The Influence of a Polyphenol-Rich Red Berry Fruit Juice on Recovery Process and Leg Strength Capacity after Six Days of Intensive Endurance Exercise in Recreational Endurance Athletes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Study Beverages
2.4. Analysis of Phenolic Ingredients
2.5. Study Day Schedule
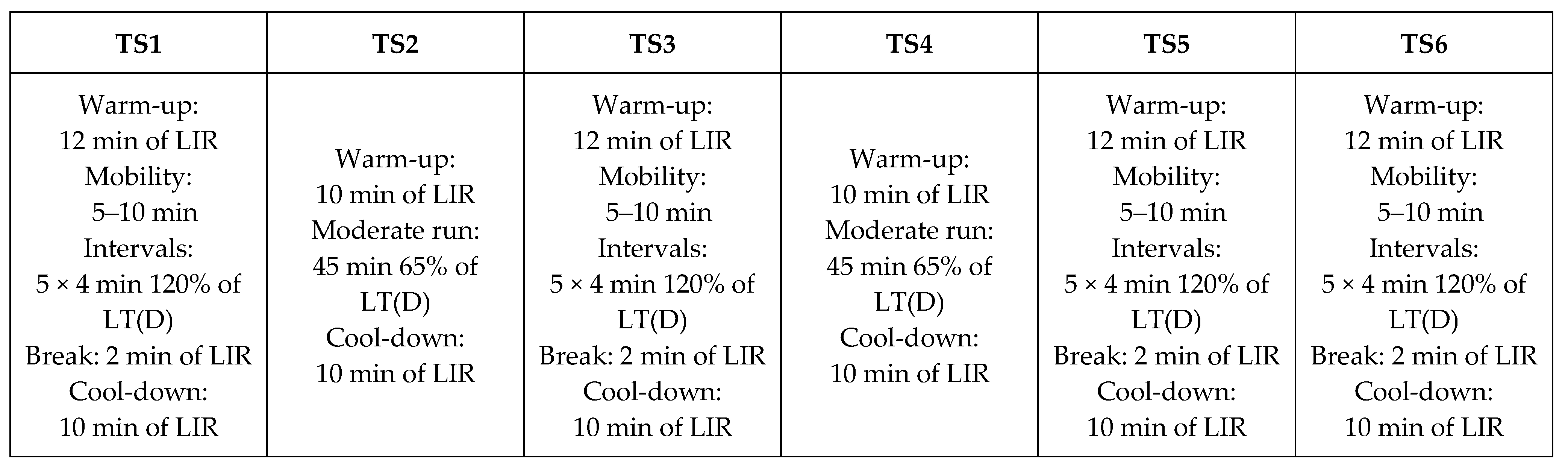
2.6. Endurance Training Protocol
2.7. Blood Analyses
2.8. Nutrition Records
2.9. Statistical Analyses
3. Results
3.1. Effects of Six-Day Running and Juice on Skeletal Muscle Damage—Creatine Kinase
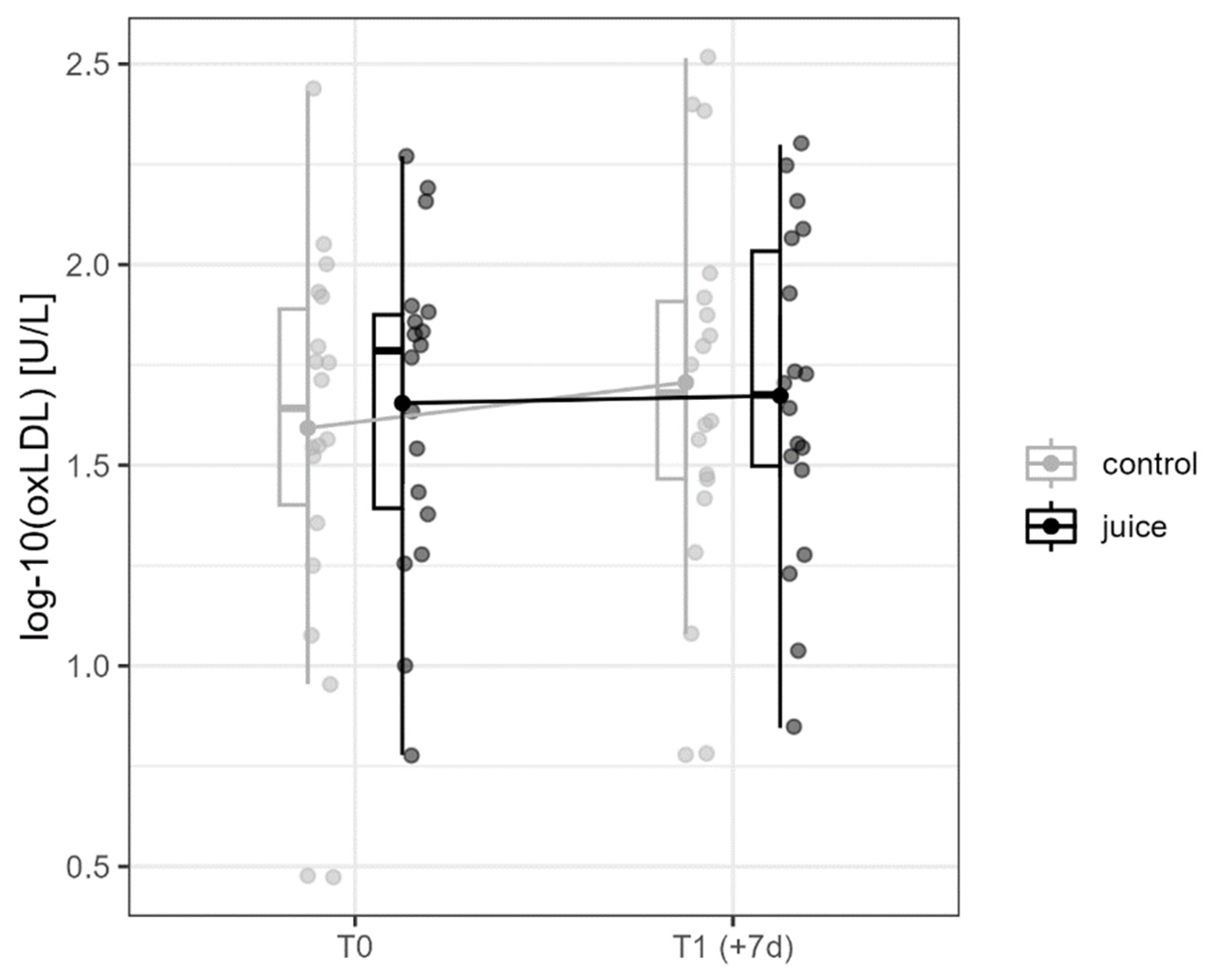
3.2. Effects of Six-Day Running and Juice on oxLDL
3.3. Effects of Juice on Recovery Processes and Strength Capacity following Six-Day Running
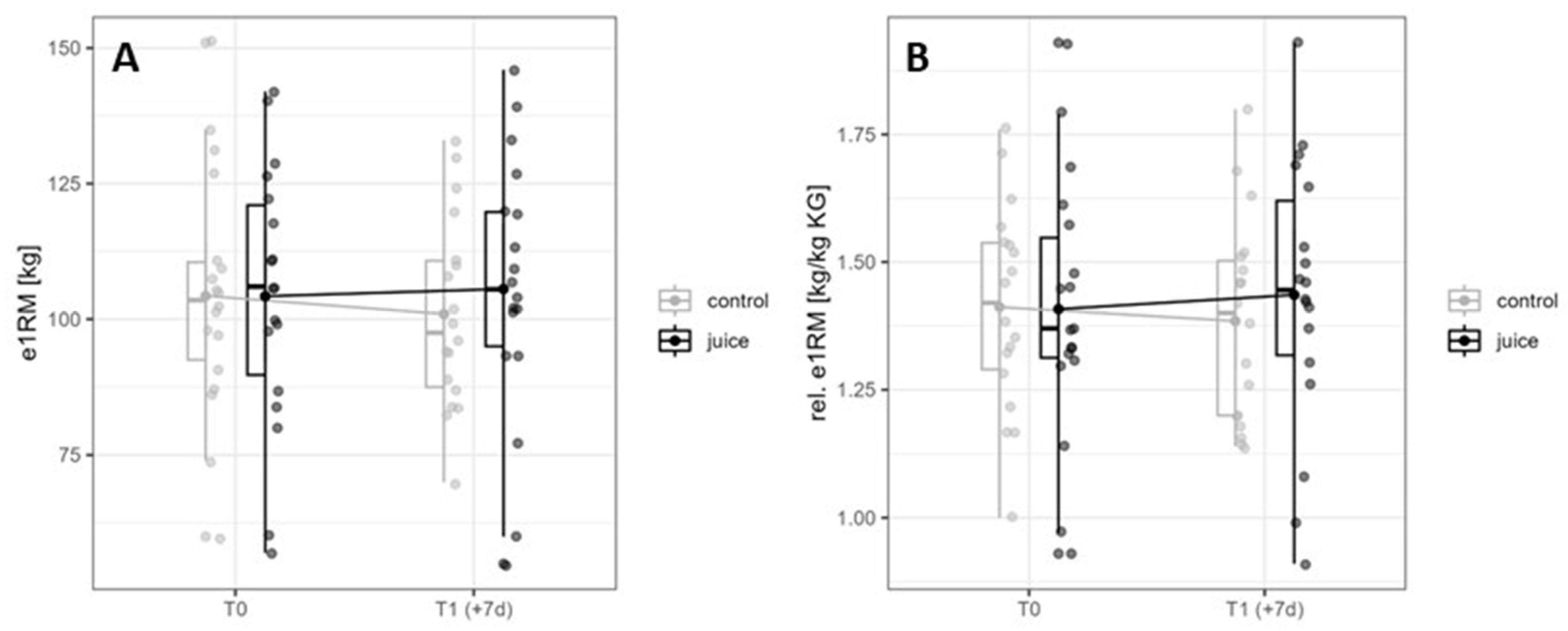
3.4. Effects of Six-Day Running and Juice on e1RM and relative e1RM in Back Squat
3.5. Evaluation and Comparison of the Nutrient Intake during the Fruit Juice Intervention and Placebo Consumption Phase
4. Discussion
Limitations/Outlook
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIC | Akaike Information Criterion |
| BIC | Bayesian Information Criterion |
| BMI | Body mass index |
| CK | Creatine kinase |
| CMJ | Countermovement jump |
| e1RM | Estimated 1RM |
| EIMD | Exercise-induced muscle damage |
| ER | Endurance run |
| GAE | Gallic acid equivalent |
| HIIT | High intensity interval training |
| IL | Interleukin |
| Log10 | Decadic logarithm |
| LT(D) | Lactate threshold calculated by the D-mod method |
| MDA | Malondialdehyde |
| oxLDL | Oxidized low-density lipoprotein |
| rel. e1RM | Relative e1RM |
| ROS | Reactive oxygen species |
| SD | Standard deviation |
| SE | Standard error |
| TAC | Total antioxidant capacity |
| TEAC | Trolox equivalence antioxidant capacity |
| TNF-α | Tumor necrosis factor alpha |
| 1RM | One-repetition maximum |
| VO2max | Maximal oxygen uptake |
References
- Souissi, W.; Bouzid, M.A.; Farjallah, M.A.; Ben Mahmoud, L.; Boudaya, M.; Engel, F.A.; Sahnoun, Z. Effect of Different Running Exercise Modalities on Post-Exercise Oxidative Stress Markers in Trained Athletes. Int. J. Environ. Res. Public Health 2020, 17, 3729. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Smuder, A.J.; Kavazis, A.N.; Hudson, M.B. Experimental guidelines for studies designed to investigate the impact of antioxidant supplementation on exercise performance. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 2–14. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef] [PubMed]
- Cooper, C.E.; Vollaard, N.B.J.; Choueiri, T.; Wilson, M.T. Exercise, free radicals and oxidative stress. Biochem. Soc. Trans. 2002, 30, 280–285. [Google Scholar] [CrossRef]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Rønnestad, B.R.; Hansen, J.; Vegge, G.; Tønnessen, E.; Slettaløkken, G. Short intervals induce superior training adaptations compared with long intervals in cyclists—An effort-matched approach. Scand. J. Med. Sci. Sports 2015, 25, 143–151. [Google Scholar] [CrossRef]
- D’Angelo, S. Polyphenols: Potential Beneficial Effects of These Phytochemicals in Athletes. Curr. Sports Med. Rep. 2020, 19, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Tolić, M.-T.; Jurčević, I.L.; Krbavčić, I.P.; Marković, K.; Vahčić, N. Phenolic Content, Antioxidant Capacity and Quality of Chokeberry (Aronia melanocarpa) Products. Food Technol. Biotechnol. 2015, 53, 171–179. [Google Scholar] [CrossRef]
- Kulling, S.E.; Rawel, H.M. Chokeberry (Aronia melanocarpa)—A review on the characteristic components and potential health effects. Planta Med. 2008, 74, 1625–1634. [Google Scholar] [CrossRef]
- Bowtell, J.; Kelly, V. Fruit-Derived Polyphenol Supplementation for Athlete Recovery and Performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Rickards, L.; Lynn, A.; Harrop, D.; Barker, M.E.; Russell, M.; Ranchordas, M.K. Effect of Polyphenol-Rich Foods, Juices, and Concentrates on Recovery from Exercise Induced Muscle Damage: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 2988. [Google Scholar] [CrossRef] [PubMed]
- Fuster-Muñoz, E.; Roche, E.; Funes, L.; Martínez-Peinado, P.; Sempere, J.M.; Vicente-Salar, N. Effects of pomegranate juice in circulating parameters, cytokines, and oxidative stress markers in endurance-based athletes: A randomized controlled trial. Nutrition 2016, 32, 539–545. [Google Scholar] [CrossRef]
- Stevanović, V.; Pantović, A.; Krga, I.; Zeković, M.; Šarac, I.; Glibetić, M.; Vidović, N. Aronia juice consumption prior to half-marathon race can acutely affect platelet activation in recreational runners. Appl. Physiol. Nutr. Metab. 2020, 45, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Pilaczynska-Szczesniak, L.; Skarpanska-Steinborn, A.; Deskur, E.; Basta, P.; Horoszkiewicz-Hassan, M. The influence of chokeberry juice supplementation on the reduction of oxidative stress resulting from an incremental rowing ergometer exercise. Int. J. Sport Nutr. Exerc. Metab. 2005, 15, 48–58. [Google Scholar] [CrossRef]
- Skarpańska-Stejnborn, A.; Basta, P.; Sadowska, J.; Pilaczyńska-Szcześniak, L. Effect of supplementation with chokeberry juice on the inflammatory status and markers of iron metabolism in rowers. J. Int. Soc. Sports Nutr. 2014, 11, 48. [Google Scholar] [CrossRef]
- Sorrenti, V.; Fortinguerra, S.; Caudullo, G.; Buriani, A. Deciphering the Role of Polyphenols in Sports Performance: From Nutritional Genomics to the Gut Microbiota toward Phytonutritional Epigenomics. Nutrients 2020, 12, 1265. [Google Scholar] [CrossRef] [PubMed]
- Isenmann, E.; Veit, S.; Flenker, U.; Lesch, A.; Lachenmeier, D.W.; Diel, P. Influence of short-term chronic oral cannabidiol application on muscle recovery and performance after an intensive training protocol—A randomized double-blind crossover study. J. Int. Soc. Sports Nutr. 2024, 21, 2337252. [Google Scholar] [CrossRef]
- Isenmann, E.; Blume, F.; Bizjak, D.A.; Hundsdörfer, V.; Pagano, S.; Schibrowski, S.; Simon, W.; Schmandra, L.; Diel, P. Comparison of Pro-Regenerative Effects of Carbohydrates and Protein Administrated by Shake and Non-Macro-Nutrient Matched Food Items on the Skeletal Muscle after Acute Endurance Exercise. Nutrients 2019, 11, 744. [Google Scholar] [CrossRef]
- Isenmann, E.; Trittel, L.; Diel, P. The effects of alpha lipoic acid on muscle strength recovery after a single and a short-term chronic supplementation—A study in healthy well-trained individuals after intensive resistance and endurance training. J. Int. Soc. Sports Nutr. 2020, 17, 61. [Google Scholar] [CrossRef] [PubMed]
- Billat, V.L.; Demarle, A.; Slawinski, J.; Paiva, M.; Koralsztein, J.P. Physical and training characteristics of top-class marathon runners. Med. Sci. Sports Exerc. 2001, 33, 2089–2097. [Google Scholar] [CrossRef]
- Haugen, T.; Sandbakk, Ø.; Enoksen, E.; Seiler, S.; Tønnessen, E. Crossing the Golden Training Divide: The Science and Practice of Training World-Class 800- and 1500-m Runners. Sports Med. 2021, 51, 1835–1854. [Google Scholar] [CrossRef] [PubMed]
- De Pauw, K.; Roelands, B.; Cheung, S.S.; de Geus, B.; Rietjens, G.; Meeusen, R. Guidelines to classify subject groups in sport-science research. Int. J. Sports Physiol. Perform. 2013, 8, 111–122. [Google Scholar] [CrossRef]
- Garcia-Ramos, A.; Jaric, S. Two-Point Method: A Quick and Fatigue-Free Procedure for Assessment of Muscle Mechanical Capacities and the 1 Repetition Maximum. Strength Cond. J. 2018, 40, 54–66. [Google Scholar] [CrossRef]
- Miller, T. NSCA’s Guide to Tests and Assessments; Human Kinetics: Champaign, IL, USA, 2012; ISBN 9780736083683. [Google Scholar]
- Dolci, F.; Kilding, A.E.; Chivers, P.; Piggott, B.; Hart, N.H. High-Intensity Interval Training Shock Microcycle for Enhancing Sport Performance: A Brief Review. J. Strength Cond. Res. 2020, 34, 1188–1196. [Google Scholar] [CrossRef]
- Bishop, D.; Jenkins, D.G.; Mackinnon, L.T. The relationship between plasma lactate parameters, Wpeak and 1-h cycling performance in women. Med. Sci. Sports Exerc. 1998, 30, 1270–1275. [Google Scholar] [CrossRef]
- The R Core Team. R: A Language and Environment for Statistical Computing: Reference Index. Available online: https://cran.r-project.org/doc/manuals/r-release/fullrefman.pdf (accessed on 10 May 2023).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models using lme4. J. Stat. Softw. 2014, 67, 1–48. [Google Scholar]
- Bowtell, J.L.; Sumners, D.P.; Dyer, A.; Fox, P.; Mileva, K.N. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med. Sci. Sports Exerc. 2011, 43, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Buonauro, R.; Limongelli, F.M. Serum enzyme monitoring in sports medicine. Clin. Sports Med. 2008, 27, 1–18, vii. [Google Scholar] [CrossRef]
- Nieman, D.C.; Capps, C.L.; Capps, C.R.; Shue, Z.L.; McBride, J.E. Effect of 4-Week Ingestion of Tomato-Based Carotenoids on Exercise-Induced Inflammation, Muscle Damage, and Oxidative Stress in Endurance Runners. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 266–273. [Google Scholar] [CrossRef]
- Neubauer, O.; König, D.; Kern, N.; Nics, L.; Wagner, K.-H. No indications of persistent oxidative stress in response to an ironman triathlon. Med. Sci. Sports Exerc. 2008, 40, 2119–2128. [Google Scholar] [CrossRef] [PubMed]
- Välimäki, I.A.; Vuorimaa, T.; Ahotupa, M.; Vasankari, T. Effect of Continuous and Intermittent Exercises on Oxidised HDL and LDL Lipids in Runners. Int. J. Sports Med. 2016, 37, 1103–1109. [Google Scholar] [CrossRef] [PubMed]
- Elosua, R.; Molina, L.; Fito, M.; Arquer, A.; Sanchez-Quesada, J.L.; Covas, M.I.; Ordoñez-Llanos, J.; Marrugat, J. Response of oxidative stress biomarkers to a 16-week aerobic physical activity program, and to acute physical activity, in healthy young men and women. Atherosclerosis 2003, 167, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Bachi, A.L.L.; Sierra, A.P.R.; Rios, F.J.O.; Gonçalves, D.A.; Ghorayeb, N.; Abud, R.L.; Victorino, A.B.; Dos Santos, J.M.B.; Kiss, M.A.D.P.; Pithon-Curi, T.C.; et al. Athletes with higher VO2max present reduced oxLDL after a marathon race. BMJ Open Sport Exerc. Med. 2015, 1, bmjsem-2015-000014. [Google Scholar] [CrossRef]
- Wang, J.-S.; Lee, T.; Chow, S.-E. Role of exercise intensities in oxidized low-density lipoprotein-mediated redox status of monocyte in men. J. Appl. Physiol. 2006, 101, 740–744. [Google Scholar] [CrossRef]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.J.; Howatson, G. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Tanner, E.A.; Gary, M.A.; Davis, A.A.; Michalik, S.; McFarlin, B.K. Alterations in Systemic Inflammatory Response Following a Half-Marathon Race with a Combined Curcumin and Pomegranate Supplement: A Feasibility Study. J. Diet. Suppl. 2021, 18, 461–477. [Google Scholar] [CrossRef]
- Lindinger, M.I.; Cairns, S.P. Regulation of muscle potassium: Exercise performance, fatigue and health implications. Eur. J. Appl. Physiol. 2021, 121, 721–748. [Google Scholar] [CrossRef]
- Doma, K.; Gahreman, D.; Connor, J. Fruit supplementation reduces indices of exercise-induced muscle damage: A systematic review and meta-analysis. Eur. J. Sport Sci. 2021, 21, 562–579. [Google Scholar] [CrossRef]
- Hooper, D.R.; Orange, T.; Gruber, M.T.; Darakjian, A.A.; Conway, K.L.; Hausenblas, H.A. Broad Spectrum Polyphenol Supplementation from Tart Cherry Extract on Markers of Recovery from Intense Resistance Exercise. J. Int. Soc. Sports Nutr. 2021, 18, 47. [Google Scholar] [CrossRef]
- Cairns, S.P.; Renaud, J.-M. The potassium-glycogen interaction on force and excitability in mouse skeletal muscle: Implications for fatigue. J. Physiol. 2023, 601, 5669–5687. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Anthony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef]
- Campbell, B.I.; La Bounty, P.M.; Wilborn, C.D. Dietary Supplements Used in Combat Sports. Strength Cond. J. 2011, 33, 50–59. [Google Scholar] [CrossRef]
- Norton, L.E.; Layman, D.K. Leucine regulates translation initiation of protein synthesis in skeletal muscle after exercise. J. Nutr. 2006, 136, 533S–537S. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, Y.; Jamurtas, A.Z.; Nikolaidis, M.G.; Fatouros, I.G.; Koutedakis, Y.; Papassotiriou, I.; Kouretas, D. Sampling time is crucial for measurement of aerobic exercise-induced oxidative stress. Med. Sci. Sports Exerc. 2007, 39, 1107–1113. [Google Scholar] [CrossRef]
- Held, S.; Rappelt, L.; Deutsch, J.-P.; Donath, L. Valid and Reliable Barbell Velocity Estimation Using an Inertial Measurement Unit. Int. J. Environ. Res. Public Health 2021, 18, 9170. [Google Scholar] [CrossRef]
- Seiler, S.; Jøranson, K.; Olesen, B.V.; Hetlelid, K.J. Adaptations to aerobic interval training: Interactive effects of exercise intensity and total work duration. Scand. J. Med. Sci. Sports 2013, 23, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, K.; Flenker, U.; Kiewardt, G.; Diel, P.R. Combinatory Effects of Training and Nutritive Administration of Carbohydrates and Protein via Food on Strength in Postmenopausal Women, and Old Men and Women. Nutrients 2023, 15, 1531. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Knaze, V.; Rothwell, J.A.; Hémon, B.; Moskal, A.; Overvad, K.; Tjønneland, A.; Kyrø, C.; Fagherazzi, G.; Boutron-Ruault, M.-C.; et al. Dietary polyphenol intake in Europe: The European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Nutr. 2016, 55, 1359–1375. [Google Scholar] [CrossRef]




| Sex | Men (14), Women (4) |
|---|---|
| Age [years] | 24.5 ± 3.1 |
| Height [m] | 1.79 ± 0.06 |
| Body weight [kg] | 73.10 ± 8.30 |
| BMI [kg/m2] | 22.84 ± 1.74 |
| Parameter | Juice | Placebo | p-Value |
|---|---|---|---|
| Training time [min] | 210.00 ± 00.00 | 210.00 ± 00.00 | --- |
| Training km [km] | 47.76 ± 9.34 | 47.40 ± 9.33 | 0.654 |
| ER km [km] | 20.09 ± 2.49 | 20.74 ± 2.40 | 0.094 |
| Interval [km] | 27.67 ± 7.24 | 26.66 ± 7.42 | 0.075 |
| Pace/total [min/km] | 4.58 ± 0.84 | 4.58 ± 0.81 | 0.981 |
| Juice Group | Placebo Group | p-Value | |
|---|---|---|---|
| Energy [kcal/d] | 2598.12 ± 723.73 | 2650.14 ± 739.60 | 0.163 |
| Carbohydrates [g/d] % of Energy | 321.79 ± 91.19 49.82 ± 6.51% | 330.79 ± 97.09 51.00 ± 4.97% | 0.044 |
| Fat [g/d] % of Energy | 96.50 ± 30.56 32.88 ± 4.11% | 97.32 ± 30.01 32.58 ± 4.20 | 0.469 |
| Protein [g/d] % of Energy | 103.87 ± 8.43 16.41 ± 2.85% | 105.76 ± 8.347 16.41 ± 2.76% | 0.095 |
| Leucine [g/d] | 8.38 ± 2.86 | 8.53 ± 2.83 | 0.101 |
| EAA [g/d] | 50.95 ± 17.139 | 51.85 ± 17.00 | 0.099 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valder, S.; Habersatter, E.; Kostov, T.; Quenzer, S.; Herzig, L.; von Bernuth, J.; Matits, L.; Herdegen, V.; Diel, P.; Isenmann, E. The Influence of a Polyphenol-Rich Red Berry Fruit Juice on Recovery Process and Leg Strength Capacity after Six Days of Intensive Endurance Exercise in Recreational Endurance Athletes. Nutrients 2024, 16, 1428. https://doi.org/10.3390/nu16101428
Valder S, Habersatter E, Kostov T, Quenzer S, Herzig L, von Bernuth J, Matits L, Herdegen V, Diel P, Isenmann E. The Influence of a Polyphenol-Rich Red Berry Fruit Juice on Recovery Process and Leg Strength Capacity after Six Days of Intensive Endurance Exercise in Recreational Endurance Athletes. Nutrients. 2024; 16(10):1428. https://doi.org/10.3390/nu16101428
Chicago/Turabian StyleValder, Sarah, Elisabeth Habersatter, Tihomir Kostov, Sina Quenzer, Lukas Herzig, Jakob von Bernuth, Lynn Matits, Volker Herdegen, Patrick Diel, and Eduard Isenmann. 2024. "The Influence of a Polyphenol-Rich Red Berry Fruit Juice on Recovery Process and Leg Strength Capacity after Six Days of Intensive Endurance Exercise in Recreational Endurance Athletes" Nutrients 16, no. 10: 1428. https://doi.org/10.3390/nu16101428
APA StyleValder, S., Habersatter, E., Kostov, T., Quenzer, S., Herzig, L., von Bernuth, J., Matits, L., Herdegen, V., Diel, P., & Isenmann, E. (2024). The Influence of a Polyphenol-Rich Red Berry Fruit Juice on Recovery Process and Leg Strength Capacity after Six Days of Intensive Endurance Exercise in Recreational Endurance Athletes. Nutrients, 16(10), 1428. https://doi.org/10.3390/nu16101428







