The Tryptophan Index Is Associated with Risk of Ischemic Stroke: A Community-Based Nested Case–Control Study
Abstract
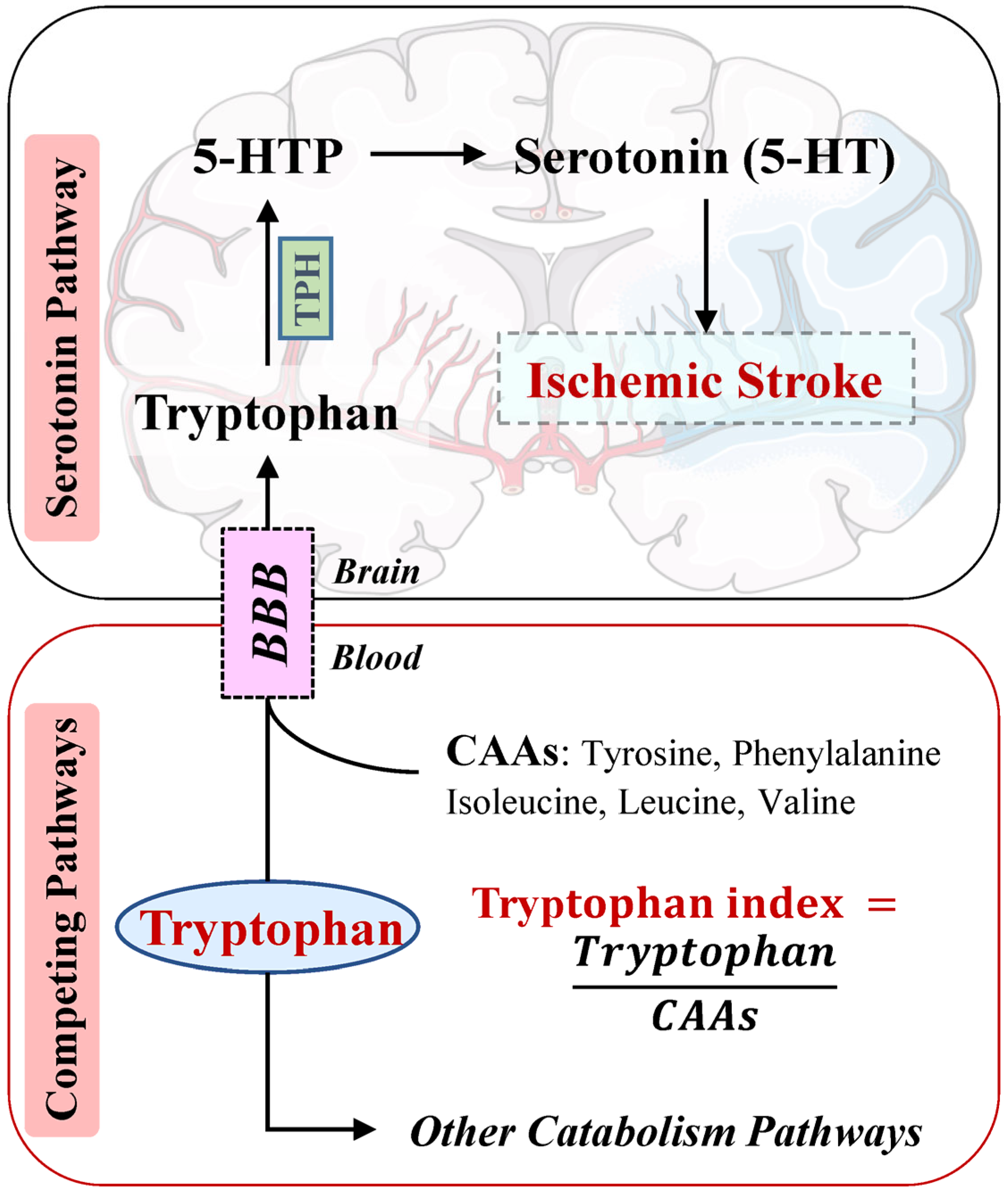
1. Introduction
2. Material and Methods
2.1. Study Participants
2.2. Follow-Up and Definition of Ischemic Stroke
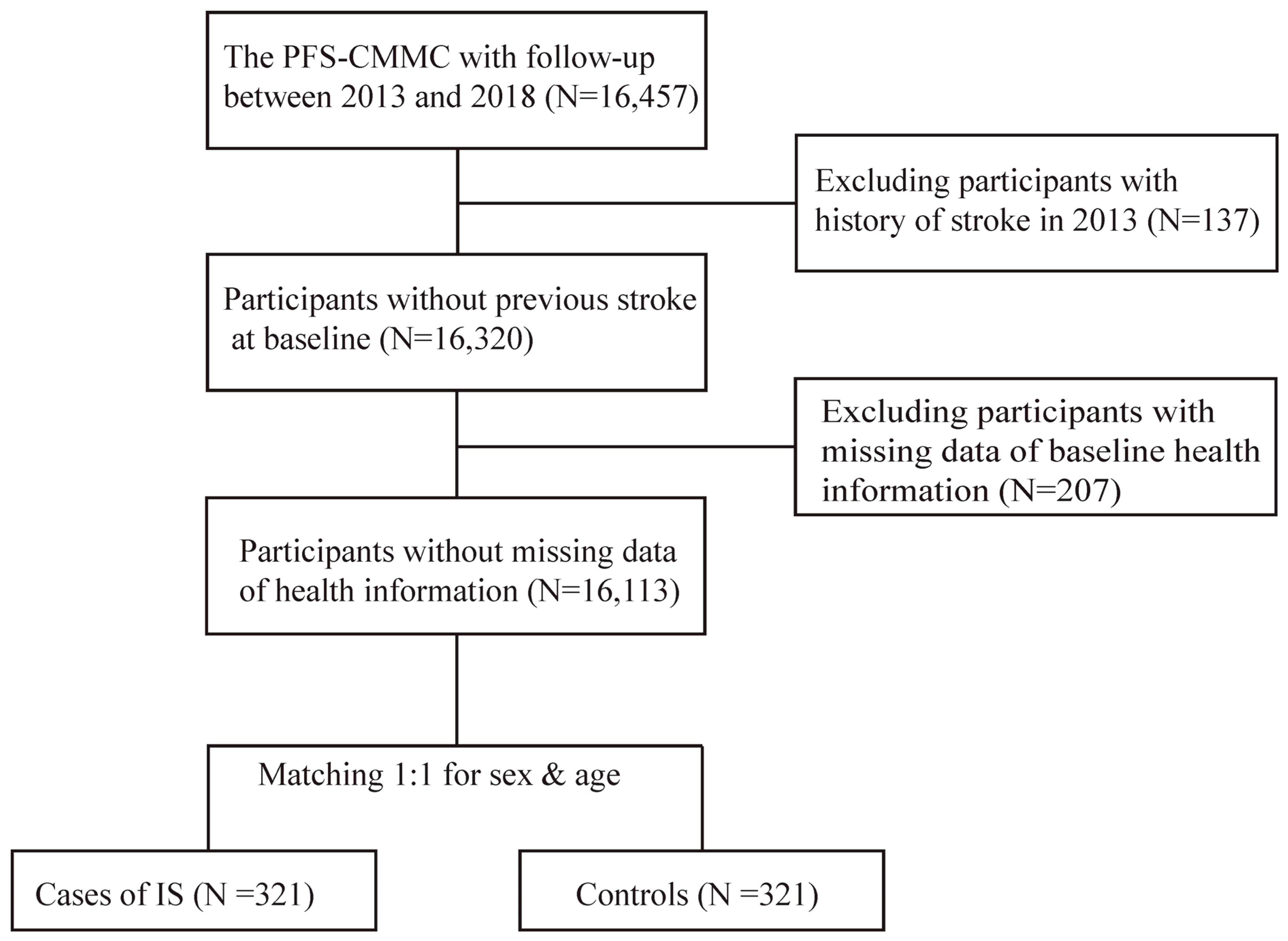
2.3. Selection of Cases and Controls
2.4. Measurements of Biomarkers
2.5. Covariates
2.6. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. The Tryptophan Index, Its Components, and the Risk of Ischemic Stroke
3.3. Sensitivity Analyses
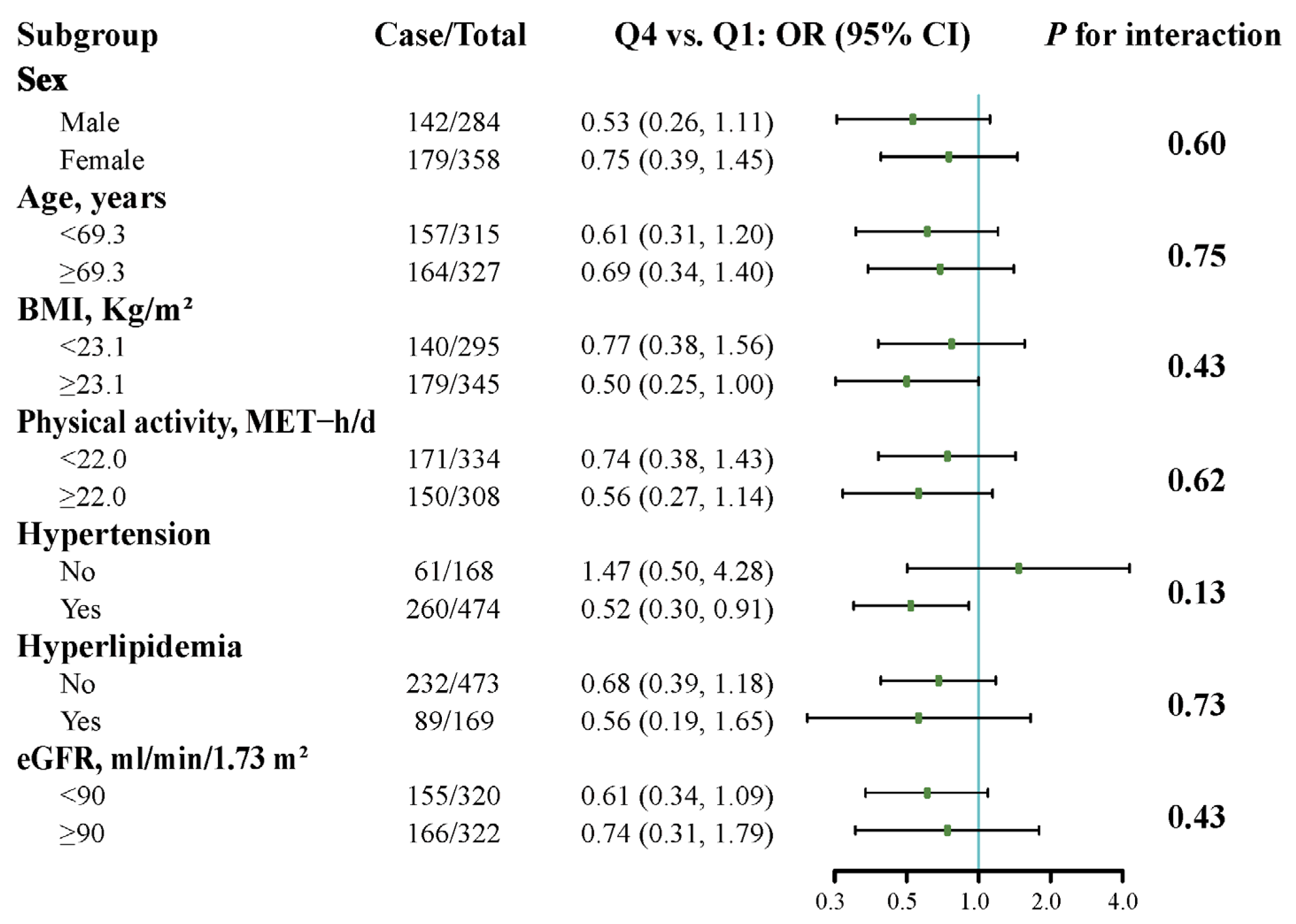
3.4. Stratified Analyses
4. Discussion
4.1. Principal Findings
4.2. The Tryptophan Index and Ischemic Stroke Risk
4.3. Mechanisms
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ma, Q.; Li, R.; Wang, L.; Yin, P.; Wang, Y.; Yan, C.; Ren, Y.; Qian, Z.; Vaughn, M.G.; McMillin, S.E.; et al. Temporal trend and attributable risk factors of stroke burden in China, 1990–2019: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2021, 6, e897–e906. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Primers 2019, 5, 70. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Hawkins, K.E.; Dore, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef] [PubMed]
- Le Floc’h, N.; Otten, W.; Merlot, E. Tryptophan metabolism, from nutrition to potential therapeutic applications. Amino Acids 2011, 41, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Wurtman, R.J. Brain serotonin content: Physiological regulation by plasma neutral amino acids. Science 1972, 178, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Moir, A.T.; Eccleston, D. The effects of precursor loading in the cerebral metabolism of 5-hydroxyindoles. J. Neurochem. 1968, 15, 1093–1108. [Google Scholar] [CrossRef]
- Ormstad, H.; Dahl, J.; Verkerk, R.; Andreassen, O.A.; Maes, M. Increased plasma levels of competing amino acids, rather than lowered plasma tryptophan levels, are associated with a non-response to treatment in major depression. Eur. Neuropsychopharmacol. 2016, 26, 1286–1296. [Google Scholar] [CrossRef]
- Ruddick, J.P.; Evans, A.K.; Nutt, D.J.; Lightman, S.L.; Rook, G.A.; Lowry, C.A. Tryptophan metabolism in the central nervous system: Medical implications. Expert Rev. Mol. Med. 2006, 8, 1–27. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Role of precursor availability in control of monoamine biosynthesis in brain. Physiol. Rev. 1983, 63, 484–546. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Wahl, D.; Clark, X.; Bagley, E.; Gregoriou, G.C.; Senior, A.M.; Wang, Q.P.; Brandon, A.E.; et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat. Metab. 2019, 1, 532–545. [Google Scholar] [CrossRef]
- Berger, M.; Gray, J.A.; Roth, B.L. The expanded biology of serotonin. Annu. Rev. Med. 2009, 60, 355–366. [Google Scholar] [CrossRef]
- Moller, S.E.; De Beurs, P.; Timmerman, L.; Tan, B.K.; Leijnse-Ybema, H.J.; Stuart, M.H.; Petersen, H.E. Plasma tryptophan and tyrosine ratios to competing amino acids in relation to antidepressant response to citalopram and maprotiline. A preliminary study. Psychopharmacology 1986, 88, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Ormstad, H.; Verkerk, R.; Aass, H.C.; Amthor, K.F.; Sandvik, L. Inflammation-induced catabolism of tryptophan and tyrosine in acute ischemic stroke. J. Mol. Neurosci. 2013, 51, 893–902. [Google Scholar] [CrossRef]
- Kim, S.W.; Park, S.Y.; Hwang, O. Up-Regulation of Tryptophan Hydroxylase Expression and Serotonin Synthesis by Sertraline. Mol. Pharmacol. 2002, 61, 778–785. [Google Scholar] [CrossRef]
- Alqdwah-Fattouh, R.; Rodriguez-Martin, S.; Barreira-Hernandez, D.; Izquierdo-Esteban, L.; Gil, M.; Gonzalez-Bermejo, D.; Fernandez-Anton, E.; Rodriguez-Miguel, A.; Garcia-Lledo, A.; Bolumar, F.; et al. Selective serotonin reuptake inhibitors and risk of noncardioembolic ischemic stroke: A nested case-control study. Stroke 2022, 53, 1560–1569. [Google Scholar] [CrossRef]
- Liu, D.; Gu, S.; Zhou, Z.; Ma, Z.; Zuo, H. Associations of plasma TMAO and its precursors with stroke risk in the general population: A nested case-control study. J. Intern. Med. 2022, 293, 110–120. [Google Scholar] [CrossRef]
- Liu, D.; Wang, J.; Xiao, L.; Gu, S.; Ma, Z.; Zhou, Z.; Gu, S.; Zuo, H. Associations of plasma carnitine, lysine, trimethyllysine and glycine with incident ischemic stroke: Findings from a nested case-control study. Clin. Nutr. 2022, 41, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.B. An incidence density sampling program for nested case-control analyses. Occup. Environ. Med. 2004, 61, e59. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, D.; Ma, Z.; Wang, C.; Gu, S.; Zhou, Z.; Zuo, H. Plasma beta-Alanine is Positively Associated With Risk of Ischemic Stroke: A Nested Case-Control Study. J. Nutr. 2023, 153, 1162–1169. [Google Scholar] [CrossRef]
- Lewis, M.R.; Pearce, J.T.; Spagou, K.; Green, M.; Dona, A.C.; Yuen, A.H.; David, M.; Berry, D.J.; Chappell, K.; Horneffer-van der Sluis, V.; et al. Development and Application of Ultra-Performance Liquid Chromatography-TOF MS for Precision Large Scale Urinary Metabolic Phenotyping. Anal. Chem. 2016, 88, 9004–9013. [Google Scholar] [CrossRef]
- Wong, J.M.; Malec, P.A.; Mabrouk, O.S.; Ro, J.; Dus, M.; Kennedy, R.T. Benzoyl chloride derivatization with liquid chromatography-mass spectrometry for targeted metabolomics of neurochemicals in biological samples. J. Chromatogr. A 2016, 1446, 78–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; Liu, D.; Zhou, Z.; Gu, S.; Zuo, H. Association of total pre-existing comorbidities with stroke risk: A large-scale community-based cohort study from China. BMC Public Health 2021, 21, 1910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, S.; Wang, C.; Liu, D.; Zhang, Q.; Yang, M.; Zhou, Z.; Zuo, H. Association between fasting blood glucose levels and stroke events: A large-scale community-based cohort study from China. BMJ Open 2021, 11, e050234. [Google Scholar] [CrossRef] [PubMed]
- Jager, K.J.; Zoccali, C.; MacLeod, A.; Dekker, F.W. Confounding: What it is and how to deal with it. Kidney Int. 2008, 73, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modelling. Fam. Med. Community Health 2020, 8, e000262. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, F.C.; Huang, K.Y.; Li, J.X.; Yang, X.L.; Wang, X.Y.; Chen, J.C.; Liu, X.Q.; Cao, J.; Shen, C.; et al. Beneficial effects of moderate to vigorous physical activity on cardiovascular disease among Chinese adults. J. Geriatr. Cardiol. 2020, 17, 85–95. [Google Scholar] [CrossRef]
- Ainsworth, B.E.; Haskell, W.L.; Herrmann, S.D.; Meckes, N.; Bassett, D.R., Jr.; Tudor-Locke, C.; Greer, J.L.; Vezina, J.; Whitt-Glover, M.C.; Leon, A.S. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med. Sci. Sports Exerc. 2011, 43, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Joint Committee for Guideline Revision. 2016 Chinese guidelines for the management of dyslipidemia in adults. J. Geriatr. Cardiol. 2018, 15, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Labrecque, J.A.; Hunink, M.M.G.; Ikram, M.A.; Ikram, M.K. Do Case-Control Studies Always Estimate Odds Ratios? Am. J. Epidemiol. 2021, 190, 318–321. [Google Scholar] [CrossRef]
- Nie, J.; Xie, L.; Zhao, B.X.; Li, Y.; Qiu, B.; Zhu, F.; Li, G.F.; He, M.; Wang, Y.; Wang, B.; et al. Serum trimethylamine N-oxide concentration is positively associated with first stroke in hypertensive patients. Stroke 2018, 49, 2021–2028. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Lee, C.H.; Yoo, D.M.; Min, C.; Choi, H.G. Association between Asthma and Meniere’s Disease: A Nested Case-Control Study. Laryngoscope 2022, 132, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Zhang, S.; Cheng, X.; An, J.; Zhang, X.; Li, P.; Li, W.; Wang, X.; Yuan, Y.; Zheng, H.; et al. Association between serum pyrethroid insecticide levels and incident type 2 diabetes risk: A nested case-control study in Dongfeng-Tongji cohort. Eur. J. Epidemiol. 2022, 37, 959–970. [Google Scholar] [CrossRef] [PubMed]
- VanderWeele, T.J.; Knol, M.J. A Tutorial on Interaction. Epidemiol. Methods 2014, 3, 33–72. [Google Scholar] [CrossRef]
- VanderWeele, T.J.; Ding, P. Sensitivity Analysis in Observational Research: Introducing the E-Value. Ann. Intern. Med. 2017, 167, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Yang, P.; Jia, Y.; Wang, Y.; Shi, M.; Zhong, C.; Peng, H.; Sun, L.; Guo, D.; Xu, Q.; et al. Plasma Amino Acid Neurotransmitters and Ischemic Stroke Prognosis: A Multicenter Prospective Study. Am. J. Clin. Nutr. 2023, 118, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Faller, D.V.; Shabshelowitz, H. Acute reduction of brain serotonin and 5-HIAA following food consumption: Correlation with the ratio of serum tryptophan to the sum of competing amino acids. J. Neural Transm. 1975, 36, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Verkerk, R.; Vandoolaeghe, E.; Van Hunsel, F.; Neels, H.; Wauters, A.; Demedts, P.; Scharpé, S. Serotonin-immune interactions in major depression: Lower serum tryptophan as a marker of an immune-inflammatory response. Eur. Arch. Psychiatry Clin. Neurosci. 1997, 247, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Wu, H.T.; Tsai, Y.T.; Huang, Y.W.; Tsai, H.J. Use of antidepressants and risk of hospitalization for acute myocardial infarction: A nationwide case-crossover study. J. Psychiatr. Res. 2017, 94, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Glymour, M.M.; Gibbons, L.E.; Gilsanz, P.; Gross, A.L.; Mez, J.; Brewster, P.W.; Marden, J.; Zahodne, L.B.; Nho, K.; Hamilton, J.; et al. Initiation of antidepressant medication and risk of incident stroke: Using the Adult Changes in Thought cohort to address time-varying confounding. Ann. Epidemiol. 2019, 35, 42–47.e41. [Google Scholar] [CrossRef]
- Steinbusch, H.W. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 1981, 6, 557–618. [Google Scholar] [CrossRef] [PubMed]
- Van Donkelaar, E.L.; Blokland, A.; Ferrington, L.; Kelly, P.A.; Steinbusch, H.W.; Prickaerts, J. Mechanism of acute tryptophan depletion: Is it only serotonin? Mol. Psychiatry 2011, 16, 695–713. [Google Scholar] [CrossRef]
- Sauer, W.H.; Berlin, J.A.; Kimmel, S.E. Effect of Antidepressants and Their Relative Affinity for the Serotonin Transporter on the Risk of Myocardial Infarction. Circulation 2003, 108, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Boyanpally, A.; Cutting, S.; Furie, K. Acute Ischemic Stroke Associated with Myocardial Infarction: Challenges and Management. Semin. Neurol. 2021, 41, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Møller, S.E. Tryptophan to competing amino acids ratio in depressive disorder: Relation to efficacy of antidepressive treatments. Acta Psychiatr. Scand. Suppl. 1985, 325, 3–31. [Google Scholar] [CrossRef]
- Zinellu, A.; Sotgia, S.; Mangoni, A.A.; Sanna, M.; Satta, A.E.; Carru, C. Impact of cholesterol lowering treatment on plasma kynurenine and tryptophan concentrations in chronic kidney disease: Relationship with oxidative stress improvement. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 153–159. [Google Scholar] [CrossRef]
| Cases (n = 321) | Controls (n = 321) | p-Value | |
|---|---|---|---|
| Age (years) | 69.5 (63.2, 75.2) | 69.6 (63.4, 75.1) | |
| Male (%) | 142 (44.2) | 142 (44.2) | |
| BMI (kg/m2) | 23.6 (21.4, 25.9) | 23.3 (21.4, 25.7) | 0.53 |
| Currently smoking (%) | 83 (25.9) | 80 (24.9) | 0.79 |
| Physical activity (MET-h/d) | 20.4 (12.3, 34.8) | 21.8 (13.5, 34.4) | 0.37 |
| Educational attainment (%) | 0.85 | ||
| 0 year | 157 (49.1) | 153 (47.7) | |
| 1–5 years | 121 (37.8) | 121 (37.7) | |
| ≥6 years | 42 (13.1) | 47 (14.6) | |
| TC (mmol/L) | 4.95 (4.30, 5.61) | 4.84 (4.21, 5.51) | 0.24 |
| TG (mmol/L) | 1.32 (0.95, 1.88) | 1.23 (0.92, 1.70) | 0.23 |
| HDL-C (mmol/L) | 1.40 (1.16, 1.69) | 1.48 (1.21, 1.71) | 0.05 |
| Fasting glucose (mmol/L) | 5.54 (5.07, 6.23) | 5.40 (4.98, 5.96) | 0.013 |
| Diabetes (%) | 61 (19.0) | 35 (10.9) | 0.004 |
| Hypertension (%) | 260 (81.0) | 214 (66.7) | <0.001 |
| eGFR (mL/min/1.73 m2) | 84.5 (72.4, 91.0) | 85.4 (74.3, 93.2) | 0.06 |
| Hyperlipidemia (%) | 89 (27.7) | 80 (24.9) | 0.42 |
| Tryptophan (μmol/L) | 76.6 (66.2, 87.9) | 79.2 (67.0, 90.3) | 0.19 |
| Tyrosine (μmol/L) | 89.5 (77.3, 106) | 91.4 (80.0, 107) | 0.23 |
| Valine (μmol/L) | 268 (237, 301) | 264 (233, 296) | 0.20 |
| Phenylalanine (μmol/L) | 69.3 (61.4, 80.1) | 69.6 (62.2, 79.9) | 0.83 |
| Isoleucine (μmol/L) | 86.8 (73.3, 106) | 83.7 (71.1, 99.4) | 0.038 |
| Leucine (μmol/L) | 115 (100, 134) | 114 (100, 129) | 0.29 |
| Total CAAs (μmol/L) | 636 (569, 700) | 626 (561, 696) | 0.28 |
| Tryptophan index (×100) | 12.1 (10.7, 13.6) | 12.5 (11.3, 13.8) | 0.010 |
| Cases/Controls | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|
| IRR (95% CI) | IRR (95% CI) | IRR (95% CI) | ||
| Tryptophan index (×100) | ||||
| Q1 (<11.2) | 101/81 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Q2 (11.3, 12.5) | 88/81 | 0.87 (0.56, 1.34) | 0.86 (0.55, 1.33) | 0.91 (0.58, 1.45) |
| Q3 (12.6, 13.7) | 68/78 | 0.68 (0.43, 1.07) | 0.62 (0.39, 1.01) | 0.63 (0.38, 1.05) |
| Q4 (>13.8) | 64/81 | 0.62 (0.40, 0.98) | 0.56 (0.35, 0.90) | 0.53 (0.31, 0.88) |
| p for trend | 0.023 | 0.008 | 0.008 | |
| Continuous | 321/321 | 0.79 (0.67, 0.93) | 0.76 (0.64, 0.90) | 0.76 (0.63, 0.93) |
| p-value | 0.005 | 0.002 | 0.006 | |
| Tryptophan (μmol/L) | ||||
| Q1 (<67.0) | 89/81 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Q2 (67.1, 79.1) | 99/80 | 1.16 (0.75, 1.79) | 1.16 (0.74, 1.82) | 1.22 (0.76, 1.79) |
| Q3 (79.2, 90.3) | 60/80 | 0.70 (0.45, 1.09) | 0.67 (0.43, 1.05) | 0.63 (0.39, 1.03) |
| Q4 (>90.4) | 73/80 | 0.82 (0.52, 1.29) | 0.75 (0.47, 1.22) | 0.72 (0.44, 1.21) |
| p for trend | 0.13 | 0.06 | 0.05 | |
| Continuous | 321/321 | 0.89 (0.76, 1.05) | 0.85 (0.72, 1.10) | 0.83 (0.69, 1.00) |
| p-value | 0.17 | 0.07 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Hong, Y.; Chen, Z.; Ma, Y.; Xia, S.; Gu, S.; Zuo, H. The Tryptophan Index Is Associated with Risk of Ischemic Stroke: A Community-Based Nested Case–Control Study. Nutrients 2024, 16, 1544. https://doi.org/10.3390/nu16111544
Liu D, Hong Y, Chen Z, Ma Y, Xia S, Gu S, Zuo H. The Tryptophan Index Is Associated with Risk of Ischemic Stroke: A Community-Based Nested Case–Control Study. Nutrients. 2024; 16(11):1544. https://doi.org/10.3390/nu16111544
Chicago/Turabian StyleLiu, Dong, Yan Hong, Zhenting Chen, Yifan Ma, Shangyu Xia, Shujun Gu, and Hui Zuo. 2024. "The Tryptophan Index Is Associated with Risk of Ischemic Stroke: A Community-Based Nested Case–Control Study" Nutrients 16, no. 11: 1544. https://doi.org/10.3390/nu16111544
APA StyleLiu, D., Hong, Y., Chen, Z., Ma, Y., Xia, S., Gu, S., & Zuo, H. (2024). The Tryptophan Index Is Associated with Risk of Ischemic Stroke: A Community-Based Nested Case–Control Study. Nutrients, 16(11), 1544. https://doi.org/10.3390/nu16111544






