Fucoidan Improves D-Galactose-Induced Cognitive Dysfunction by Promoting Mitochondrial Biogenesis and Maintaining Gut Microbiome Homeostasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Reagents
2.2. Animals
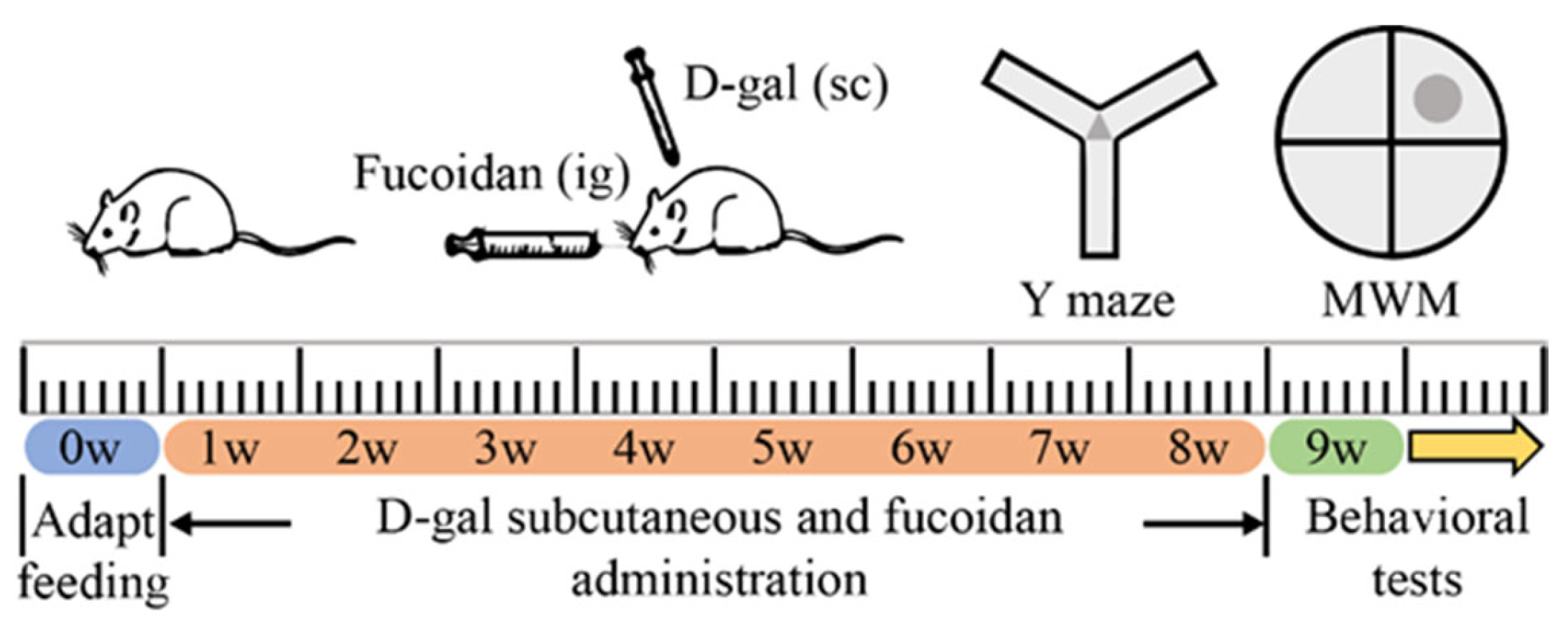
2.3. Experimental Design
2.4. Behavioral Test
2.4.1. Y-Maze
2.4.2. Morris Water Maze
2.5. HE Staining and Nissl Staining
2.6. Oxidative Stress and Inflammation Detection
2.7. ATP and mtDNA Copy Number Detection
2.8. Immunofluorescence Staining
2.9. Western Blotting
2.10. 16S rRNA Gene Microbiome Sequencing Analysis
2.11. Statistical Analysis
3. Results
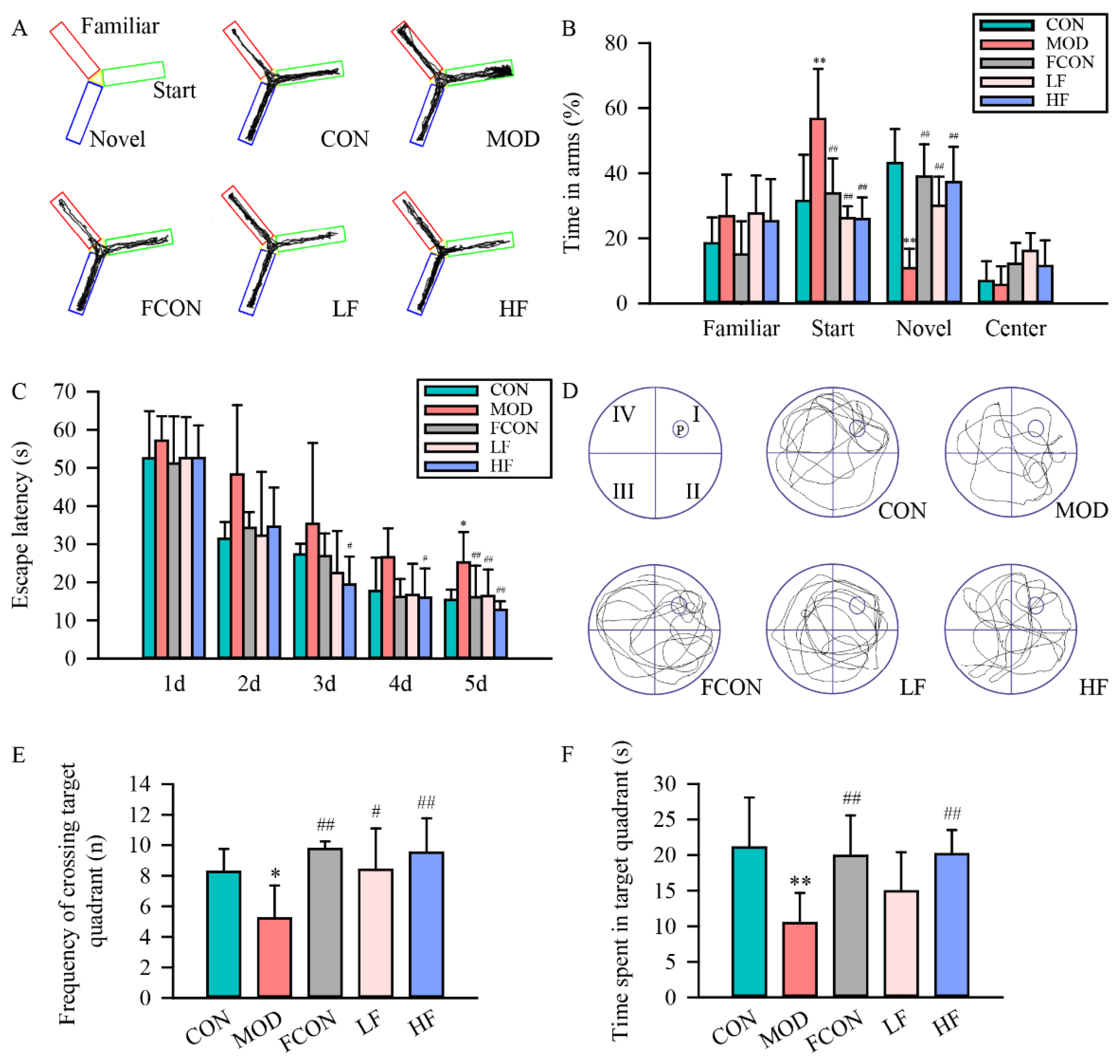
3.1. Effects of Fucoidan on Impairment of Learning and Memory Abilities in D-Gal-Exposed Rats
3.2. Effects of Fucoidan on Histopathological Changes in Hippocampus Region in D-Gal-Exposed Rats
3.3. Effects of Fucoidan on Oxidative Stress and Inflammation in D-Gal-Exposed Rats
3.4. Effects of Fucoidan on Mitochondrial Damage in D-Gal-Exposed Rats
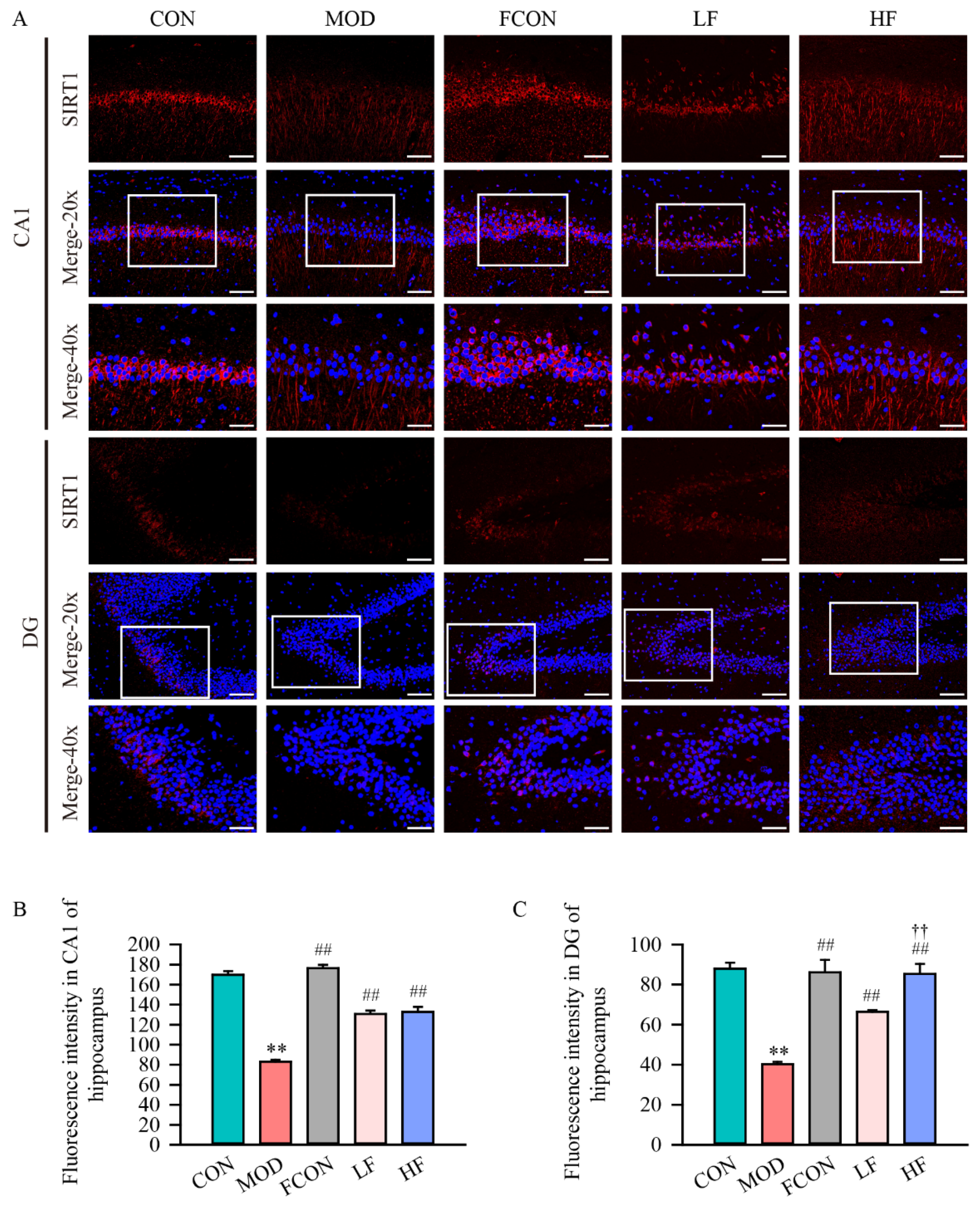
3.5. Effects of Fucoidan on Mitochondrial Biogenesis of Hippocampal Tissue of D-Gal-Exposed Rats
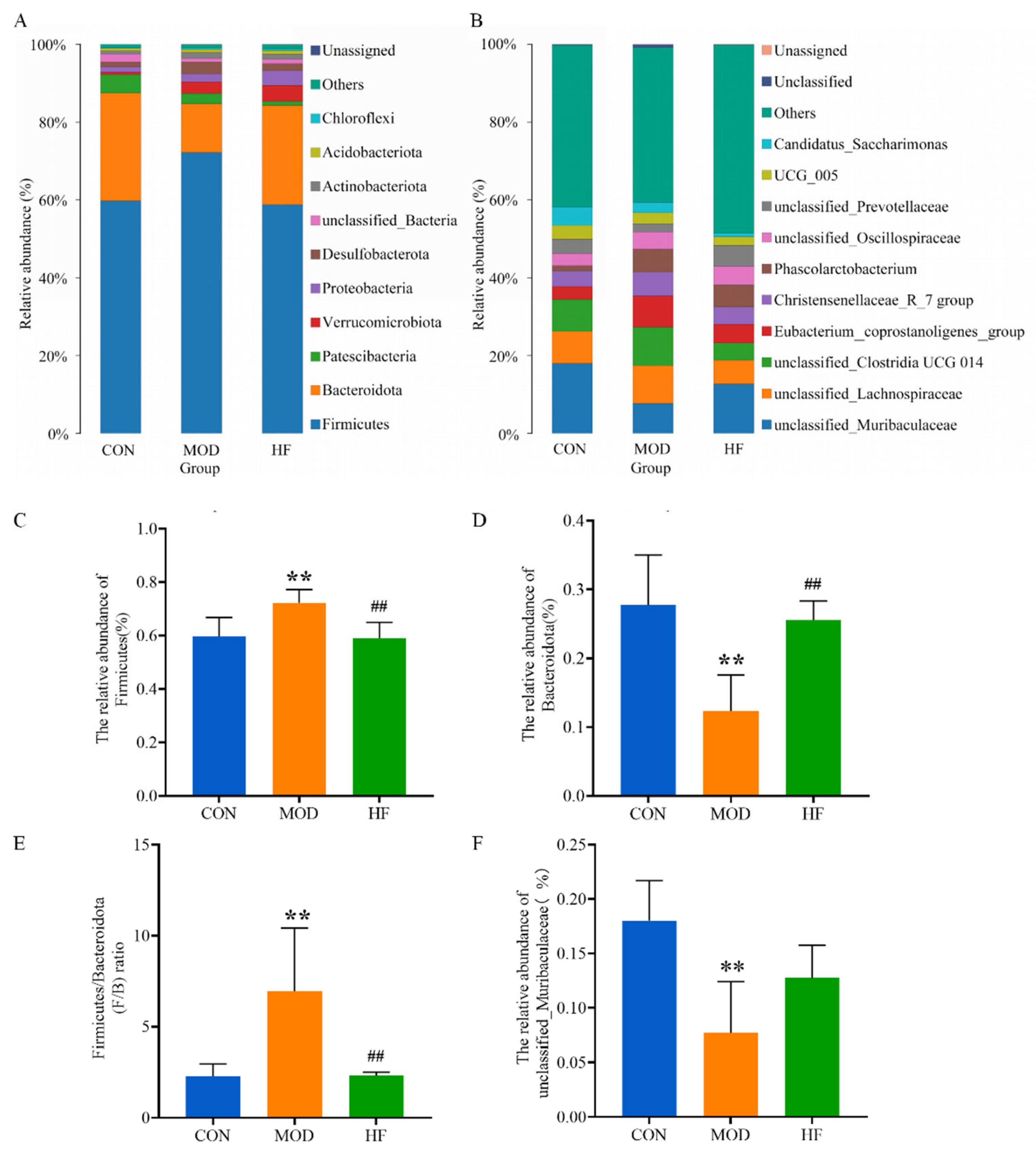
3.6. Effects of Fucoidan on Gut Microbiota in D-Gal-Exposed Rats
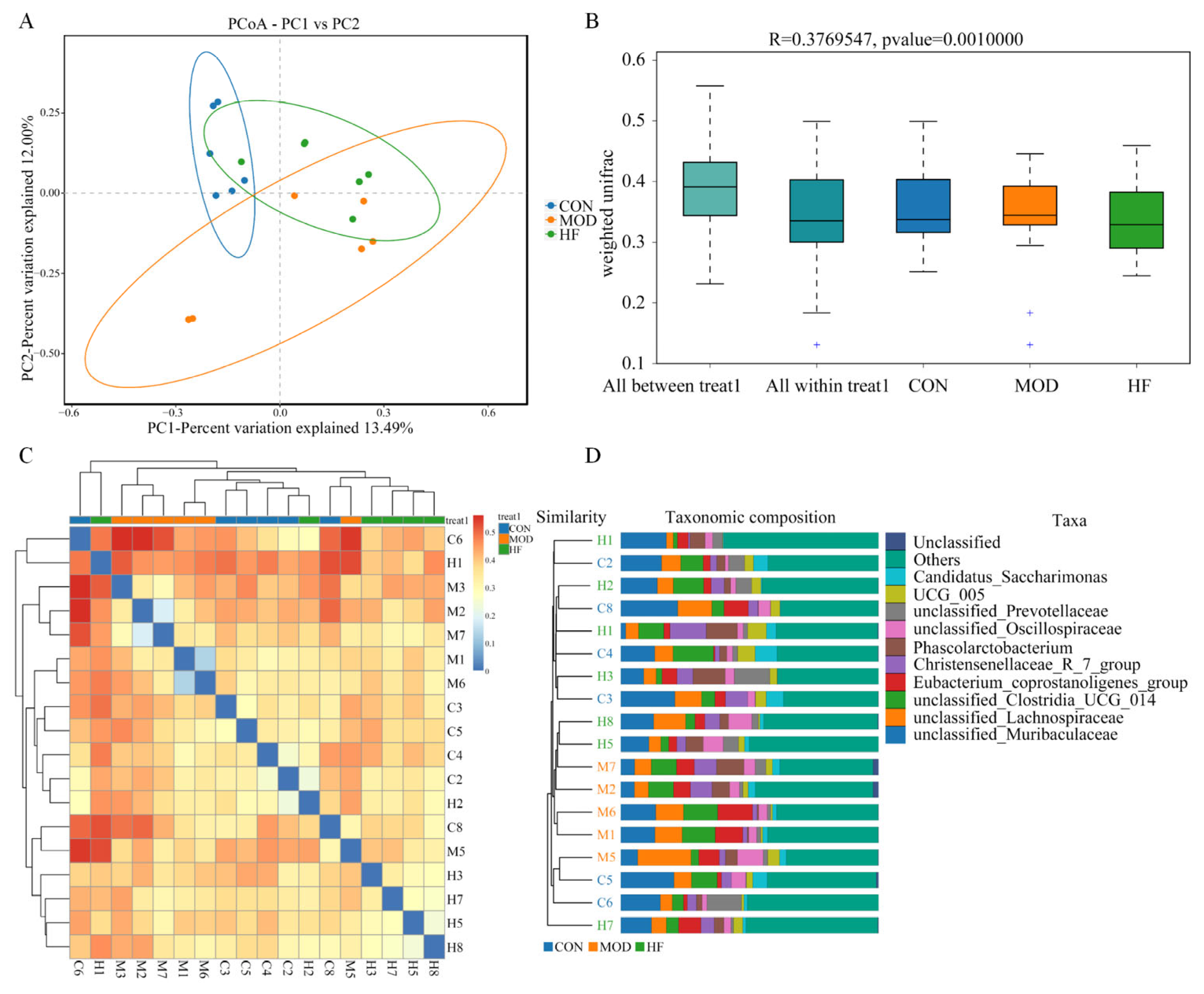
3.6.1. α-Diversity Analysis
3.6.2. β-Diversity Analysis
3.6.3. Microbial Composition of the Phylum and Genus Level
3.6.4. LEfSe Analysis
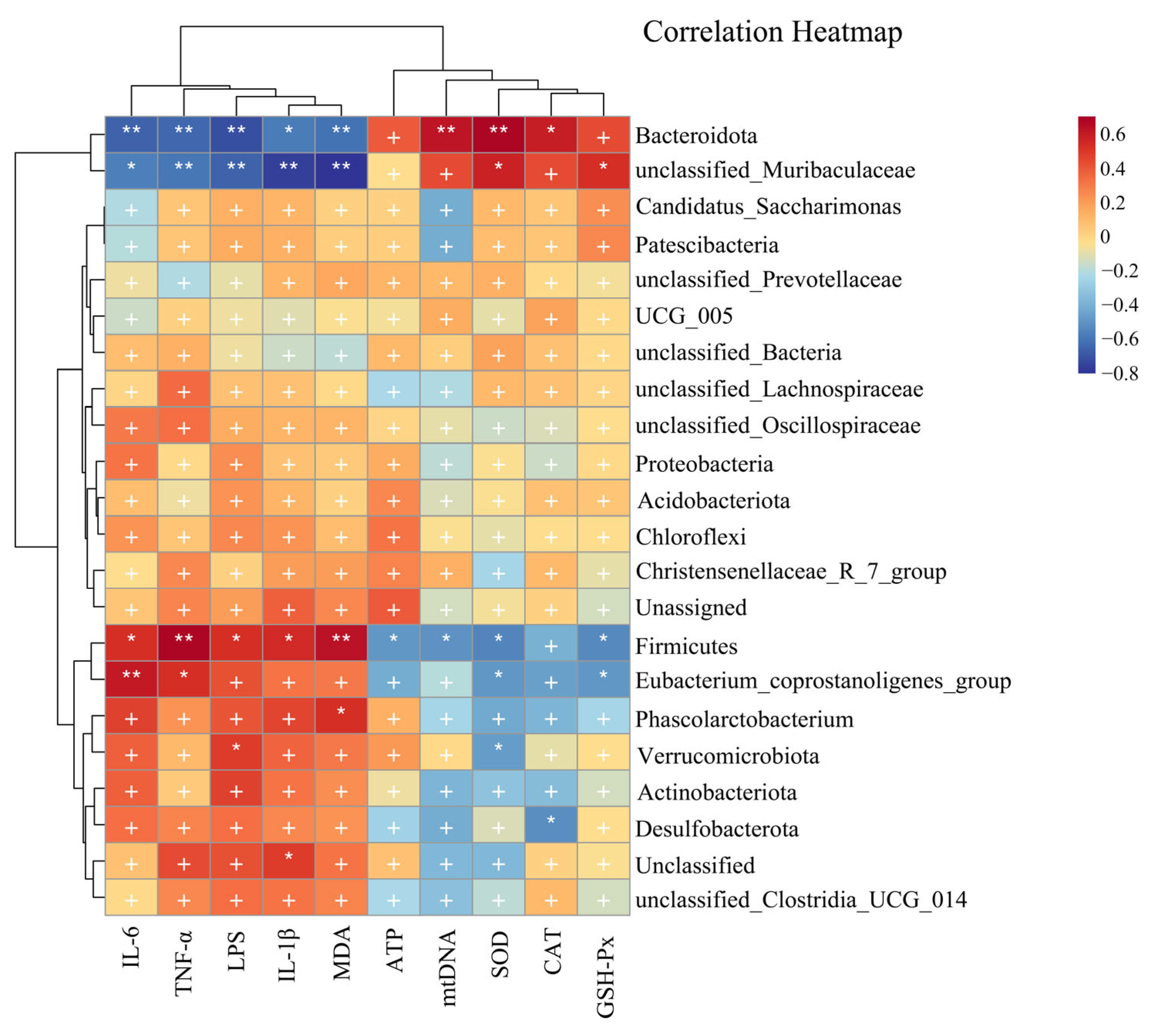
3.6.5. Correlations among Mitochondrial-Dysfunction-Related Indices, Oxidative-Stress-Related Indices, Inflammation Factors and Gut Microbiota
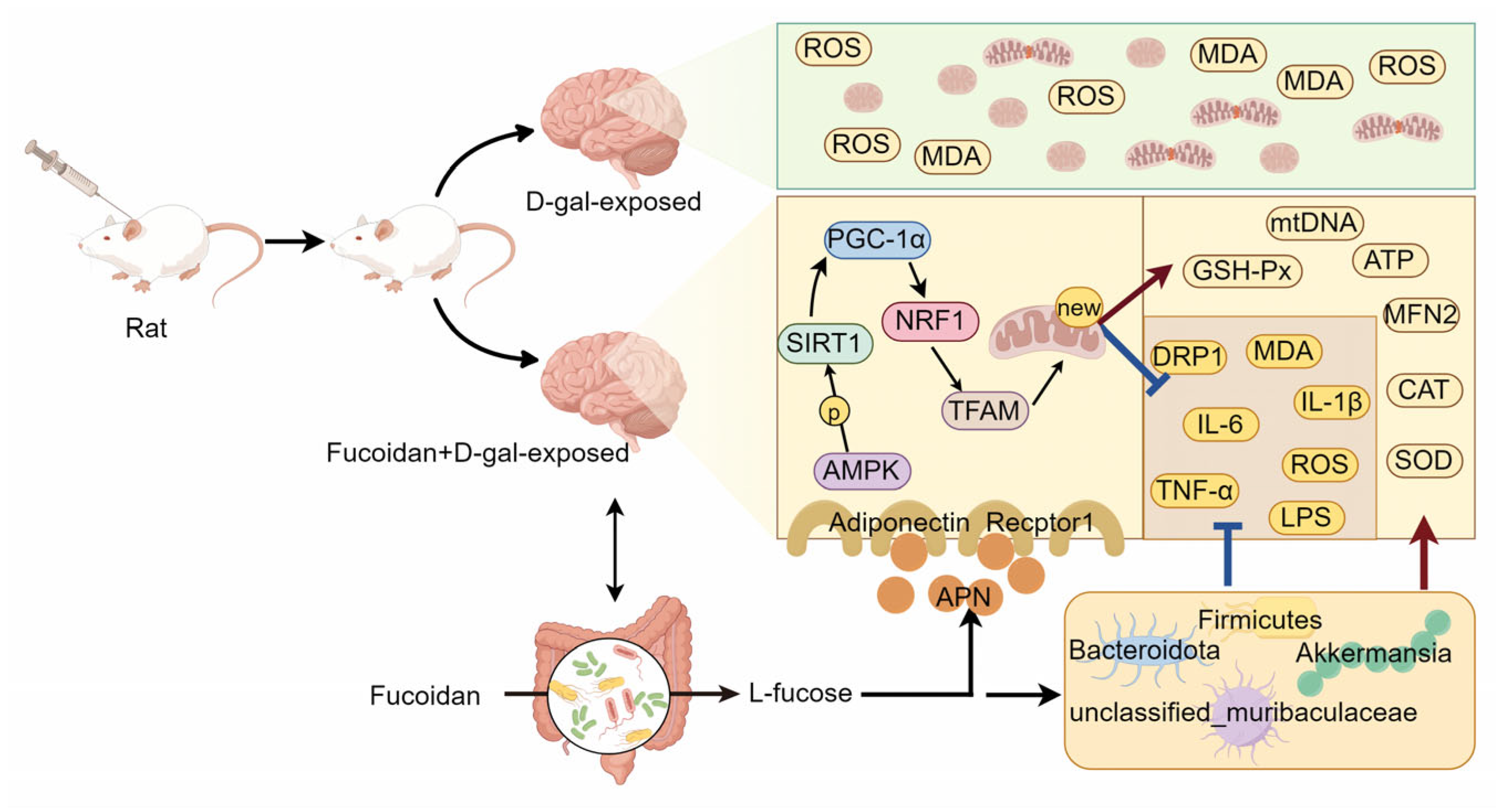
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raz, N.; Ghisletta, P.; Rodrigue, K.M.; Kennedy, K.M.; Lindenberger, U. Trajectories of brain aging in middle-aged and older adults: Regional and individual differences. NeuroImage 2010, 51, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.H.; Hof, P.R. Life and death of neurons in the aging brain. Science 1997, 278, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Perry, G.; Cash, A.D.; Smith, M.A. Alzheimer Disease and Oxidative Stress. J. Biomed. Biotechnol. 2002, 2, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Scheff, S.W.; Ansari, M.A.; Mufson, E.J. Oxidative stress and hippocampal synaptic protein levels in elderly cognitively intact individuals with Alzheimer’s disease pathology. Neurobiol. Aging 2016, 42, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Yan, X. Oxidation of Potassium Channels in Neurodegenerative Diseases: A Mini-Review. CNS Neurol. Disord. Drug Targets 2018, 17, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Tabassum, R.; Jeong, N.Y. Potential for therapeutic use of hydrogen sulfide in oxidative stress-induced neurodegenerative diseases. Int. J. Med. Sci. 2019, 16, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Hussien, R.; Brooks, G.A. H2O2-induced mitochondrial fragmentation in C2C12 myocytes. Free Radic. Biol. Med. 2010, 49, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-C.; Wei, Y.-H. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int. J. Biochem. Cell Biol. 2005, 37, 822–834. [Google Scholar] [CrossRef] [PubMed]
- Medala, V.K.; Gollapelli, B.; Dewanjee, S.; Ogunmokun, G.; Kandimalla, R.; Vallamkondu, J. Mitochondrial dysfunction, mitophagy, and role of dynamin-related protein 1 in Alzheimer’s disease. J. Neurosci. Res. 2021, 99, 1120–1135. [Google Scholar] [CrossRef]
- Sheng, B.; Wang, X.; Su, B.; Lee, H.g.; Casadesus, G.; Perry, G.; Zhu, X. Impaired mitochondrial biogenesis contributes to mitochondrial dysfunction in Alzheimer’s disease. J. Neurochem. 2011, 120, 419–429. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W.; Li, L.; Perry, G.; Lee, H.-g.; Zhu, X. Oxidative stress and mitochondrial dysfunction in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 1240–1247. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Q.; Li, H.; Lan, X.; Kan, M.; Lin, J.; Wang, J.; Zhang, Z.; Ming, S.; Li, Z. The anti-aging effect of velvet antler polypeptide is dependent on modulation of the gut microbiota and regulation of the PPARα/APOE4 pathway. J. Integr. Neurosci. 2021, 20, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Liu, Y.; Ma, F.; Sun, M.; Song, Y.; Xu, D.; Mu, G.; Tuo, Y. Lactobacillus plantarum Y44 alleviates oxidative stress by regulating gut microbiota and colonic barrier function in Balb/C mice with subcutaneous d-galactose injection. Food Funct. 2021, 12, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Hsia, C.-H.; Wang, C.-H.; Kuo, Y.-W.; Ho, Y.-J.; Chen, H.-L. Fructo-oligosaccharide systemically diminishedd-galactose-induced oxidative molecule damages in BALB/cJ mice. Br. J. Nutr. 2011, 107, 1787–1792. [Google Scholar] [CrossRef] [PubMed]
- Azman, K.F.; Zakaria, R. d-Galactose-induced accelerated aging model: An overview. Biogerontology 2019, 20, 763–782. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-L.; Wang, C.-H.; Kuo, Y.-W.; Tsai, C.-H. Antioxidative and hepatoprotective effects of fructo-oligosaccharide ind-galactose-treated Balb/cJ mice. Br. J. Nutr. 2010, 105, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Yao, H.; Xiang, Y.; Chen, L.; Xiao, M.; Wang, Z.; Xiao, H.; Wang, L.; Wang, S.; Wang, Y. Effect of Angelica polysaccharide on brain senescence of Nestin-GFP mice induced by D-galactose. Neurochem. Int. 2019, 122, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Shwe, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp. Gerontol. 2018, 101, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Zuo, P.; Zhang, Q.; Li, X.; Hu, Y.; Long, J.; Packer, L.; Liu, J. Chronic systemic D-galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: Protective effects of R-α-lipoic acid. J. Neurosci. Res. 2006, 84, 647–654. [Google Scholar] [CrossRef]
- Ho, S.C.; Liu, J.H.; Wu, R.Y. Establishment of the mimetic aging effect in mice caused by D-galactose. Biogerontology 2003, 4, 15–18. [Google Scholar] [CrossRef]
- Hao, L.; Huang, H.; Gao, J.; Marshall, C.; Chen, Y.; Xiao, M. The influence of gender, age and treatment time on brain oxidative stress and memory impairment induced by d-galactose in mice. Neurosci. Lett. 2014, 571, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xue, M.; Sun, T.; Yang, J.; Pei, Z.; Qin, K. Fucoidan as an Autophagy Regulator: Mechanisms and Therapeutic Potentials for Cancer and Other Diseases. Nutr. Cancer 2021, 74, 1568–1579. [Google Scholar] [CrossRef]
- Kuznetsova, T.A.; Besednova, N.N.; Mamaev, A.N.; Momot, A.P.; Shevchenko, N.M.; Zvyagintseva, T.N. Anticoagulant activity of fucoidan from brown algae Fucus evanescens of the Okhotsk Sea. Bull. Exp. Biol. Med. 2003, 136, 471–473. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Zorofchian Moghadamtousi, S.; Abubakar, S.; Zandi, K. Antiviral Potential of Algae Polysaccharides Isolated from Marine Sources: A Review. BioMed Res. Int. 2015, 2015, 825203. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Liang, H.; Ji, X.; Liu, Y.; Ge, Y.; Hou, L.; Sun, T. Fucoidan prevent murine autoimmune diabetes via suppression TLR4-signaling pathways, regulation DC/Treg induced immune tolerance and improving gut microecology. Nutr. Metab. 2019, 16, 87. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova-Shumkovska, J.; Krstanoski, L.; Veenman, L. Potential Beneficial Actions of Fucoidan in Brain and Liver Injury, Disease, and Intoxication—Potential Implication of Sirtuins. Mar. Drugs 2020, 18, 242. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, C.; Yin, J.; Shen, J.; Wang, H.; Wu, Y.; Jin, H. Fucoidan, a sulfated polysaccharide from brown algae, improves cognitive impairment induced by infusion of Aβ peptide in rats. Environ. Toxicol. Pharmacol. 2012, 33, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-S.; Lee, J.H.; Lee, S.H. Fucoidan Suppresses Mitochondrial Dysfunction and Cell Death against 1-Methyl-4-Phenylpyridinum-Induced Neuronal Cytotoxicity via Regulation of PGC-1α Expression. Mar. Drugs 2019, 17, 518. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Gao, Z.; Zheng, L.; Zhang, C.; Liu, Z.; Yang, Y.; Teng, H.; Hou, L.; Yin, Y.; Zou, X. Protective Effects of Fucoidan on Aβ25–35 and d-Gal-Induced Neurotoxicity in PC12 Cells and d-Gal-Induced Cognitive Dysfunction in Mice. Mar. Drugs 2017, 15, 77. [Google Scholar] [CrossRef]
- Xue, M.; Teng, X.; Liang, H.; Zhao, J.; Jiang, Y.; Qiu, X.; Zhang, Z.; Pei, Z.; Zhang, N.; Qin, Y. Neuroprotective effect of fucoidan by regulating gut-microbiota-brain axis in alcohol withdrawal mice. J. Funct. Foods 2021, 86, 104726. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Q.; Zhang, Y.; Xue, M.; Yan, H.; Qiu, X.; Tian, Y.; Zhang, H.; Liang, H. Fucoidan from Saccharina japonica Alleviates Hyperuricemia-Induced Renal Fibrosis through Inhibiting the JAK2/STAT3 Signaling Pathway. J. Agric. Food Chem. 2023, 71, 11454–11465. [Google Scholar] [CrossRef] [PubMed]
- Blackmore, T.; Meftah, S.; Murray, T.K.; Craig, P.J.; Blockeel, A.; Phillips, K.; Eastwood, B.; O’Neill, M.J.; Marston, H.; Ahmed, Z.; et al. Tracking progressive pathological and functional decline in the rTg4510 mouse model of tauopathy. Alzheimer’s Res. Ther. 2017, 9, 77. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.G.; Temido-Ferreira, M.; Vicente Miranda, H.; Batalha, V.L.; Coelho, J.E.; Szegö, É.M.; Marques-Morgado, I.; Vaz, S.H.; Rhee, J.S.; Schmitz, M.; et al. α-synuclein interacts with PrPC to induce cognitive impairment through mGluR5 and NMDAR2B. Nat. Neurosci. 2017, 20, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Guo, P.; Wang, Y.; Teng, X.; Zhang, H.; Sun, L.; Xue, M.; Liang, H. Propolis Ameliorates Alcohol-Induced Depressive Symptoms in C57BL/6J Mice by Regulating Intestinal Mucosal Barrier Function and Inflammatory Reaction. Nutrients 2022, 14, 1213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Zhang, X.; Zhang, L.; Zhao, X.; Xu, Y.; Wang, P.; Liang, X.; Xue, M.; Liang, H. Maternal Folic Acid Supplementation during Pregnancy Prevents Hepatic Steatosis in Male Offspring of Rat Dams Fed High-Fat Diet, Which Is Associated with the Regulation of Gut Microbiota. Nutrients 2023, 15, 4726. [Google Scholar] [CrossRef] [PubMed]
- Serrano, F.; Klann, E. Reactive oxygen species and synaptic plasticity in the aging hippocampus. Ageing Res. Rev. 2004, 3, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, K.H.; Khabour, O.F.; Salah, H.A.; Hasan, Z. Vitamin E prevents high-fat high-carbohydrates diet-induced memory impairment: The role of oxidative stress. Physiol. Behav. 2013, 119, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, R.H. Brain aging, Alzheimer’s disease, and mitochondria. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2011, 1812, 1630–1639. [Google Scholar] [CrossRef] [PubMed]
- Friedland-Leuner, K.; Stockburger, C.; Denzer, I.; Eckert, G.P.; Müller, W.E. Mitochondrial Dysfunction. In The Mitochondrion in Aging and Disease; Progress in Molecular Biology and Translational Science; Hayle Medical: New York, NY, USA, 2014; pp. 183–210. [Google Scholar]
- Swerdlow, R.H.; Burns, J.M.; Khan, S.M. The Alzheimer’s disease mitochondrial cascade hypothesis: Progress and perspectives. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 1219–1231. [Google Scholar] [CrossRef]
- Wirz, K.T.S.; Keitel, S.; Swaab, D.F.; Verhaagen, J.; Bossers, K. Early Molecular Changes in Alzheimer Disease: Can We Catch the Disease in its Presymptomatic Phase? J. Alzheimer’s Dis. 2013, 38, 719–740. [Google Scholar] [CrossRef]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Ruggiero, F.M. Mitochondrial dysfunction in brain aging: Role of oxidative stress and cardiolipin. Neurochem. Int. 2011, 58, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.H. Mitochondrial Medicine for Aging and Neurodegenerative Diseases. Neuromolecular Med. 2008, 10, 291–315. [Google Scholar] [CrossRef] [PubMed]
- Chiroma, S.M.; Mohd Moklas, M.A.; Mat Taib, C.N.; Baharuldin, M.T.H.; Amon, Z. d-galactose and aluminium chloride induced rat model with cognitive impairments. Biomed. Pharmacother. 2018, 103, 1602–1608. [Google Scholar] [CrossRef]
- Guo, Y.-R.; Lee, H.-C.; Lo, Y.-C.; Yu, S.-C.; Huang, S.-Y. n-3 polyunsaturated fatty acids preventd-galactose-induced cognitive deficits in prediabetic rats. Food Funct. 2018, 9, 2228–2239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Ma, Y.-M.; Jing, L.; Wang, Y.-L.; Zhang, J.-Z. Synaptic remodeling and reduced expression of the transcription factors, HES1 and HES5, in the cortex neurons of cognitively impaired hyperhomocysteinemic mice. Pathol. Res. Pract. 2020, 216, 152953. [Google Scholar] [CrossRef] [PubMed]
- He, L.-L.; Wang, Y.-C.; Ai, Y.-T.; Wang, L.; Gu, S.-M.; Wang, P.; Long, Q.-H.; Hu, H. Qiangji Decoction Alleviates Neurodegenerative Changes and Hippocampal Neuron Apoptosis Induced by D-Galactose via Regulating AMPK/SIRT1/NF-κB Signaling Pathway. Front. Pharmacol. 2021, 12, 735812. [Google Scholar] [CrossRef]
- Jo, M.G.; Ikram, M.; Jo, M.H.; Yoo, L.; Chung, K.C.; Nah, S.-Y.; Hwang, H.; Rhim, H.; Kim, M.O. Gintonin Mitigates MPTP-Induced Loss of Nigrostriatal Dopaminergic Neurons and Accumulation of α-Synuclein via the Nrf2/HO-1 Pathway. Mol. Neurobiol. 2018, 56, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Park, S.H.; Kwon, B.S.; Kim, G.-H.; Heo, H.J. Protective Effect of Fucoidan Extract from Ecklonia cava on Hydrogen Peroxide-Induced Neurotoxicity. J. Microbiol. Biotechnol. 2018, 28, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Chung, D.H.; Kim, D.-O.; Kim, G.-H.; Heo, H.J. Fucoidan-Rich Substances from Ecklonia cava Improve Trimethyltin-Induced Cognitive Dysfunction via Down-Regulation of Amyloid β Production/Tau Hyperphosphorylation. Mar. Drugs 2019, 17, 591. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, S.; Zhao, H.; Liu, Y.; Xue, M.; Zhang, H.; Qiu, X.; Sun, Z.; Liang, H. Protective effects of fucoidan against ethanol-induced liver injury through maintaining mitochondrial function and mitophagy balance in rats. Food Funct. 2021, 12, 3842–3854. [Google Scholar] [CrossRef]
- Chen, Y.; Csordás, G.; Jowdy, C.; Schneider, T.G.; Csordás, N.; Wang, W.; Liu, Y.; Kohlhaas, M.; Meiser, M.; Bergem, S.; et al. Mitofusin 2-Containing Mitochondrial-Reticular Microdomains Direct Rapid Cardiomyocyte Bioenergetic Responses Via Interorganelle Ca2+ Crosstalk. Circ. Res. 2012, 111, 863–875. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.T. A pivotal role for PINK1 and autophagy in mitochondrial quality control: Implications for Parkinson disease. Hum. Mol. Genet. 2010, 19, R28–R37. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Elorza, A.; Molina, A.J.A.; Mohamed, H.; Wikstrom, J.D.; Walzer, G.; Stiles, L.; Haigh, S.E.; Katz, S.; Las, G.; et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008, 27, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Manczak, M.; Calkins, M.J.; Reddy, P.H. Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: Implications for neuronal damage. Hum. Mol. Genet. 2011, 20, 2495–2509. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Li, J.; Liu, X.; Chang, J.; Zhao, Q.; Jia, S.; Fan, J.; Chen, N. Swimming attenuates d-galactose-induced brain aging via suppressing miR-34a-mediated autophagy impairment and abnormal mitochondrial dynamics. J. Appl. Physiol. 2017, 122, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Austin, S.; St-Pierre, J. PGC1α and mitochondrial metabolism—Emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012, 125, 4963–4971. [Google Scholar] [CrossRef] [PubMed]
- Uguccioni, G.; Hood, D.A. The importance of PGC-1α in contractile activity-induced mitochondrial adaptations. Am. J. Physiol. -Endocrinol. Metab. 2011, 300, E361–E371. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, J.; Ji, C.; Wang, L. MiR-144-3p and Its Target Gene β-Amyloid Precursor Protein Regulate 1-Methyl-4-Phenyl-1,2-3,6-Tetrahydropyridine-Induced Mitochondrial Dysfunction. Mol. Cells 2016, 39, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Kuwashiro, T.; Ago, T.; Kamouchi, M.; Matsuo, R.; Hata, J.; Kuroda, J.; Fukuda, K.; Sugimori, H.; Fukuhara, M.; Awano, H.; et al. Significance of plasma adiponectin for diagnosis, neurological severity and functional outcome in ischemic stroke—Research for Biomarkers in Ischemic Stroke (REBIOS). Metabolism 2014, 63, 1093–1103. [Google Scholar] [CrossRef]
- Thundyil, J.; Pavlovski, D.; Sobey, C.G.; Arumugam, T.V. Adiponectin receptor signalling in the brain. Br. J. Pharmacol. 2011, 165, 313–327. [Google Scholar] [CrossRef]
- Guillod-Maximin, E.; Roy, A.F.; Vacher, C.M.; Aubourg, A.; Bailleux, V.; Lorsignol, A.; Pénicaud, L.; Parquet, M.; Taouis, M. Adiponectin receptors are expressed in hypothalamus and colocalized with proopiomelanocortin and neuropeptide Y in rodent arcuate neurons. J. Endocrinol. 2009, 200, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhang, Y.; Doycheva, D.M.; Ding, Y.; Zhang, Y.; Tang, J.; Guo, H.; Zhang, J.H. Adiponectin attenuates neuronal apoptosis induced by hypoxia-ischemia via the activation of AdipoR1/APPL1/LKB1/AMPK pathway in neonatal rats. Neuropharmacology 2018, 133, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wu, X.; Wang, J.; Shi, Y.; Hu, Q.; Cui, W.; Bai, H.; Zhou, J.; Du, Y.; Han, L.; et al. Adiponectin/AdiopR1 signaling prevents mitochondrial dysfunction and oxidative injury after traumatic brain injury in a SIRT3 dependent manner. Redox Biol. 2022, 54, 102390. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.-H.; Shiue, S.-J.; Chen, C.-N.; Cheng, S.-W.; Lin, H.-Y.; Wu, L.-W.; Wu, M.-S. Fucoidan and Fucoxanthin Attenuate Hepatic Steatosis and Inflammation of NAFLD through Modulation of Leptin/Adiponectin Axis. Mar. Drugs 2021, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, T.; Wang, Z.; Xu, Y.; Zhang, Q.; Luo, D. Low molecular weight fucoidan attenuates liver injury via SIRT1/AMPK/PGC1α axis in db/db mice. Int. J. Biol. Macromol. 2018, 112, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.Z.A.; Zhao, D.; Hussain, T.; Yang, L. Role of the AMPK pathway in promoting autophagic flux via modulating mitochondrial dynamics in neurodegenerative diseases: Insight into prion diseases. Ageing Res. Rev. 2017, 40, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Stern, M.; McNew, J.A. A transition to degeneration triggered by oxidative stress in degenerative disorders. Mol. Psychiatry 2020, 26, 736–746. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, K.; Feng, Y.; Fan, C.; Wang, F.; Yan, J.; Yang, J.; Pei, H.; Liang, Z.; Jiang, S.; et al. Novel role of silent information regulator 1 in acute endothelial cell oxidative stress injury. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 2246–2256. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Bartlett, J.D. Sirtuin1 and autophagy protect cells from fluoride-induced cell stress. Biochim. Biophys. Acta Mol. Basis Dis. 2014, 1842, 245–255. [Google Scholar] [CrossRef]
- Ota, H.; Eto, M.; Kano, M.R.; Ogawa, S.; Iijima, K.; Akishita, M.; Ouchi, Y. Cilostazol Inhibits Oxidative Stress–Induced Premature Senescence Via Upregulation of Sirt1 in Human Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1634–1639. [Google Scholar] [CrossRef]
- Huang, B.; Cheng, X.; Wang, D.; Peng, M.; Xue, Z.; Da, Y.; Zhang, N.; Yao, Z.; Li, M.; Xu, A.; et al. Adiponectin promotes pancreatic cancer progression by inhibiting apoptosis via the activation of AMPK/Sirt1/PGC-1α signaling. Oncotarget 2014, 5, 4732–4745. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.E.; Vincent, G.; Sodhi, C.P.; Novak, E.A.; Ranganathan, S.; Egan, C.E.; Stolz, D.B.; Rogers, M.B.; Firek, B.; Morowitz, M.J.; et al. Peroxisome Proliferator-activated Receptor-γ Coactivator 1-α (PGC1α) Protects against Experimental Murine Colitis. J. Biol. Chem. 2016, 291, 10184–10200. [Google Scholar] [CrossRef] [PubMed]
- Bárcena, C.; Valdés-Mas, R.; Mayoral, P.; Garabaya, C.; Durand, S.; Rodríguez, F.; Fernández-García, M.T.; Salazar, N.; Nogacka, A.M.; Garatachea, N.; et al. Healthspan and lifespan extension by fecal microbiota transplantation into progeroid mice. Nat. Med. 2019, 25, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Yuan, X.; Cheng, G.; Jiao, S.; Feng, C.; Zhao, X.; Yin, H.; Du, Y.; Liu, H. Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr. Polym. 2018, 190, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Summanen, P.H.; Henning, S.M.; Hsu, M.; Lam, H.; Huang, J.; Tseng, C.-H.; Dowd, S.E.; Finegold, S.M.; Heber, D.; et al. Xylooligosaccharide supplementation alters gut bacteria in both healthy and prediabetic adults: A pilot study. Front. Physiol. 2015, 6, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, S.; Cao, H.; Shen, P.; Liu, J.; Fu, Y.; Cao, Y.; Zhang, N. The protective role of phloretin against dextran sulfate sodium-induced ulcerative colitis in mice. Food Funct. 2019, 10, 422–431. [Google Scholar] [CrossRef]
- Shao, M.; Yuan, F.; Liu, J.; Luo, H. Mast Cell Specific Receptor Mrgprb2 Regulating Experimental Colitis is Associated with the Microbiota-Gut-Brain Axis. J. Inflamm. Res. 2022, 15, 6137–6151. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhou, N.; Lu, M.; Wang, Q.; Zhao, C.; Wang, J.; Zhou, M.; Xu, Y. Effects of electroacupuncture on urinary metabolome and microbiota in presenilin1/2 conditional double knockout mice. Front. Microbiol. 2023, 13, 1047121. [Google Scholar] [CrossRef]
- Shin, J.; Noh, J.-R.; Choe, D.; Lee, N.; Song, Y.; Cho, S.; Kang, E.-J.; Go, M.-J.; Ha, S.K.; Chang, D.-H.; et al. Ageing and rejuvenation models reveal changes in key microbial communities associated with healthy ageing. Microbiome 2021, 9, 240. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Xue, M.; Li, J.; Ma, Y.; Wang, Y.; Zhang, H.; Liang, H. Fucoidan Improves D-Galactose-Induced Cognitive Dysfunction by Promoting Mitochondrial Biogenesis and Maintaining Gut Microbiome Homeostasis. Nutrients 2024, 16, 1512. https://doi.org/10.3390/nu16101512
Xu Y, Xue M, Li J, Ma Y, Wang Y, Zhang H, Liang H. Fucoidan Improves D-Galactose-Induced Cognitive Dysfunction by Promoting Mitochondrial Biogenesis and Maintaining Gut Microbiome Homeostasis. Nutrients. 2024; 16(10):1512. https://doi.org/10.3390/nu16101512
Chicago/Turabian StyleXu, Yan, Meilan Xue, Jing Li, Yiqing Ma, Yutong Wang, Huaqi Zhang, and Hui Liang. 2024. "Fucoidan Improves D-Galactose-Induced Cognitive Dysfunction by Promoting Mitochondrial Biogenesis and Maintaining Gut Microbiome Homeostasis" Nutrients 16, no. 10: 1512. https://doi.org/10.3390/nu16101512
APA StyleXu, Y., Xue, M., Li, J., Ma, Y., Wang, Y., Zhang, H., & Liang, H. (2024). Fucoidan Improves D-Galactose-Induced Cognitive Dysfunction by Promoting Mitochondrial Biogenesis and Maintaining Gut Microbiome Homeostasis. Nutrients, 16(10), 1512. https://doi.org/10.3390/nu16101512





