Strontium Ranelate and Strontium Chloride Supplementation Influence on Bone Microarchitecture and Bone Turnover Markers—A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
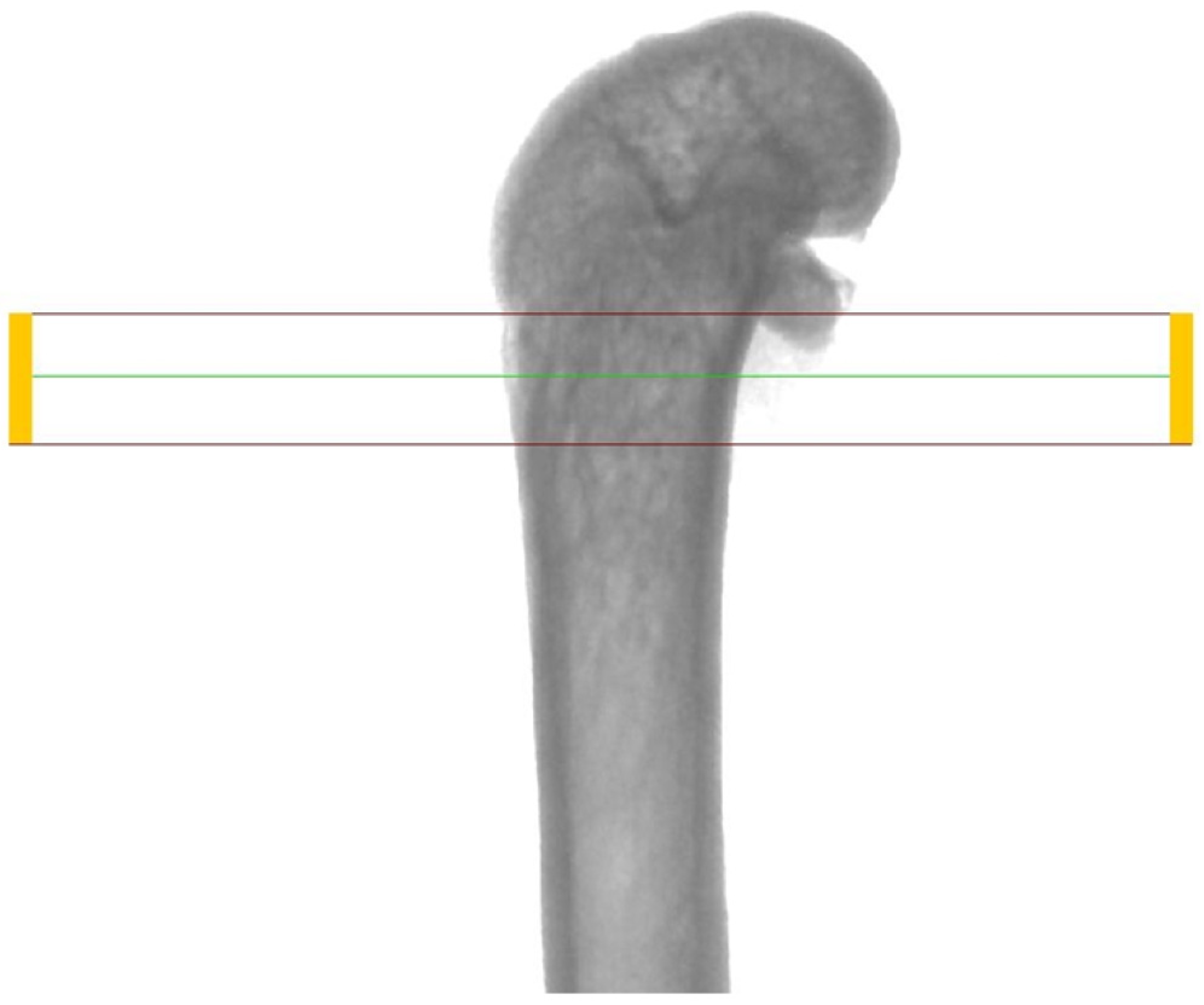
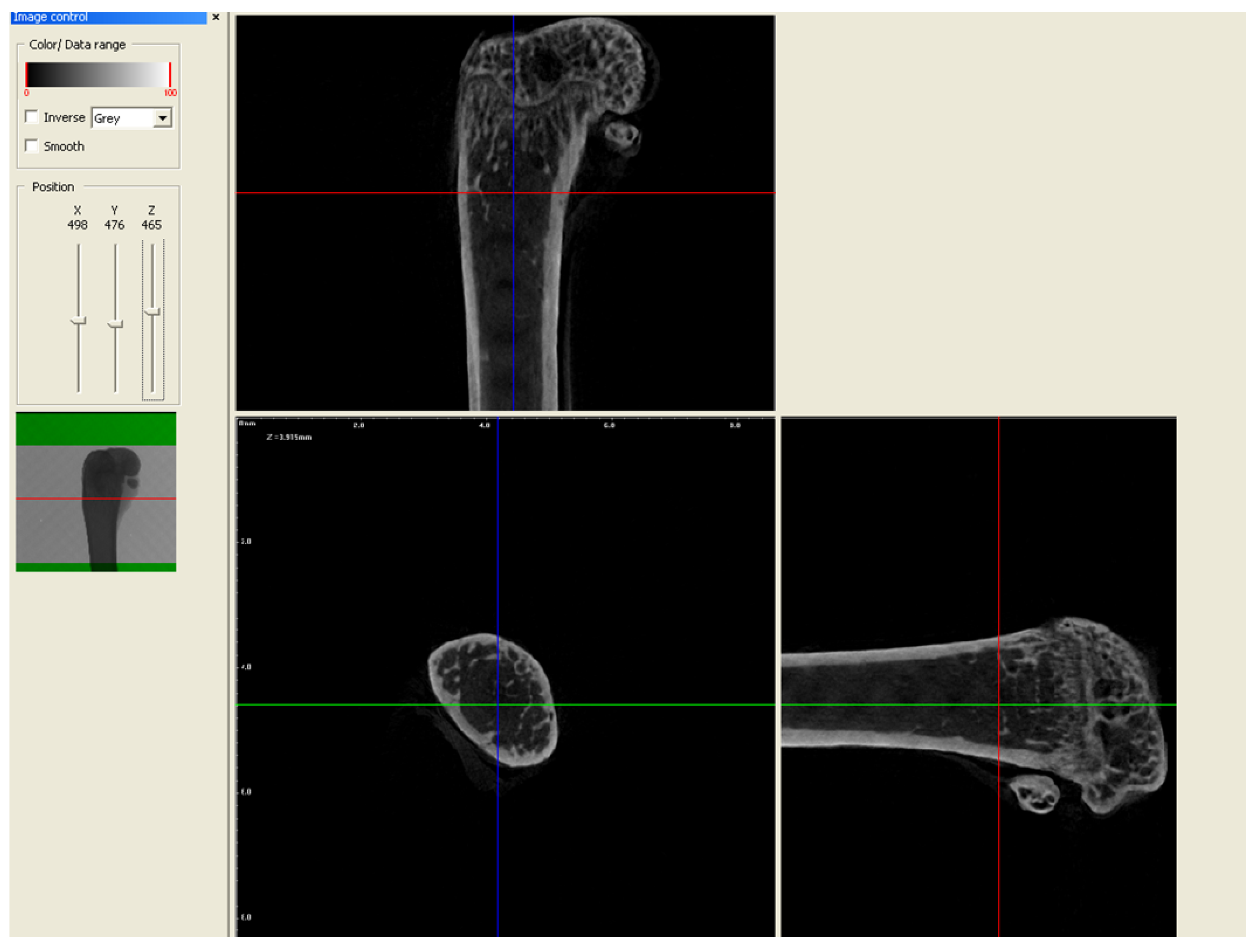
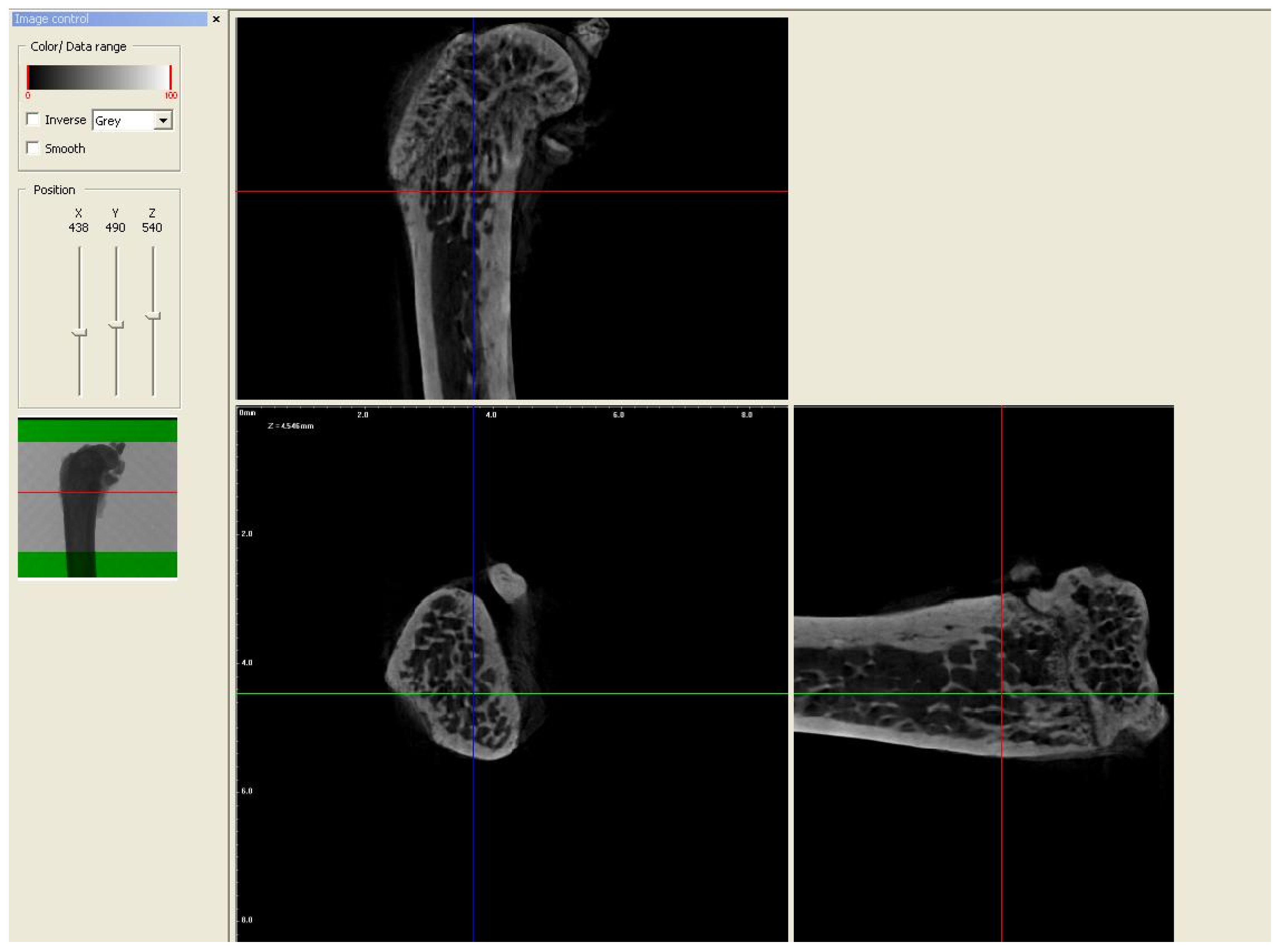
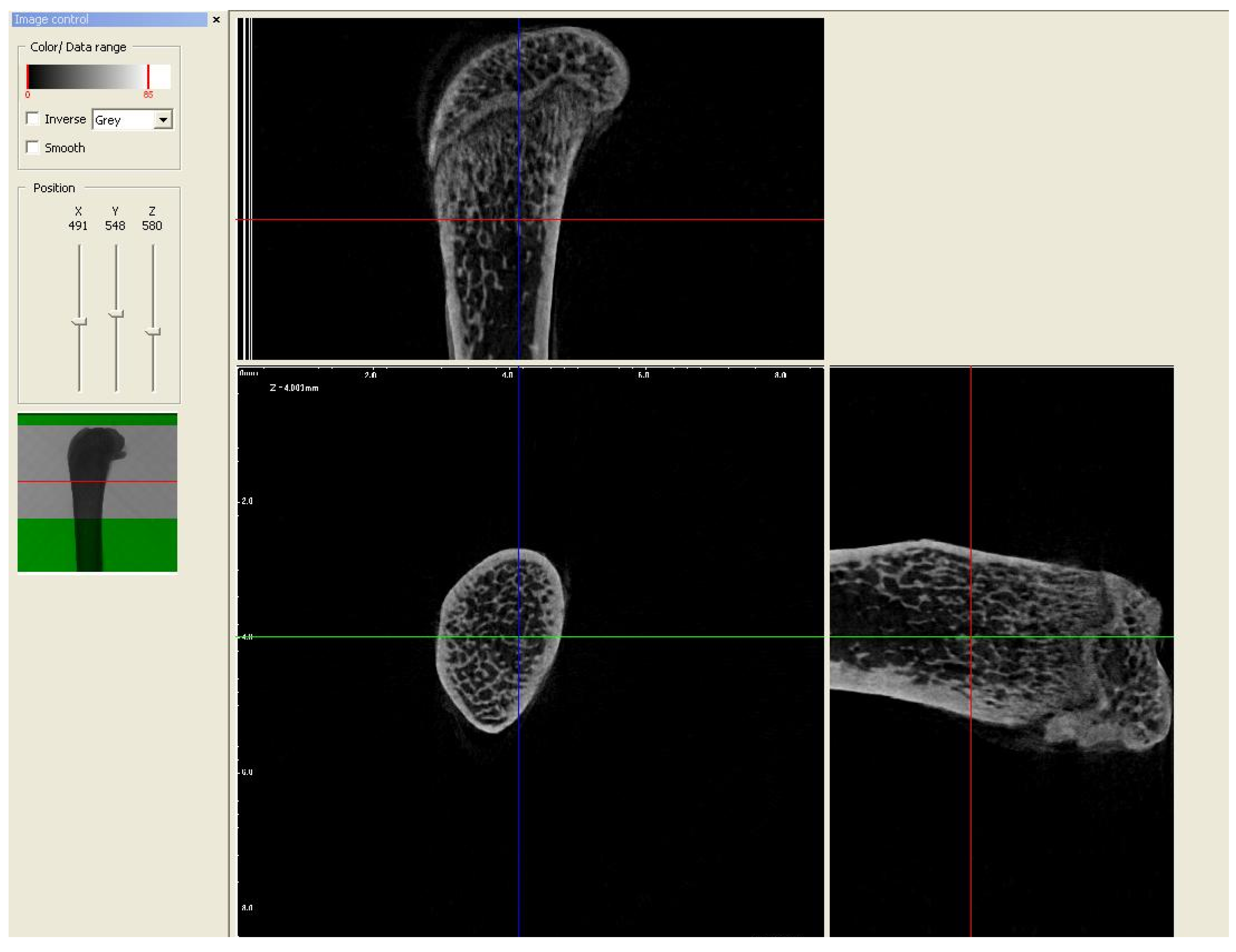
2.2. Micro-CT Analysis
2.3. Examination of the Level of Bone Turnover Markers in the Blood Serum
2.4. Statistical Analysis
3. Results
3.1. General Observations
3.2. Body Mass
3.3. Trabecular Micro-Architecture
3.3.1. Trabecular Thickness (Tb.Th), Trabecular Number (Tb.N), and Percent of Bone Volume (BV/TV)
3.3.2. Trabecular Separation
3.4. Cortical Bone Area
3.5. Bone Turnover Markers
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arceo-Mendoza, R.M.; Camacho, P.M. Postmenopausal Osteoporosis: Latest Guidelines. Endocrinol. Metab. Clin. N. Am. 2021, 50, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Golden, N.H. Bones and Birth Control in Adolescent Girls. J. Pediatr. Adolesc. Gynecol. 2020, 33, 249–254. [Google Scholar]
- Hadji, P.; Colli, E.; Regidor, P.A. Bone Health in Estrogen-Free Contraception. Osteoporos. Int. 2019, 30, 2391–2400. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zheng, H. Factors influencing peak bone mass gain. Front. Med. 2021, 15, 53–69. [Google Scholar] [CrossRef] [PubMed]
- McDevitt, H.; McGowan, A.; Ahmed, S.F. Establishing good bone health in children. Paediatr. Child. Health 2014, 24, 78–82. [Google Scholar] [CrossRef]
- Karlsson, M.K.; Rosengren, B.E. Exercise and Peak Bone Mass. Curr. Osteoporos. Rep. 2020, 18, 285–290. [Google Scholar] [CrossRef]
- Guthold, R.; Stevens, G.A.; Riley, L.M.; Bull, F.C. Worldwide trends in insufficient physical activity from 2001 to 2016: A pooled analysis of 358 population-based surveys with 1.9 million participants. Lancet Glob. Health 2018, 6, 1077–1086. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Carter, D.R.; Beaupré, G.S. A Theoretical Analysis of the Relative Influences of Peak BMD, Age-Related Bone Loss and Menopause on the Development of Osteoporosis. Osteoporos. Int. 2003, 14, 843–847. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Barrile, G.C.; Cavioni, A.; Mansueto, F.; Mazzola, G.; Oberto, L.; Patelli, Z.; Pirola, M.; Tartara, A.; et al. Nutrition, Physical Activity, and Dietary Supplementation to Prevent Bone Mineral Density Loss: A Food Pyramid. Nutrients 2021, 14, 74. [Google Scholar] [CrossRef]
- Marx, D.; Rahimnejad Yazdi, A.; Papini, M.; Towler, M. A review of the latest insights into the mechanism of action of strontium in bone. Bone Rep. 2020, 24, 100273. [Google Scholar] [CrossRef]
- Pilmane, M.; Salma-Ancane, K.; Loca, D.; Locs, J.; Berzina-Cimdina, L. Strontium and strontium ranelate: Historical review of some of their functions. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 1, 1222–1230. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Berencsi, K.; Marinier, K.; Deltour, N.; Perez-Guthann, S.; Pedersen, L.; Rijnbeek, P.; Lapi, F.; Simonetti, M.; Reyes, C.; et al. Comparative cardiovascular safety of strontium ranelate and bisphosphonates: A multi-database study in 5 EU countries by the EU-ADR Alliance. Osteoporos. Int. 2020, 31, 2425–2438. [Google Scholar] [CrossRef] [PubMed]
- Strontium Ranelate Returns to UK as an Osteoporosis Drug Treatment. Available online: https://www.osteodorset.org.uk/2019/02/strontium-ranelate-returns-to-the-uk-as-an-osteoporosis-drug-treatment/ (accessed on 5 October 2023).
- Westberg, S.M.; Awker, A.; Torkelson, C.J. Use of Strontium Chloride for the Treatment of Osteoporosis: A Case Report. Altern. Ther. Health Med. 2016, 22, 66–70. [Google Scholar] [PubMed]
- Tordoff, M.G.; Bachmanov, A.A.; Reed, D.R. Forty mouse strain survey of water and sodium intake. Physiol. Behav. 2007, 91, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Bachmanov, A.A.; Reed, D.R.; Beauchamp, G.K.; Tordoff, M.G. Food intake, water intake, and drinking spout side preference of 28 mouse strains. Behav. Genet. 2002, 32, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Apostoaei, A.I. Absorption of strontium from the gastrointestinal tract into plasma in healthy human adults. Health Phys. 2002, 83, 56–65. [Google Scholar] [CrossRef]
- Jabłoński, M.B.; Stefaniak, E.A.; Darchuk, L.; Turżańska, K.; Gorzelak, M.; Kuduk, R.; Dorriné, W.; Van Grieken, R. Microchemical investigation of bone derived from mice treated with strontium in different chemical forms using scanning electron microscopy and micro-Raman spectroscopy. Microchem. J. 2013, 108, 168–173. [Google Scholar] [CrossRef]
- Rosales Rocabado, J.M.; Kaku, M.; Nozaki, K.; Ida, T.; Kitami, M.; Aoyagi, Y.; Uoshima, K. A multi-factorial analysis of bone morphology and fracture strength of rat femur in response to ovariectomy. J. Orthop. Surg. Res. 2018, 13, 318. [Google Scholar] [CrossRef]
- Aveline, P.; Cesaro, A.; Mazor, M.; Best, T.M.; Lespessailles, E.; Toumi, H. Cumulative Effects of Strontium Ranelate and Impact Exercise on Bone Mass in Ovariectomized Rats. Int. J. Mol. Sci. 2021, 22, 3040. [Google Scholar] [CrossRef]
- Delannoy, P.; Bazot, D.; Marie, P.J. Long-term treatment with strontium ranelate increases vertebral bone mass without deleterious effect in mice. Metabolism 2002, 51, 906–911. [Google Scholar] [CrossRef]
- Ammann, P.; Badoud, I.; Barraud, S.; Dayer, R.; Rizzoli, R. Strontium ranelate treatment improves trabecular and cortical intrinsic bone tissue quality, a determinant of bone strength. J. Bone Miner. Res. 2007, 22, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Wohl, G.R.; Chettle, D.R.; Pejović-Milić, A.; Druchok, C.; Webber, C.E.; Adachi, J.D.; Beattie, K.A. Accumulation of bone strontium measured by in vivo XRF in rats supplemented with strontium citrate and strontium ranelate. Bone 2013, 52, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Hu, B.; Guo, L.; Cao, P.; Tian, Y.; Ma, J.; Chen, Y.; Wu, H.; Hu, J.; Deng, L.; et al. Strontium Ranelate Reduces the Fracture Incidence in a Growing Mouse Model of Osteogenesis Imperfecta. J. Bone Miner. Res. 2016, 31, 1003–1014. [Google Scholar] [CrossRef] [PubMed]
- Camcı, H.; Doruk, C.; Saraydın, S.Ü. Effect of Strontium Ranelate on Condylar Growth during Mandibular Advancement in Rats. Turk. J. Orthod. 2020, 33, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Pors Nielsen, S. The biological role of strontium. Bone 2004, 35, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Newman, F.W.; Newman, M.W. The Chemical Dynamics of Bone Mineral; The University of Chicago Press: Chicago, IL, USA, 1958. [Google Scholar]
- Ressler, A.; Žužić, A.; Ivanišević, I.; Kamboj, N.; Ivanković, H. Ionic substituted hydroxyapatite for bone regeneration applications: A review. Open Ceram. 2021, 6, 100–122. [Google Scholar] [CrossRef]
- Cardemil, C.; Elgali, I.; Xia, W.; Emanuelsson, L.; Norlindh, B.; Omar, O.; Thomsen, P. Strontium-doped calcium phosphate and hydroxyapatite granules promote different inflammatory and bone remodelling responses in normal and ovariectomised rats. PLoS ONE 2013, 8, e84932. [Google Scholar] [CrossRef]
- Boivin, G.; Deloffre, P.; Perrat, B.; Panczer, G.; Boudeulle, M.; Mauras, Y.; Allain, P.; Tsouderos, Y.; Meunier, P.J. Strontium distribution and interactions with bone mineral in monkey iliac bone after strontium salt (S 12911) administration. J. Bone Miner. Res. 1996, 11, 1302–1311. [Google Scholar] [CrossRef]
- Marie, P.J.; Ammann, P.; Boivin, G.; Rey, C. Mechanisms of action and therapeutic potential of strontium in bone. Calcif. Tissue Int. 2001, 69, 121–129. [Google Scholar] [CrossRef]
- Cabrera, W.E.; Schrooten, I.; De Broe, M.E.; D’Haese, P.C. Strontium and bone. J. Bone Miner. Res. 1999, 14, 661–668. [Google Scholar] [CrossRef]
- Oste, L.; Bervoets, A.R.; Behets, G.J.; Dams, G.; Marijnissen, R.L.; Geryl, H.; Lamberts, L.V.; Verberckmoes, S.C.; Van Hoof, V.O.; De Broe, M.E.; et al. Time-evolution and reversibility of strontium-induced osteomalacia in chronic renal failure rats. Kidney Int. 2005, 67, 920–930. [Google Scholar] [CrossRef] [PubMed]
- El-Hajj Fuleihan, G. Strontium ranelate-a novel therapy for osteoporosis or a permutation of the same? N. Engl. J. Med. 2004, 350, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Grynpas, M.D.; Marie, P.J. Effects of low doses of strontium on bone quality and quantity in rats. Bone 1990, 11, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Bunger, M.H.; Oxlund, H.; Hansen, T.K.; Sørensen, S.; Bibby, B.M.; Thomsen, J.S.; Langdahl, B.L.; Besenbacher, F.; Pedersen, J.S.; Birkedal, H. Strontium and bone nanostructure in normal and ovariectomized rats investigated by scanning small-angle x-ray scattering. Calcif. Tissue Int. 2010, 86, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P.; Naylor, K.; Hoyle, N.R.; Eastell, R.; Leary, E.T. National Bone Health Alliance Bone Turnover Marker Project. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos. Int. 2017, 28, 2541–2556. [Google Scholar] [CrossRef] [PubMed]
- Lorentzon, M.; Branco, J.; Brandi, M.L.; Bruyère, O.; Chapurlat, R.; Cooper, C.; Cortet, B.; Diez-Perez, A.; Ferrari, S.; Gasparik, A.; et al. Algorithm for the Use of Biochemical Markers of Bone Turnover in the Diagnosis, Assessment and Follow-Up of Treatment for Osteoporosis. Adv. Ther. 2019, 36, 2811–2824. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, J.P.; Suominen, M.I.; Peng, Z.; Morko, J.; Rasi, S.; Risteli, J.; Halleen, J.M. Short-term changes in serum PINP predict long-term changes in trabecular bone in the rat ovariectomy model. Calcif. Tissue Int. 2008, 82, 155–161. [Google Scholar] [CrossRef]
- Hale, L.V.; Galvin, R.S.; Risteli, J.; Ma, Y.; Harvey, A.; Yang, X.; Cain, R.; Zeng, Q.; Frolik, C.; Sato, M.; et al. PINP: A serum biomarker of bone formation in the rat. Bone 2007, 40, 1103–1109. [Google Scholar] [CrossRef]
- Mackenzie, N.C.; Zhu, D.; Milne, E.M.; van’t Hof, R.; Martin, A.; Quarles, D.L. Altered bone development and an increase in FGF-23 expression in Enpp1(−/−) mice. PLoS ONE 2012, 7, 32177. [Google Scholar] [CrossRef]
- Pennisi, P.; Clementi, G.; Prato, A.; Luca, T.; Martinez, G.; Mangiafico, R.A.; Pulvirenti, I.; Muratore, F.; Fiore, C.E. L-arginine supplementation normalizes bone turnover and preserves bone mass in streptozotocin-induced diabetic rats. J. Endocrinol. Investig. 2009, 32, 546–551. [Google Scholar] [CrossRef]
- Marie, P.J. Strontium ranelate: A novel mode of action optimizing bone formation and resorption. Osteoporos. Int. 2005, 16 (Suppl. S1), 7–10. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, N.A. Strontium ranelate improves bone microarchitecture in osteoporosis. Rheumatology 2009, 48 (Suppl. S4), 9–13. [Google Scholar]
- Brun, L.R.; Galich, A.M.; Vega, E.; Salerni, H.; Maffei, L.; Premrou, V. Strontium ranelate effect on bone mineral density is modified by previous bisphosphonate treatment. Springer Plus 2014, 3, 676. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Laroche, M.; Krieg, M.A.; Frieling, I.; Thomas, T.; Delmas, P.; Felsenberg, D. Strontium ranelate and alendronate have differing effects on distal tibia bone microstructure in women with osteoporosis. Rheumatol. Int. 2010, 30, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Gómez, J.M.; Muschitz, C.; Gómez-Reino, J.; Greisen, H.; Andersen, H.S.; Dimai, H.P. The effect of PTH(1-84) or strontium ranelate on bone formation markers in postmenopausal women with primary osteoporosis: Results of a randomized, open-label clinical trial. Osteoporos. Int. 2011, 22, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Maria, S.; Swanson, M.H.; Enderby, L.T.; D’Amico, F.; Enderby, B.; Samsonraj, R.M.; Dudakovic, A.; van Wijnen, A.J.; Witt-Enderby, P.A. Melatonin-micronutrients Osteopenia Treatment Study (MOTS): A translational study assessing melatonin, strontium (citrate), vitamin D3 and vitamin K2 (MK7) on bone density, bone marker turnover and health related quality of life in postmenopausal osteopenic women following a one-year double-blind RCT and on osteoblast-osteoclast co-cultures. Aging 2017, 9, 256–285. [Google Scholar]
- Recker, R.R.; Marin, F.; Ish-Shalom, S.; Möricke, R.; Hawkins, F.; Kapetanos, G.; de la Peña, M.P.; Kekow, J.; Farrerons, J.; Sanz, B.; et al. Comparative effects of teriparatide and strontium ranelate on bone biopsies and biochemical markers of bone turnover in postmenopausal women with osteoporosis. J. Bone Miner. Res. 2009, 24, 1358–1368. [Google Scholar] [CrossRef]
- Abboskhujaeva, L.S.; Ismailov, S.I.; Alikhanova, N.M. Efficacy of strontium ranelate in combination with a D-hormone analog for the treatment of postmenopausal osteoporosis. Drugs R&D 2014, 14, 315–324. [Google Scholar]
- Gomez-Chinchón, M.; Martin-Fernandez, M.; Diaz-Curiel, M.; De la Piedra, C. Effects of a preventive long duration treatment of ovariectomized rats with strontium ranelate and zoledronic acid on bone mass and bone remodeling. Bone Abstr. 2014, 3, 328. [Google Scholar]
- Tsai, T.T.; Tai, C.L.; Ho, N.Y.; Lai, P.L.; Fu, T.S.; Niu, C.C.; Chen, L.H.; Chen, W.J. Effects of Strontium Ranelate on Spinal Interbody Fusion Surgery in an Osteoporotic Rat Model. PLoS ONE 2017, 12, e0167296. [Google Scholar] [CrossRef]
- Oestergaard, S.; Chouinard, L.; Doyle, N.; Karsdal, M.A.; Smith, S.Y.; Qvist, P.; Tankó, L.B. The utility of measuring C-terminal telopeptides of collagen type II (CTX-II) in serum and synovial fluid samples for estimation of articular cartilage status in experimental models of destructive joint diseases. Osteoarthr. Cartil. 2006, 14, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Oestergaard, S.; Chouinard, L.; Doyle, N.; Smith, S.Y.; Tankó, L.B.; Qvist, P. Early elevation in circulating levels of C-telopeptides of type II collagen predicts structural damage in articular cartilage in the rodent model of collagen-induced arthritis. Arthritis Rheum. 2006, 54, 2886–2890. [Google Scholar] [CrossRef] [PubMed]
- Høegh-Andersen, P.; Tankó, L.B.; Andersen, T.L.; Lundberg, C.V.; Mo, J.A.; Heegaard, A.-M.; Delaissé, J.-M.; Christgau, S. Ovariectomized rats as a model of postmenopausal osteoarthritis: Validation and application. Arthritis Res. Ther. 2004, 6, R169. [Google Scholar] [CrossRef] [PubMed]
- Boeth, H.; MacMahon, A.; Poole, A.R.; Buttgereit, F.; Önnerfjord, P.; Lorenzo, P.; Klint, C.; Pramhed, A.; Duda, G.N. Differences in biomarkers of cartilage matrix turnover and their changes over 2 years in adolescent and adult volleyball athletes. J. Exp. Ortop. 2017, 4, 7. [Google Scholar] [CrossRef] [PubMed]
- Brinckmann, P.; Biggemann, M.; Hilweg, D. Prediction of the compressive strength of human lumbar vertebrae. Clin. Biomech. 1989, 4, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Ferreni, J.L.; Capozza, R.F.; Mondelo, N.; Zanchetta, J.R. Interrelationships between densitometrical, geometric and mechanical properties of rat femurs. Inferences concerning mechanical regulation of bone modeling. J. Bone Miner. Res. 1993, 8, 1389–1396. [Google Scholar]
- Francisco, J.I.; Yu, Y.; Oliver, R.A.; Walsh, W.R. Relationship between age, skeletal site, and time post-ovariectomy on bone mineral and trabecular microarchitecture in rats. J. Orthop. Res. 2011, 29, 189–196. [Google Scholar] [CrossRef]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Ammann, P.; Shen, V.; Robin, B.; Mauras, Y.; Bonjour, J.P.; Rizzoli, R. Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female rats. J. Bone Miner. Res. 2004, 19, 2012–2020. [Google Scholar]
- Peng, S.; Liu, X.S.; Wang, T.; Li, Z.; Zhou, G.; Luk, K.D.; Lu, W.W. In vivo anabolic effect of strontium on trabecular bone was associated with increased osteoblastogenesis of bone marrow stromal cells. J. Orthop. Res. 2010, 28, 1208–1214. [Google Scholar] [CrossRef]
- Marie, P.J.; Hott, M.; Modrowski, D.; De Pollak, C.; Guillemain, J.; Deloffre, P.; Tsouderos, Y. An uncoupling agent containing strontium prevents bone loss by depressing bone resorption and maintaining bone formation in estrogen-deficient rats. J. Bone Miner. Res. 1993, 8, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Meunier, P.J.; Slosman, D.O.; Delmas, P.D.; Sebert, J.L.; Brandi, M.L.; Albanese, C.; Lorenc, R.; Pors-Nielsen, S.; De Vernejoul, M.C.; Roces, A.; et al. Strontium ranelate: Dose-dependent effects in established postmenopausal vertebral osteoporosis--a 2-year randomized placebo controlled trial. J. Clin. Endocrinol. Metab. 2002, 87, 2060–2066. [Google Scholar] [PubMed]
- Arlot, M.E.; Jiang, Y.; Genant, H.K.; Zhao, J.; Burt-Pichat, B.; Roux, J.P.; Delmas, P.D.; Meunier, P.J. Histomorphometric and microCT analysis of bone biopsies from postmenopausal osteoporotic women treated with strontium ranelate. J. Bone Miner. Res. 2008, 23, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Zacchetti, G.; Dayer, R.; Rizzoli, R.; Ammann, P. Systemic treatment with strontium ranelate accelerates the filling of a bone defect and improves the material level properties of the healing bone. Biomed. Res. Int. 2014, 2014, 549785. [Google Scholar] [CrossRef] [PubMed]
- Johannesdottir, F.; Putman, M.S.; Burnett-Bowie, S.M.; Finkelstein, J.S.; Yu, E.W.; Bouxsein, M.L. Age-Related Changes in Bone Density, Microarchitecture, and Strength in Postmenopausal Black and White Women: The SWAN Longitudinal HR-pQCT Study. J. Bone Miner. Res. 2022, 37, 41–51. [Google Scholar] [CrossRef]
- Blake, G.M.; Fogelman, I. Effect of bone strontium on BMD measurements. J. Clin. Densitom. 2007, 10, 34–38. [Google Scholar] [CrossRef]
- Pemmer, B.; Hofstaetter, J.G.; Meirer, F.; Smolek, S.; Wobrauschek, P.; Simon, R.; Fuchs, R.K.; Allen, M.R.; Condon, K.W.; Reinwald, S.; et al. Increased strontium uptake in trabecular bone of ovariectomized calcium-deficient rats treated with strontium ranelate or strontium chloride. J. Synchrotron Radiat. 2011, 18 Pt 6, 835–841. [Google Scholar] [CrossRef]
- Kołodziejska, B.; Stępień, N.; Kolmas, J. The Influence of Strontium on Bone Tissue Metabolism and Its Application in Osteoporosis Treatment. Int. J. Mol. Sci. 2021, 22, 6564. [Google Scholar] [CrossRef]

| Group | Weight Gain (g) |
|---|---|
| Control | 11.993 ± 1.028 |
| Strontium chloride | 11.673 ± 1.653 |
| Strontium ranelate | 11.674 ± 1.268 |
| Group | N | Tb.Th (µm) Mean | Tb.Th (µm) Std.Dev. | Tb. Th (µm) Std.Err |
|---|---|---|---|---|
| Control | 14 | 6.289619 | 1.605175 | 0.429001 |
| Strontium chloride | 14 | 8.033544 * | 1.837590 | 0.491117 |
| Strontium ranelate | 14 | 8.665711 ** | 1.869460 | 0.499634 |
| Tukey HSD Test; Variable Tb.Th (µm) Approximate Probabilities for Post Hoc Tests Error: Between MS = 3.1494, df = 39.000 | ||||
|---|---|---|---|---|
| Group | (1) 6.2896 | (2) 8.0335 | (3) 8.6657 | |
| 1 | Control | 0.034361 * | 0.003032 ** | |
| 2 | Strontium chloride | 0.034361 * | 0.617156 | |
| 3 | Strontium ranelate | 0.003032 ** | 0.617156 | |
| Group | N | Tb.N (1/mm) Mean | Tb.N (1/mm) Std.Dev. | Tb.N (1/mm) Std.Err |
|---|---|---|---|---|
| Control | 14 | 0.008873 | 0.008141 | 0.002176 |
| Strontium chloride | 14 | 0.022479 ** | 0.009416 | 0.002517 |
| Strontium ranelate | 14 | 0.027754 *** | 0.012664 | 0.003385 |
| Tukey HSD Test; Variable Tb.N (1/mm) Approximate Probabilities for Post Hoc Tests Error: Between MS = 0.00011, df = 39.000 | ||||
|---|---|---|---|---|
| Group | (1) 0.00887 | (2) 0.02248 | (3) 0.02775 | |
| 1 | Control | 0.003299 ** | 0.000170 *** | |
| 2 | Strontium chloride | 0.003299 ** | 0.371000 | |
| 3 | Strontium ranelate | 0.000170 *** | 0.371000 | |
| Group | N | BV/TV (%) Mean | BV/TV (%) Std.Dev. | BV/TV (%) Std.Err |
|---|---|---|---|---|
| Control | 14 | 6.16186 | 5.74342 | 1.534992 |
| Strontium chloride | 14 | 18.54775 ** | 10.27522 | 2.746167 |
| Strontium ranelate | 14 | 23.70778 *** | 11.46758 | 3.064840 |
| Tukey HSD Test; Variable BV/TV (%) Approximate Probabilities for Post Hoc Tests Error: Between MS = 90.024, df = 39.000 | ||||
|---|---|---|---|---|
| Group | (1) 6.1619 | (2) 18.548 | (3) 23.708 | |
| 1 | Control | 0.003861 ** | 0.000167 *** | |
| 2 | Strontium chloride | 0.003861 ** | 0.331406 | |
| 3 | Strontium ranelate | 0.000167 *** | 0.331406 | |
| Group | N | Tb.Sp (µm) Mean | Tb.Sp (µm) Std.Dev. | Tb.Sp (µm) Std.Err |
|---|---|---|---|---|
| Control | 14 | 37.971 | 13.366 | 0.068 |
| Strontium chloride | 14 | 25.119 * | 9.075 | 0.046 |
| Strontium ranelate | 14 | 21.255 ** | 7.36 | 0.037 |
| Multiple Comparisons p Values (2-Tailed); Tb.Sp. (µm) Independent (Grouping) Variable: Group Kruskal–Wallis test: H (2, N = 42) = 11.44850 p = 0.0033 | |||
|---|---|---|---|
| Control R: 30.357 | Strontium Chloride R: 18.714 | Strontium Ranelate R: 15.429 | |
| Control | 0.036121 * | 0.003851 ** | |
| Strontium chloride | 0.036121 * | 1.000000 | |
| Strontium ranelate | 0.003851 ** | 1.000000 | |
| Group | N | Cortical Area (µm2) Mean | Cortical Area (µm2) Std.Dev. | Cortical Area (µm2) Std.Err |
|---|---|---|---|---|
| Control | 14 | 14,802.05 | 2139.508 | 571.807 |
| Strontium chloride | 14 | 19,856.42 *** | 4972.380 | 1328.924 |
| Strontium ranelate | 14 | 19,246.10 ** | 2496.255 | 667.152 |
| Tukey HSD Test; Variable Cortical Area (µm2) Approximate Probabilities for Post Hoc Tests Error: Between MS = 1184 × 104 df = 39.000 | ||||
|---|---|---|---|---|
| Group | (1) 14,802. | (2) 19,856. | (3) 19,246. | |
| 1 | Control | 0.001199 *** | 0.004270 ** | |
| 2 | Strontium chloride | 0.001199 *** | 0.886159 | |
| 3 | Strontium ranelate | 0.004270 ** | 0.886159 | |
| Group | N | PINP (µg/L) Mean | PINP (µg/L) Std.Dev. | PINP (µg/L) Std.Err |
|---|---|---|---|---|
| Control | 9 | 8.679778 | 1.167847 | 0.389282 |
| Strontium chloride | 9 | 9.643667 | 2.670709 | 0.890236 |
| Strontium ranelate | 9 | 7.850000 | 1.582566 | 0.527522 |
| Group | N | CTXI (µg/L) Mean | CTXI (µg/L) Std.Dev. | CTXI (µg/L) Std.Err |
|---|---|---|---|---|
| Control | 9 | 21.95556 | 4.496855 | 1.498952 |
| Strontium chloride | 9 | 19.32400 | 5.389408 | 1.796469 |
| Strontium ranelate | 9 | 20.06567 | 8.385476 | 2.795159 |
| Group | N | CTXII (µg/L) Mean | CTXII (µg/L) Std.Dev. | CTXII (µg/L) Std.Err |
|---|---|---|---|---|
| Control | 9 | 153.8659 | 76.30417 | 25.43472 |
| Strontium chloride | 9 | 145.1188 | 72.77673 | 24.25891 |
| Strontium ranelate | 9 | 126.4287 | 58.02944 | 19.34315 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turżańska, K.; Tomczyk-Warunek, A.; Dobrzyński, M.; Jarzębski, M.; Patryn, R.; Niezbecka-Zając, J.; Wojciechowska, M.; Mela, A.; Zarębska-Mróz, A. Strontium Ranelate and Strontium Chloride Supplementation Influence on Bone Microarchitecture and Bone Turnover Markers—A Preliminary Study. Nutrients 2024, 16, 91. https://doi.org/10.3390/nu16010091
Turżańska K, Tomczyk-Warunek A, Dobrzyński M, Jarzębski M, Patryn R, Niezbecka-Zając J, Wojciechowska M, Mela A, Zarębska-Mróz A. Strontium Ranelate and Strontium Chloride Supplementation Influence on Bone Microarchitecture and Bone Turnover Markers—A Preliminary Study. Nutrients. 2024; 16(1):91. https://doi.org/10.3390/nu16010091
Chicago/Turabian StyleTurżańska, Karolina, Agnieszka Tomczyk-Warunek, Maciej Dobrzyński, Maciej Jarzębski, Rafał Patryn, Joanna Niezbecka-Zając, Monika Wojciechowska, Aneta Mela, and Aneta Zarębska-Mróz. 2024. "Strontium Ranelate and Strontium Chloride Supplementation Influence on Bone Microarchitecture and Bone Turnover Markers—A Preliminary Study" Nutrients 16, no. 1: 91. https://doi.org/10.3390/nu16010091








