiovascular disease- a review of the recent literature. Curr. Cardiol. Rev. 2010, 6, 54–61. [Google Scholar] [CrossRef]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H.B. Dietary Sources and Bioactivities of Melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef]
- Cortés-Montaña, D.; Bernalte-García, M.J.; Velardo-Micharet, B.; Serrano, M.; Serradilla, M.J. Impact of Pre-Storage Melatonin Application on the Standard, Sensory, and Bioactive Quality of Early Sweet Cherry. Foods 2023, 12, 1723. [Google Scholar] [CrossRef]
- USDA. Food Data Central. Available online: https://fdc.nal.usda.gov/fdc-app.html#/?component=1210 (accessed on 20 November 2023).
- Zuraikat, F.M.; Wood, R.A.; Barragán, R.; St-Onge, M.P. Sleep and Diet: Mounting Evidence of a Cyclical Relationship. Annu. Rev. Nutr. 2021, 41, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Asgari Mehrabadi, M.; Azimi, I.; Sarhaddi, F.; Axelin, A.; Niela-Vilen, H.; Myllyntausta, S.; Stenholm, S.; Dutt, N.; Liljeberg, P.; Rahmani, A.M. Sleep Tracking of a Commercially Available Smart Ring and Smartwatch Against Medical-Grade Actigraphy in Everyday Settings: Instrument Validation Study. JMIR Mhealth Uhealth 2020, 8, e20465. [Google Scholar] [CrossRef] [PubMed]
- Tobin, S.Y.; Williams, P.G.; Baron, K.G.; Halliday, T.M.; Depner, C.M. Challenges and Opportunities for Applying Wearable Technology to Sleep. Sleep Med. Clin. 2021, 16, 607–618. [Google Scholar] [CrossRef] [PubMed]
| Food/Raw Material | Tryptophan Content (mg/100 g) |
|---|---|
| Cheddar cheese | 574 |
| Tuna | 313 |
| Salmon | 285 |
| Oat flakes | 234 |
| Buckwheat | 192 |
| Tofu | 235 |
| Kidney beans | 104 |
| Pumpkin seeds | 576 |
| Sunflower seeds | 295 |
| Almond | 209 |
| Peanut | 193 |
| Lamb (shoulders) | 415 |
| Chicken breast | 404 |
| Ground pork, lean | 326 |
| Turkey breast | 287 |
| Egg, whole | 167 |
| Body composition |
| Use body composition analysis tools to assess nutritional status Keep the weight in healthy range with adequate nutrients intake |
| Manage overweight, obesity: reduce current energy by 500–700 kcal/day Regularly physical activity |
| Dietary recommendations |
| Protein—0.8–1–1.2 g/bwkg/day |
| Calcium, vitamin D, vitamin C, vitamin B |
| n-3 LCPUFA, omega-3 fatty acids |
| Vegetables: 300–400 g/day, 3–4 portions/day |
| Fruits: 100–200 g, 1–2 portions/day |
| Legumes: beans, peas, lentils, chickpeas, soy/at least once a week |
| Low-fat dairy products, half a liter of milk—calcium Red meat: 350–500 g boiled/steamed/fried—per week Deep-sea fish: 100–120 g/occasion/at least two servings per week 30 g unsalted nuts, oily seeds/per day 30–45 g/day dietary fiber: whole grain, fiber-rich cereals |
| To be avoided |
| Simple, fast-acting sugars |
| Smoking Sugary and alcoholic beverages Sedentary life Salt (max. 5 g/day) Saturated fat—not exceed 10% of the total energy intake |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Erdélyi, A.; Pálfi, E.; Tűű, L.; Nas, K.; Szűcs, Z.; Török, M.; Jakab, A.; Várbíró, S. The Importance of Nutrition in Menopause and Perimenopause—A Review. Nutrients 2024, 16, 27. https://doi.org/10.3390/nu16010027
Erdélyi A, Pálfi E, Tűű L, Nas K, Szűcs Z, Török M, Jakab A, Várbíró S. The Importance of Nutrition in Menopause and Perimenopause—A Review. Nutrients. 2024; 16(1):27. https://doi.org/10.3390/nu16010027
Chicago/Turabian StyleErdélyi, Aliz, Erzsébet Pálfi, László Tűű, Katalin Nas, Zsuzsanna Szűcs, Marianna Török, Attila Jakab, and Szabolcs Várbíró. 2024. "The Importance of Nutrition in Menopause and Perimenopause—A Review" Nutrients 16, no. 1: 27. https://doi.org/10.3390/nu16010027
APA StyleErdélyi, A., Pálfi, E., Tűű, L., Nas, K., Szűcs, Z., Török, M., Jakab, A., & Várbíró, S. (2024). The Importance of Nutrition in Menopause and Perimenopause—A Review. Nutrients, 16(1), 27. https://doi.org/10.3390/nu16010027