Evaluation of Physicochemical Properties and Prebiotics Function of a Bioactive Pleurotus eryngii Aqueous Extract Powder Obtained by Spray Drying
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of SPAE and PEP
2.3. Characterization of SPAE and PEP
2.3.1. SEM
2.3.2. FTIR
2.3.3. TGA and DSC
2.4. Determination of Antioxidant Activity of SPAE and PEP In Vitro
2.5. In Vitro Digestion of SPAE and PEP
2.6. Animal Experiments
2.6.1. Experimental Design for Animals
2.6.2. Determination of Organ Indexes in Mice
2.6.3. Determination of Biochemical Indices in Mice
2.6.4. Determination of SCFAs in Mice Intestines
2.6.5. Cecal Contents DNA Extraction and 16S High-Throughput Sequencing
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of SPAE
3.1.1. Morphological Properties
3.1.2. FT-IR Spectra Analysis
3.1.3. Thermogravimetric Analysis
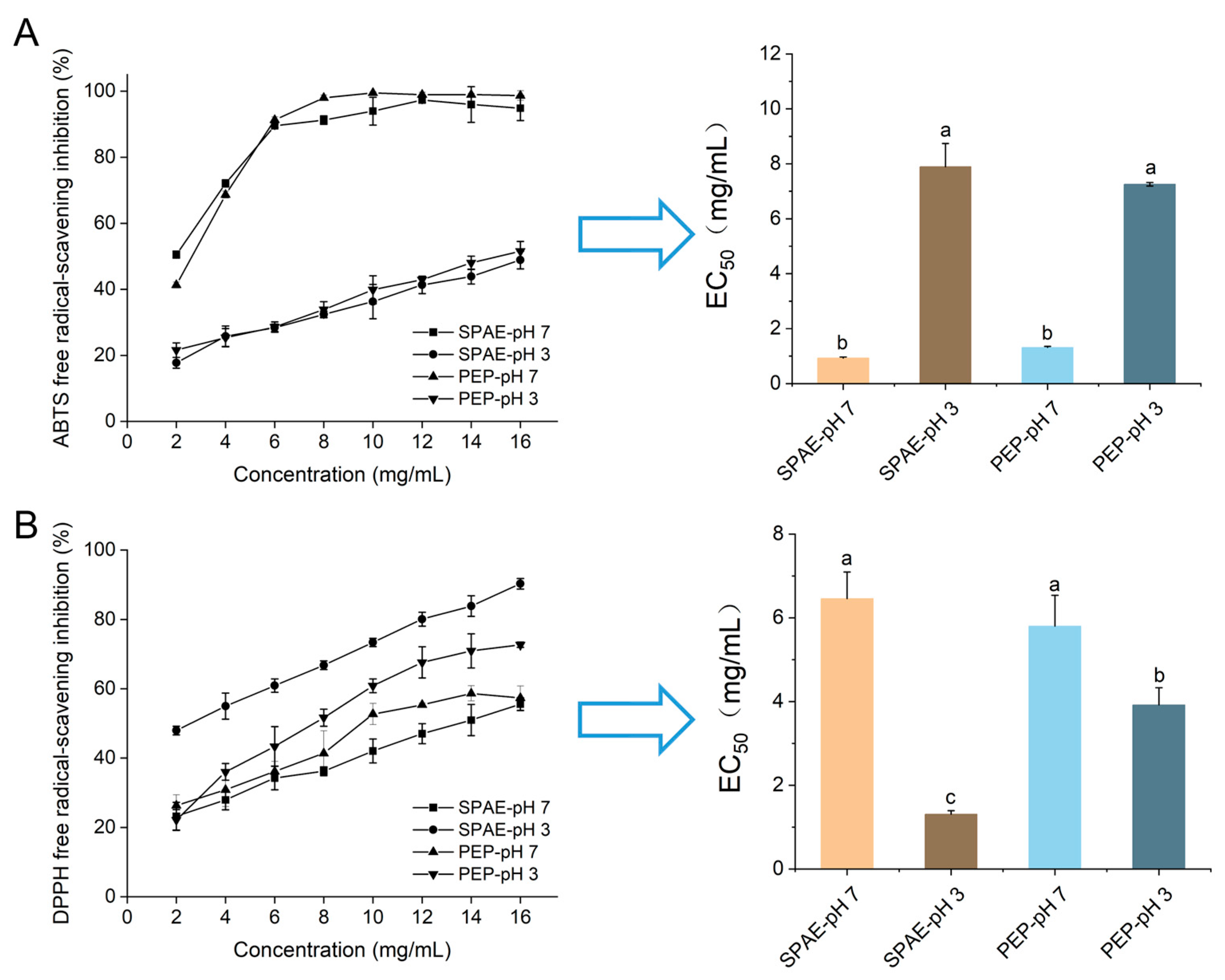
3.2. Antioxidative Activity In Vitro
3.3. Variations in Reducing Sugar of SPAE during Dynamic Digestion In Vitro
3.4. Effects of SPAE on the Gross Growth and Organ Indexes in Mice
3.5. Effects of SPAE on Inflammatory Parameters in Serum of Mice
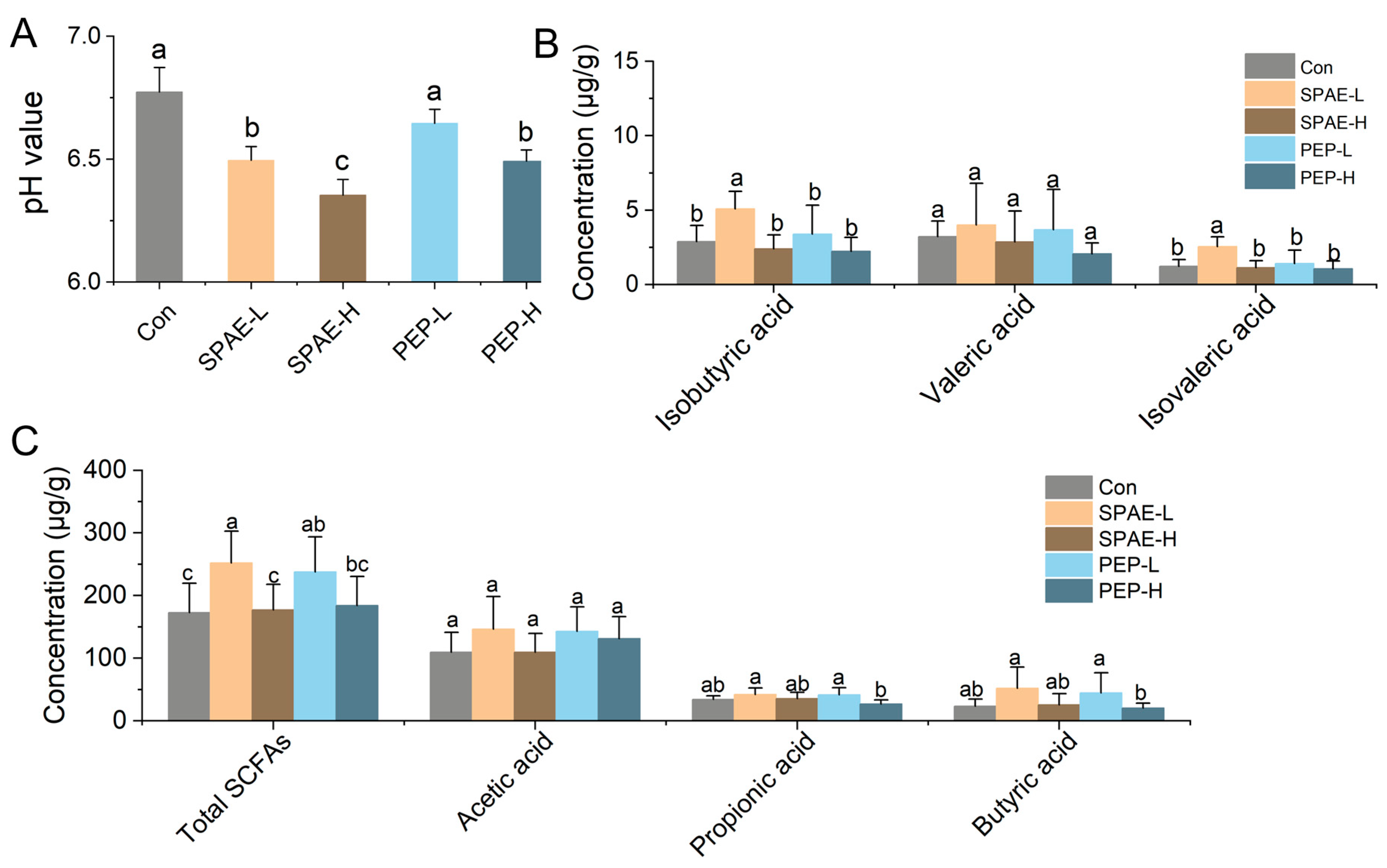
3.6. Effects of SPAE on Intestinal pH and SCFAs Concentration
3.7. Effects of SPAE on Intestinal Microbiota Composition Analysis
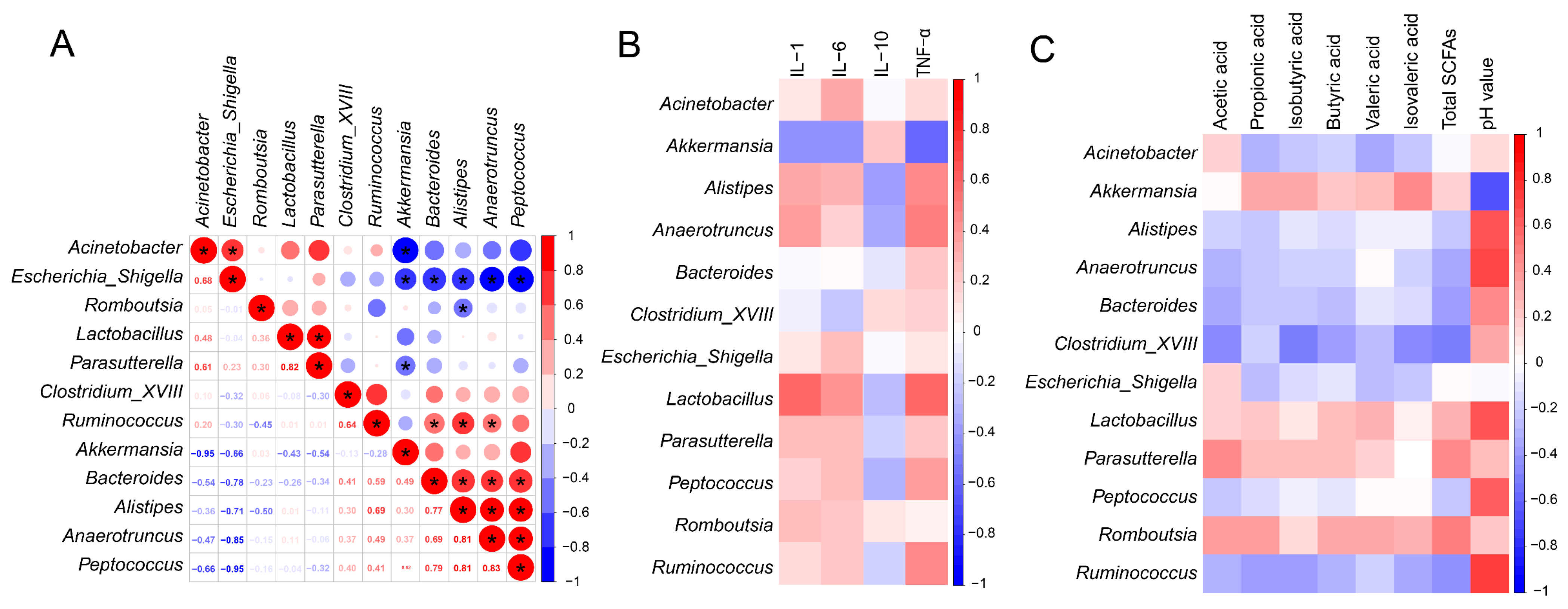
3.8. Relationship between Inflammatory Parameters, SCFAs and Microbiota
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Zhao, R. A review on nutritional advantages of edible mushrooms and its industrialization development situation in protein meat analogues. J. Future Foods 2023, 3, 1–7. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Sha, O.; Xu, W.; Wang, S. Hypolipidaemic and hypoglycaemic activities of polysaccharide from Pleurotus eryngii in Kunming mice. Int. J. Biol. Macromol. 2016, 93, 1206–1209. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Zhang, F.; Linhardt, R.J.; Zeng, G.; Zhang, A. Extraction, structure and bioactivities of the polysaccharides from Pleurotus eryngii: A review. Int. J. Biol. Macromol. 2020, 150, 1342–1347. [Google Scholar] [CrossRef]
- Li, L.; Cao, X.; Huang, J.; Zhang, T.; Wu, Q.; Xiang, P.; Shen, C.; Zou, L.; Li, J.; Li, Q. Effect of Pleurotus eryngii mycelial fermentation on the composition and antioxidant properties of tartary buckwheat. Heliyon 2024, 10, e25980. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Xu, Q.; Du, H.; Muinde Kimatu, B.; Su, A.; Yang, W.; Hu, Q.; Xiao, H. Characterization of polysaccharide from Pleurotus eryngii during simulated gastrointestinal digestion and fermentation. Food Chem. 2022, 370, 131303. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Mohite, A.M.; Sharma, N. Influence of particle size on physical, mechanical, thermal, and morphological properties of tamarind- fenugreek mucilage biodegradable films. Polym. Bull. 2022, 80, 3119–3133. [Google Scholar] [CrossRef]
- Shen, H.; Yu, J.; Bai, J.; Liu, Y.; Ge, X.; Li, W.; Zheng, J. A new pre-gelatinized starch preparing by spray drying and electron beam irradiation of oat starch. Food Chem. 2023, 398, 133938. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Fu, N.; Chen, X. The extent and mechanism of the effect of protectant material in the production of active lactic acid bacteria powder using spray drying: A review. Curr. Opin. Food Sci. 2022, 44, 100807. [Google Scholar] [CrossRef]
- Zhao, Q.; Dong, B.; Chen, J.; Zhao, B.; Wang, X.; Wang, L.; Zha, S.; Wang, Y.; Zhang, J.; Wang, Y. Effect of drying methods on physicochemical properties and antioxidant activities of wolfberry (Lycium barbarum) polysaccharide. Carbohydr. Polym. 2015, 127, 176–181. [Google Scholar] [CrossRef]
- Bai, J.; Wang, Y.; Cai, J.; Zhang, L.; Dai, Y.; Tian, X.; Xiao, H. Three-Dimensional Appearance and Physicochemical Properties of Pleurotus eryngii under Different Drying Methods. Foods 2023, 12, 1999. [Google Scholar] [CrossRef]
- Yang, R.; Li, Q.; Hu, Q. Physicochemical properties, microstructures, nutritional components, and free amino acids of Pleurotus eryngii as affected by different drying methods. Sci. Rep. 2020, 10, 121. [Google Scholar] [CrossRef]
- Levy, M.; Blacher, E.; Elinav, E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 2017, 35, 8–15. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, Y.; Zhou, W.; Chen, D.; Huang, K.; Yu, S.; Mi, J.; Lu, L.; Zeng, X.; Cao, Y. Effects of polysaccharides from bee collected pollen of Chinese wolfberry on immune response and gut microbiota composition in cyclophosphamide-treated mice. J. Funct. Foods 2020, 72, 104057. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Wang, X.; Huang, M.; Yang, F.; Sun, H.; Zhou, X.; Guo, Y.; Wang, X.; Zhang, M. Rapeseed polysaccharides as prebiotics on growth and acidifying activity of probiotics in vitro. Carbohydr. Polym. 2015, 125, 232–240. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Loo, J.V.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef]
- Cao, X.; Li, N.; Qi, G.; Sun, X.S.; Wang, D. Effect of spray drying on the properties of camelina gum isolated from camelina seeds. Ind. Crops Prod. 2018, 117, 278–285. [Google Scholar] [CrossRef]
- Zhao, R.; Cheng, N.; Nakata, P.; Zhao, L.; Hu, Q. Consumption of polysaccharides from Auricularia auricular modulates the intestinal microbiota in mice. Food Res. Int. 2019, 123, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.V.L.; de Castro, E.V.R.; Muri, E.J.B.; Loureiro, B.V.; Costalonga, M.L.; Filgueiras, P.R. Thermal and spectroscopic analyses of guar gum degradation submitted to turbulent flow. Int. J. Biol. Macromol. 2019, 131, 43–49. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Li, J.; Xie, J.; Yu, M.; Zhou, M.; Xi, M.; Sun, S. Physicochemical properties and in vitro digestion behavior of a new bioactive Tremella fuciformis gum. Int. J. Biol. Macromol. 2022, 207, 611–621. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Wu, P.; Bhattarai, R.; Dhital, S.; Deng, R.; Chen, X.; Gidley, M.J. In vitro digestion of pectin- and mango-enriched diets using a dynamic rat stomach-duodenum model. J. Food Eng. 2017, 202, 65–78. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Liu, C.; Du, P.; Guo, Y.; Xie, Y.; Yu, H.; Yao, W.; Cheng, Y.; Qian, H. Extraction, characterization of aloe polysaccharides and the in-depth analysis of its prebiotic effects on mice gut microbiota. Carbohydr. Polym. 2021, 261, 117874. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; An, S.; Luo, Z.; Zhou, P.; Wang, L.; Feng, R. Polysaccharides from the hard shells of Juglans regia L. modulate intestinal function and gut microbiota in vivo. Food Chem. 2023, 412, 135592. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, J.; Wang, C.; Xiao, M.; Zhu, Y.; Li, N.; Huang, Z.; Liu, B.; Huang, Y. Antidiabetic potential of Chlorella pyrenoidosa functional formulations in streptozocin-induced type 2 diabetic mice. J. Funct. Foods 2023, 103, 105489. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Zhang, W.; Wang, C.; Wang, P.; Niu, L.; Wu, H. Homogeneous selection dominates the microbial community assembly in the sediment of the Three Gorges Reservoir. Sci. Total Environ. 2019, 690, 50–60. [Google Scholar] [CrossRef]
- An, K.; Wu, J.; Xiao, H.; Hu, T.; Yu, Y.; Yang, W.; Xiao, G.; Xu, Y. Effect of various drying methods on the physicochemical characterizations, antioxidant activities and hypoglycemic activities of lychee (Litchi chinensis Sonn.) pulp polysaccharides. Int. J. Biol. Macromol. 2022, 220, 510–519. [Google Scholar] [CrossRef]
- Guo, B.; Zhu, C.; Huang, Z.; Yang, R.; Liu, C. Microcapsules with slow-release characteristics prepared by soluble small molecular starch fractions through the spray drying method. Int. J. Biol. Macromol. 2022, 200, 34–41. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Yu, X.; Li, H.; Ji, L.; Sun, Y.; Jiang, X.; Li, X.; Liu, H. Effects of freeze-drying and spray-drying on the physical and chemical properties of Perinereis aibuhitensis hydrolysates: Sensory characteristics and antioxidant activities. Food Chem. 2022, 382, 132317. [Google Scholar] [CrossRef]
- Jha, N.; Sivagnanavelmurugan, M.; Prasad, P.; Lakra, A.; Ayyanna, R.; Domdi, L.; Arul, V. Physicochemical properties, preliminary characterization, and assessment of potential bioactivities of polysaccharide purified from the leaves of Avicennia marina. Biocatal. Agric. Biotechnol. 2021, 35, 102110. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, G.; Peng, Y.; Rui, Y.; Zeng, X.; Ye, H. Simulated digestion and fermentation in vitro with human gut microbiota of polysaccharides from Coralline pilulifera. LWT—Food Sci. Technol. 2019, 100, 167–174. [Google Scholar] [CrossRef]
- Wu, D.; Nie, X.; Gan, R.; Guo, H.; Fu, Y.; Yuan, Q.; Zhang, Q.; Qin, W. In vitro digestion and fecal fermentation behaviors of a pectic polysaccharide from okra (Abelmoschus esculentus) and its impacts on human gut microbiota. Food Hydrocoll. 2021, 114, 106577. [Google Scholar] [CrossRef]
- Muralidharan, S.; Mandrekar, P. Cellular stress response and innate immune signaling: Integrating pathways in host defense and inflammation. J. Leukoc. Biol. 2013, 94, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, W.; Zhao, L. Regulatory Effects of Ganoderma lucidum, Grifola frondosa, and American ginseng extract formulation on gut microbiota and fecal metabolomics in mice. Foods 2023, 12, 3804. [Google Scholar] [CrossRef] [PubMed]
- Yin, C.; Noratto, G.D.; Fan, X.; Chen, Z.; Yao, F.; Shi, D.; Gao, H. The impact of mushroom polysaccharides on gut microbiota and its beneficial effects to host: A review. Carbohydr. Polym. 2020, 250, 116942. [Google Scholar] [CrossRef] [PubMed]
- Gullón, B.; Gullón, P.; Tavaria, F.; Pintado, M.; Gomes, A.M.; Alonso, J.L.; Parajó, J.C. Structural features and assessment of prebiotic activity of refined arabinoxylooligosaccharides from wheat bran. J. Funct. Foods 2014, 6, 438–449. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Xu, Q.; Yang, W.; Zhao, J.; Ren, Y.; Yu, Z.; Ma, L. Taxifolin alleviates DSS-induced ulcerative colitis by acting on gut microbiome to produce butyric acid. Nutrients 2022, 14, 1069. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.M.; Kim, Y.S.; Lee, M.; Lee, H.Y.; Bae, Y.S. Isovaleric acid ameliorates ovariectomy-induced osteoporosis by inhibiting osteoclast differentiation. J. Cell. Mol. Med. 2021, 25, 4287–4297. [Google Scholar] [CrossRef]
- Miao, M.; Jia, X.; Jiang, B.; Wu, S.; Cui, S.W.; Li, X. Elucidating molecular structure and prebiotics properties of bioengineered α-D-glucan from Leuconostoc citreum SK24.002. Food Hydrocoll. 2016, 54, 227–233. [Google Scholar] [CrossRef]
- Chen, J.; Liang, R.; Liu, W.; Liu, C.; Li, T.; Tu, Z.; Wan, J. Degradation of high-methoxyl pectin by dynamic high pressure microfluidization and its mechanism. Food Hydrocoll. 2012, 28, 121–129. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, X. Lentinula edodes-derived polysaccharide alters the spatial structure of gut microbiota in mice. PLoS ONE 2015, 10, e0115037. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.L.; Heaver, S.L.; Walters, W.A.; Ley, R.E. Microbiome and metabolic disease: Revisiting the bacterial phylum Bacteroidetes. J. Mol. Med. 2017, 95, 1–8. [Google Scholar] [CrossRef]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos Willem, M. The mucin degrader Akkermansia muciniphila is an abundant resident of the human intestinal tract. Appl. Environ. Microb. 2008, 74, 1646–1648. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Q.; Zhang, C.; Zhao, J.; Zhang, H.; Chen, W.; Zhai, Q. Strain-specific effects of Akkermansia muciniphila on the regulation of intestinal barrier. Food Sci. Hum. Wellness 2023, 12, 1526–1537. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, K.; Jia, Y.; Shi, J.; Tong, Z.; Fang, D.; Yang, B.; Su, C.; Li, R.; Xiao, X.; et al. Gut microbiome alterations in high-fat-diet-fed mice are associated with antibiotic tolerance. Nat. Microbiol. 2021, 6, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Reque, P.M.; Brandelli, A. Encapsulation of probiotics and nutraceuticals: Applications in functional food industry. Trends Food Sci. Technol. 2021, 114, 1–10. [Google Scholar] [CrossRef]
- Zhong, Y.; Fu, D.; Deng, Z.; Tang, W.; Mao, J.; Zhu, T.; Zhang, Y.; Liu, J.; Wang, H. Lactic acid bacteria mixture isolated from wild pig alleviated the gut inflammation of mice challenged by Escherichia coli. Front. Immunol. 2022, 13, 822754. [Google Scholar] [CrossRef] [PubMed]
- Mangifesta, M.; Mancabelli, L.; Milani, C.; Gaiani, F.; de’Angelis, N.; de’Angelis, G.L.; van Sinderen, D.; Ventura, M.; Turroni, F. Mucosal microbiota of intestinal polyps reveals putative biomarkers of colorectal cancer. Sci. Rep. 2018, 8, 13974. [Google Scholar] [CrossRef]
- Ye, X.; Liu, Y.; Hu, J.; Gao, Y.; Ma, Y.; Wen, D. Chlorogenic acid-induced gut microbiota improves metabolic endotoxemia. Front. Endocrinol. 2021, 12, 762691. [Google Scholar] [CrossRef]
- Xu, W.; Huang, Y.; Zhou, W.; Peng, Y.; Kan, X.; Dong, W.; Chen, G.; Zeng, X.; Liu, Z. Theasinensin A attenuated diabetic development by restoring glucose homeostasis, improving hepatic steatosis and modulating gut microbiota in high-fat-diet/streptozotocin-induced diabetic mice. Food Sci. Hum. Wellness 2023, 12, 2073–2086. [Google Scholar] [CrossRef]
- Ju, T.; Kong, J.Y.; Stothard, P.; Willing, B.P. Defining the role of Parasutterella, a previously uncharacterized member of the core gut microbiota. ISME J. 2019, 13, 1520–1534. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.; Ma, L.; Xu, Y.; Wu, J.; Yu, Y.; Peng, J.; Tang, D.; Zou, B.; Li, L. Effects of probiotic litchi juice on immunomodulatory function and gut microbiota in mice. Food Res. Int. 2020, 137, 109433. [Google Scholar] [CrossRef] [PubMed]

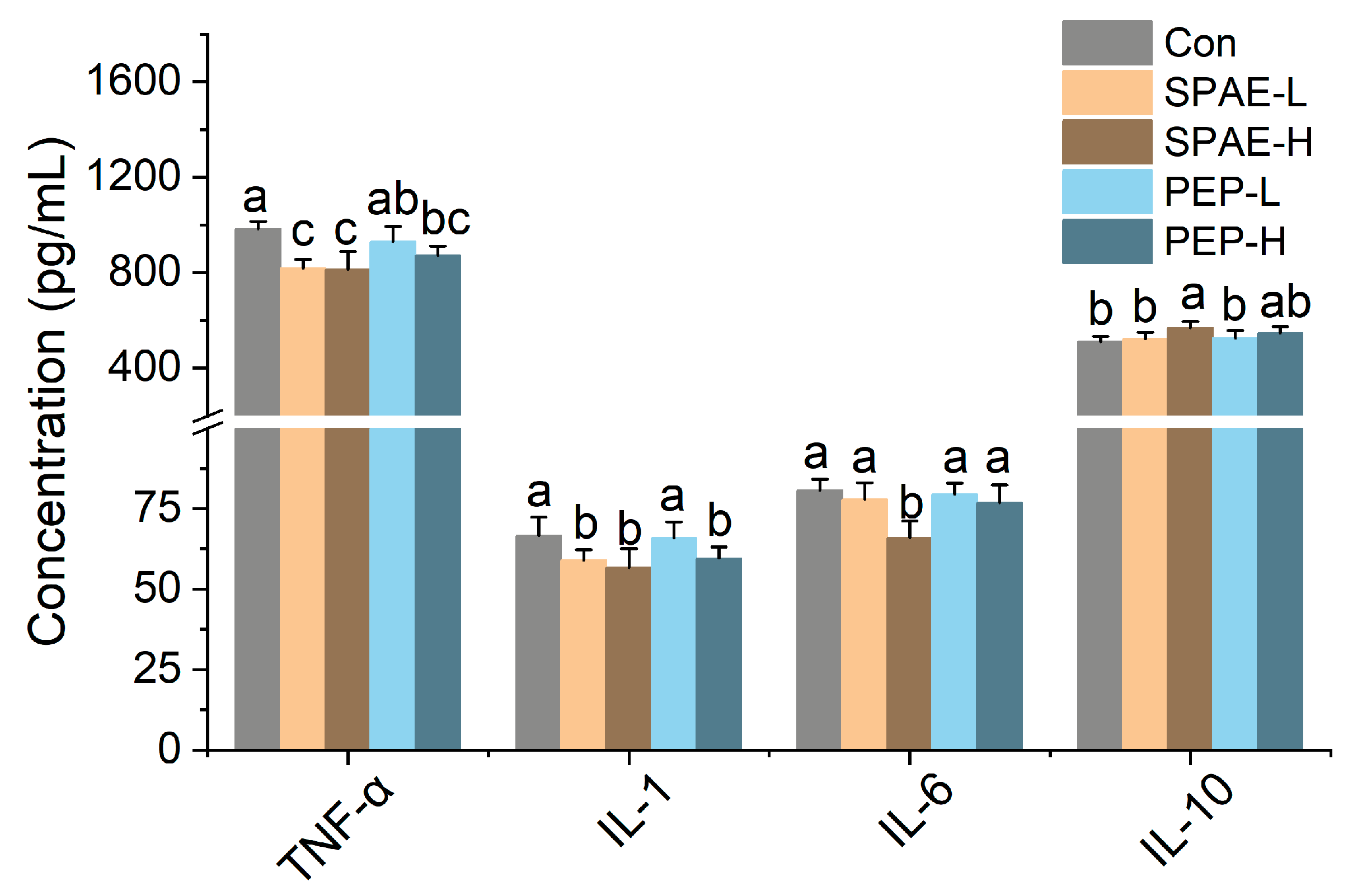

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhou, M.; Chen, L.; Yang, C.; Deng, Y.; Li, J.; Sun, S. Evaluation of Physicochemical Properties and Prebiotics Function of a Bioactive Pleurotus eryngii Aqueous Extract Powder Obtained by Spray Drying. Nutrients 2024, 16, 1555. https://doi.org/10.3390/nu16111555
Chen J, Zhou M, Chen L, Yang C, Deng Y, Li J, Sun S. Evaluation of Physicochemical Properties and Prebiotics Function of a Bioactive Pleurotus eryngii Aqueous Extract Powder Obtained by Spray Drying. Nutrients. 2024; 16(11):1555. https://doi.org/10.3390/nu16111555
Chicago/Turabian StyleChen, Jianqiu, Mengling Zhou, Liding Chen, Chengfeng Yang, Yating Deng, Jiahuan Li, and Shujing Sun. 2024. "Evaluation of Physicochemical Properties and Prebiotics Function of a Bioactive Pleurotus eryngii Aqueous Extract Powder Obtained by Spray Drying" Nutrients 16, no. 11: 1555. https://doi.org/10.3390/nu16111555
APA StyleChen, J., Zhou, M., Chen, L., Yang, C., Deng, Y., Li, J., & Sun, S. (2024). Evaluation of Physicochemical Properties and Prebiotics Function of a Bioactive Pleurotus eryngii Aqueous Extract Powder Obtained by Spray Drying. Nutrients, 16(11), 1555. https://doi.org/10.3390/nu16111555





