Unfavorable Mealtime, Meal Skipping, and Shiftwork Are Associated with Circadian Syndrome in Adults Participating in NHANES 2005–2016
Abstract
1. Introduction
2. Materials and Methods
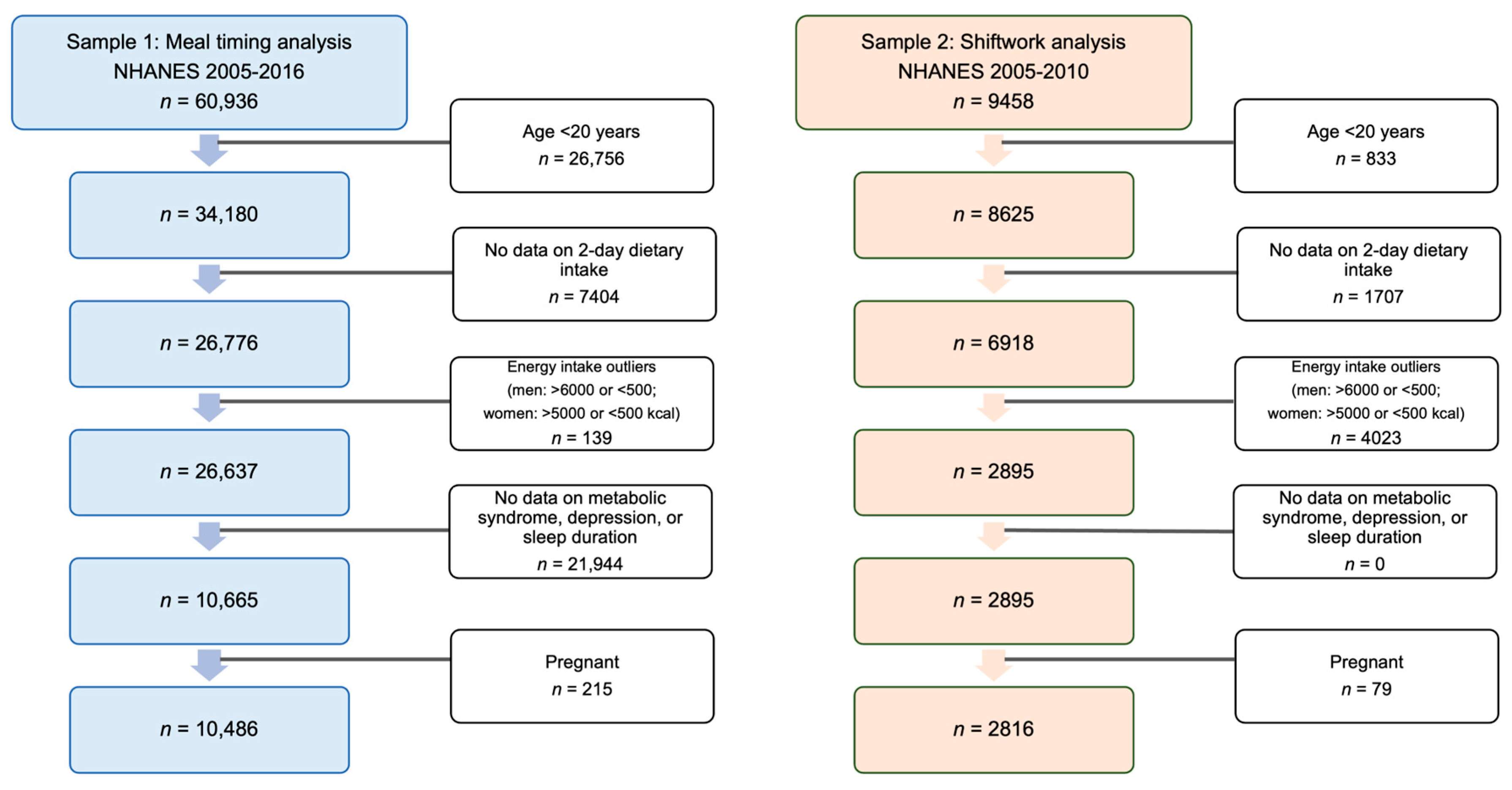
2.1. Study Design and Sample
2.2. Outcome Variable
Circadian Syndrome
2.3. Exposure Variables
2.3.1. Meal Timing and Meal Skipping
2.3.2. Shiftwork
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Sample Characteristics
3.2. Meal Timing, Meal Skipping, and Circadian Syndrome
3.3. Shiftwork and Circadian Syndrome
3.4. Subgroup Analyses
4. Discussion
4.1. Comparison with Other Studies
4.2. Potential Mechanisms
4.3. Implications of Meal Timing and Skipping Meals
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allada, R.; Bass, J. Circadian Mechanisms in Medicine. N. Engl. J. Med. 2021, 384, 550–561. [Google Scholar] [CrossRef] [PubMed]
- The Nobel Prize in Physiology or Medicine 2017. Available online: https://www.nobelprize.org/prizes/medicine/2017/advanced-information/ (accessed on 15 March 2023).
- Berson, D.M.; Dunn, F.A.; Takao, M. Phototransduction by Retinal Ganglion Cells That Set the Circadian Clock. Science 2002, 295, 1070–1073. [Google Scholar] [CrossRef] [PubMed]
- Potter, G.D.M.; Skene, D.J.; Arendt, J.; Cade, J.E.; Grant, P.J.; Hardie, L.J. Circadian Rhythm and Sleep Disruption: Causes, Metabolic Consequences, and Countermeasures. Endocr. Rev. 2016, 37, 584–608. [Google Scholar] [CrossRef] [PubMed]
- Dibner, C.; Schibler, U.; Albrecht, U. The Mammalian Circadian Timing System: Organization and Coordination of Central and Peripheral Clocks. Annu. Rev. Physiol. 2010, 72, 517–549. [Google Scholar] [CrossRef] [PubMed]
- Pickel, L.; Sung, H.-K. Feeding Rhythms and the Circadian Regulation of Metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Panda, S. Circadian Physiology of Metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Zimmet, P.; Alberti, K.G.M.M.; Stern, N.; Bilu, C.; El-Osta, A.; Einat, H.; Kronfeld-Schor, N. The Circadian Syndrome: Is the Metabolic Syndrome and Much More! J. Intern. Med. 2019, 286, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Crnko, S.; Du Pré, B.C.; Sluijter, J.P.G.; Van Laake, L.W. Circadian Rhythms and the Molecular Clock in Cardiovascular Biology and Disease. Nat. Rev. Cardiol. 2019, 16, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Reinke, H.; Asher, G. Circadian Clock Control of Liver Metabolic Functions. Gastroenterology 2016, 150, 574–580. [Google Scholar] [CrossRef]
- Mason, I.C.; Qian, J.; Adler, G.K.; Scheer, F.A.J.L. Impact of Circadian Disruption on Glucose Metabolism: Implications for Type 2 Diabetes. Diabetologia 2020, 63, 462–472. [Google Scholar] [CrossRef]
- Kronfeld-Schor, N.; Einat, H. Circadian Rhythms and Depression: Human Psychopathology and Animal Models. Neuropharmacology 2012, 62, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Logan, R.W.; McClung, C.A. Rhythms of Life: Circadian Disruption and Brain Disorders across the Lifespan. Nat. Rev. Neurosci. 2019, 20, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Crouse, J.J.; Carpenter, J.S.; Song, Y.J.C.; Hockey, S.J.; Naismith, S.L.; Grunstein, R.R.; Scott, E.M.; Merikangas, K.R.; Scott, J.; Hickie, I.B. Circadian Rhythm Sleep-Wake Disturbances and Depression in Young People: Implications for Prevention and Early Intervention. Lancet Psychiatry 2021, 8, 813–823. [Google Scholar] [CrossRef]
- Even, C.; Schröder, C.M.; Friedman, S.; Rouillon, F. Efficacy of Light Therapy in Nonseasonal Depression: A Systematic Review. J. Affect. Disord. 2008, 108, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-H.; Liu, C.-Y.; Chen, S.-J.; Tsai, H.-C. Efficacy of Light Therapy on Nonseasonal Depression among Elderly Adults: A Systematic Review and Meta-Analysis. Neuropsychiatr. Dis. Treat. 2018, 14, 3091–3102. [Google Scholar] [CrossRef] [PubMed]
- Almoosawi, S.; Vingeliene, S.; Gachon, F.; Voortman, T.; Palla, L.; Johnston, J.D.; Van Dam, R.M.; Darimont, C.; Karagounis, L.G. Chronotype: Implications for Epidemiologic Studies on Chrono-Nutrition and Cardiometabolic Health. Adv. Nutr. 2019, 10, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Akbar, Z.; Shi, Z. Dietary Patterns and Circadian Syndrome among Adults Attending NHANES 2005–2016. Nutrients 2023, 15, 3396. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, L.; Zhang, Y.; Liu, J. Relationship between Circadian Syndrome and Stroke: A Cross-Sectional Study of the National Health and Nutrition Examination Survey. Front. Neurol. 2022, 13, 946172. [Google Scholar] [CrossRef]
- Xiao, Y.; Yin, S.; Bai, Y.; Yang, Z.; Wang, J.; Cui, J.; Wang, J. Association between Circadian Syndrome and the Prevalence of Kidney Stones in Overweight Adults: A Cross-Sectional Analysis of NHANES 2007–2018. BMC Public Health 2023, 23, 960. [Google Scholar]
- Xiao, Y.; Yin, S.; Wang, J.; Cui, J.; Yang, Z.; Wang, J.; Bai, Y. A Positive Association between the Prevalence of Circadian Syndrome and Overactive Bladder in United States Adults. Front. Public Health 2023, 11. [Google Scholar] [CrossRef]
- Xiao, Y.; Yin, S.; Cui, J.; Bai, Y.; Yang, Z.; Wang, J.; Wang, J. Association between the Prevalence Rates of Circadian Syndrome and Testosterone Deficiency in US Males: Data from NHANES (2011–2016). Front. Nutr. 2023, 10, 1137668. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Nie, Z.; Ou, Y.; Lin, L.; Qian, Z.; Vaughn, M.G.; McMillin, S.E.; Zhou, Y.; Wu, Y.; Dong, G.; et al. Long-Term Exposure to Ambient Air Pollution, Circadian Syndrome and Cardiovascular Disease: A Nationwide Study in China. Sci. Total Environ. 2023, 868, 161696. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Tuomilehto, J.; Kronfeld-Schor, N.; Alberti, G.K.; Stern, N.; El-Osta, A.; Bilu, C.; Einat, H.; Zimmet, P. The Circadian Syndrome Predicts Cardiovascular Disease Better than Metabolic Syndrome in Chinese Adults. J. Intern. Med. 2021, 289, 851–860. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Zhang, F.; Wu, C.; Zhang, Y.; Huang, X.; Qin, F.; Yuan, J. The Circadian Syndrome Predicts Lower Urinary Tract Symptoms Suggestive of Benign Prostatic Hyperplasia Better Than Metabolic Syndrome in Aging Males: A 4-Year Follow-Up Study. Front. Med. 2021, 8, 715830. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Fernández, O.H.; Liu, J.A.; Nelson, R.J. Circadian Rhythms Disrupted by Light at Night and Mistimed Food Intake Alter Hormonal Rhythms and Metabolism. Int. J. Mol. Sci. 2023, 24, 3392. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.A. Alterations in Sleep during Pregnancy and Postpartum: A Review of 30 Years of Research. Sleep Med. Rev. 1998, 2, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Minguez, J.; Dashti, H.S.; Madrid-Valero, J.J.; Madrid, J.A.; Saxena, R.; Scheer, F.A.J.L.; Ordoñana, J.R.; Garaulet, M. Heritability of the Timing of Food Intake. Clin. Nutr. 2019, 38, 767–773. [Google Scholar] [CrossRef] [PubMed]
- Billingsley, H.E.; Canada, J.M.; Dixon, D.L.; Kirkman, D.L.; Bohmke, N.; Rotelli, B.; Kadariya, D.; Markley, R.; Van Tassell, B.W.; Celi, F.S.; et al. Midpoint of Energy Intake, Non-Fasting Time and Cardiorespiratory Fitness in Heart Failure with Preserved Ejection Fraction and Obesity. Int. J. Cardiol. 2022, 355, 23–27. [Google Scholar] [CrossRef]
- Dashti, H.S.; Gómez-Abellán, P.; Qian, J.; Esteban, A.; Morales, E.; Scheer, F.A.J.L.; Garaulet, M. Late Eating Is Associated with Cardiometabolic Risk Traits, Obesogenic Behaviors, and Impaired Weight Loss. Am. J. Clin. Nutr. 2020, 113, 154–161. [Google Scholar] [CrossRef]
- Kennedy, E.T.; Ohls, J.; Carlson, S.; Fleming, K. The Healthy Eating Index: Design and Applications. J. Am. Diet. Assoc. 1995, 95, 1103–1108. [Google Scholar] [CrossRef]
- Ali, M.; Reutrakul, S.; Petersen, G.; Knutson, K.L. Associations between Timing and Duration of Eating and Glucose Metabolism: A Nationally Representative Study in the U.S. Nutrients 2023, 15, 729. [Google Scholar] [CrossRef]
- Iao, S.I.; Jansen, E.; Shedden, K.; O’Brien, L.M.; Chervin, R.D.; Knutson, K.L.; Dunietz, G.L. Associations between Bedtime Eating or Drinking, Sleep Duration and Wake after Sleep Onset: Findings from the American Time Use Survey. Br. J. Nutr. 2021, 127, 1888–1897. [Google Scholar] [CrossRef]
- Gu, C.; Brereton, N.; Schweitzer, A.; Cotter, M.; Duan, D.; Børsheim, E.; Wolfe, R.R.; Pham, L.V.; Polotsky, V.Y.; Jun, J.C. Metabolic Effects of Late Dinner in Healthy Volunteers-A Randomized Crossover Clinical Trial. J. Clin. Endocrinol. Metab. 2020, 105, 2789–2802. [Google Scholar] [CrossRef]
- Yoshida, J.; Eguchi, E.; Nagaoka, K.; Ito, T.; Ogino, K. Association of Night Eating Habits with Metabolic Syndrome and Its Components: A Longitudinal Study. BMC Public Health 2018, 18, 1366. [Google Scholar] [CrossRef]
- Park, H.; Shin, D.; Lee, K.W. Association of Main Meal Frequency and Skipping with Metabolic Syndrome in Korean Adults: A Cross-Sectional Study. Nutr. J. 2023, 22, 24. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Rong, S.; Liu, B.; Du, Y.; Wu, Y.; Chen, L.; Xiao, Q.; Snetselaar, L.; Wallace, R.; Bao, W. Meal Skipping and Shorter Meal Intervals Are Associated with Increased Risk of All-Cause and Cardiovascular Disease Mortality among US Adults. J. Acad. Nutr. Diet. 2023, 123, 417–426.e3. [Google Scholar] [CrossRef]
- Zeballos, E.; Todd, J.E. The Effects of Skipping a Meal on Daily Energy Intake and Diet Quality. Public Health Nutr. 2020, 23, 3346–3355. [Google Scholar] [CrossRef]
- Jung, J.; Kim, A.-S.; Ko, H.-J.; Choi, H.-I.; Hong, H.-E. Association between Breakfast Skipping and the Metabolic Syndrome: The Korea National Health and Nutrition Examination Survey, 2017. Medicina 2020, 56, 396. [Google Scholar] [CrossRef] [PubMed]
- Mohd Azmi, N.A.S.; Juliana, N.; Mohd Fahmi Teng, N.I.; Azmani, S.; Das, S.; Effendy, N. Consequences of Circadian Disruption in Shift Workers on Chrononutrition and Their Psychosocial Well-Being. Int. J. Environ. Res. Public Health 2020, 17, 2043. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves Insulin Sensitivity, Blood Pressure, and Oxidative Stress Even without Weight Loss in Men with Prediabetes. Cell Metab. 2018, 27, 1212–1221.e3. [Google Scholar] [CrossRef]
- Jones, R.; Pabla, P.; Mallinson, J.; Nixon, A.; Taylor, T.; Bennett, A.; Tsintzas, K. Two Weeks of Early Time-Restricted Feeding (ETRF) Improves Skeletal Muscle Insulin and Anabolic Sensitivity in Healthy Men. Am. J. Clin. Nutr. 2020, 112, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Ravussin, E.; Beyl, R.A.; Poggiogalle, E.; Hsia, D.S.; Peterson, C.M. Early Time-Restricted Feeding Reduces Appetite and Increases Fat Oxidation but Does Not Affect Energy Expenditure in Humans. Obesity 2019, 27, 1244–1254. [Google Scholar] [CrossRef]
- Jamshed, H.; Beyl, R.A.; Della Manna, D.L.; Yang, E.S.; Ravussin, E.; Peterson, C.M. Early Time-Restricted Feeding Improves 24-Hour Glucose Levels and Affects Markers of the Circadian Clock, Aging, and Autophagy in Humans. Nutrients 2019, 11, 1234. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Yi, P.; Liu, F. The Effect of Early Time-Restricted Eating vs. Later Time-Restricted Eating on Weight Loss and Metabolic Health: A Network Meta-Analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2023, 108, 1824–1834. [Google Scholar] [CrossRef]
- Bellet, M.M.; Orozco-Solis, R.; Sahar, S.; Eckel-Mahan, K.; Sassone-Corsi, P. The Time of Metabolism: NAD+, SIRT1, and the Circadian Clock. Cold Spring Harb. Symp. Quant. Biol. 2011, 76, 31–38. [Google Scholar] [CrossRef]
- BaHammam, A.S.; Pirzada, A. Timing Matters: The Interplay between Early Mealtime, Circadian Rhythms, Gene Expression, Circadian Hormones, and Metabolism—A Narrative Review. Clocks Sleep 2023, 5, 34. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Wainstein, J.; Landau, Z.; Raz, I.; Ahren, B.; Chapnik, N.; Ganz, T.; Menaged, M.; Barnea, M.; Bar-Dayan, Y.; et al. Influences of Breakfast on Clock Gene Expression and Postprandial Glycemia in Healthy Individuals and Individuals with Diabetes: A Randomized Clinical Trial. Diabetes Care 2017, 40, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Carlson, O.; Martin, B.; Stote, K.S.; Golden, E.; Maudsley, S.; Najjar, S.S.; Ferrucci, L.; Ingram, D.K.; Longo, D.L.; Rumpler, W.V.; et al. Impact of Reduced Meal Frequency without Caloric Restriction on Glucose Regulation in Healthy, Normal-Weight Middle-Aged Men and Women. Metabolism 2007, 56, 1729–1734. [Google Scholar] [CrossRef]
- Laguzzi, F.; Salleber, S.; Gigante, B.; De Faire, U.; Hellenius, M.L.; Leander, K. Irregular Eating Behavior and Incidence of Cardiovascular Disease: Results from a Swedish 60-Year-Old Cohort of Men and Women. Eur. Heart J. 2019, 40, ehz746.0018. [Google Scholar] [CrossRef]
- Sierra-Johnson, J.; Undén, A.-L.; Linestrand, M.; Rosell, M.; Sjogren, P.; Kolak, M.; De Faire, U.; Fisher, R.M.; Hellénius, M.-L. Eating Meals Irregularly: A Novel Environmental Risk Factor for the Metabolic Syndrome. Obesity 2008, 16, 1302–1307. [Google Scholar] [CrossRef]
- Fanelli, S.; Walls, C.; Taylor, C. Skipping Breakfast Is Associated with Nutrient Gaps and Poorer Diet Quality among Adults in the United States. Proc. Nutr. Soc. 2021, 80, E48. [Google Scholar] [CrossRef]
- Wennberg, M.; Gustafsson, P.E.; Wennberg, P.; Hammarström, A. Irregular Eating of Meals in Adolescence and the Metabolic Syndrome in Adulthood: Results from a 27-Year Prospective Cohort. Public Health Nutr. 2016, 19, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Djuric, Z.; Nikolic, M.; Zekovic, M.; Plegue, M.; Glibetic, M. Association of Meal Timing with Dietary Quality in a Serbian Population Sample. BMC Nutr. 2020, 6, 45. [Google Scholar] [CrossRef]
- Lima, M.T.M.; Nunes, F.S.M.; Custódio, I.D.D.; Carvalho, K.P.; Canto, P.P.L.; Paiva, C.E.; Crispim, C.A.; Paiva Maia, Y.C. Eating Earlier and More Frequently Is Associated with Better Diet Quality in Female Brazilian Breast Cancer Survivors Using Tamoxifen. J. Acad. Nutr. Diet. 2022, 122, 1688–1702.e3. [Google Scholar] [CrossRef] [PubMed]
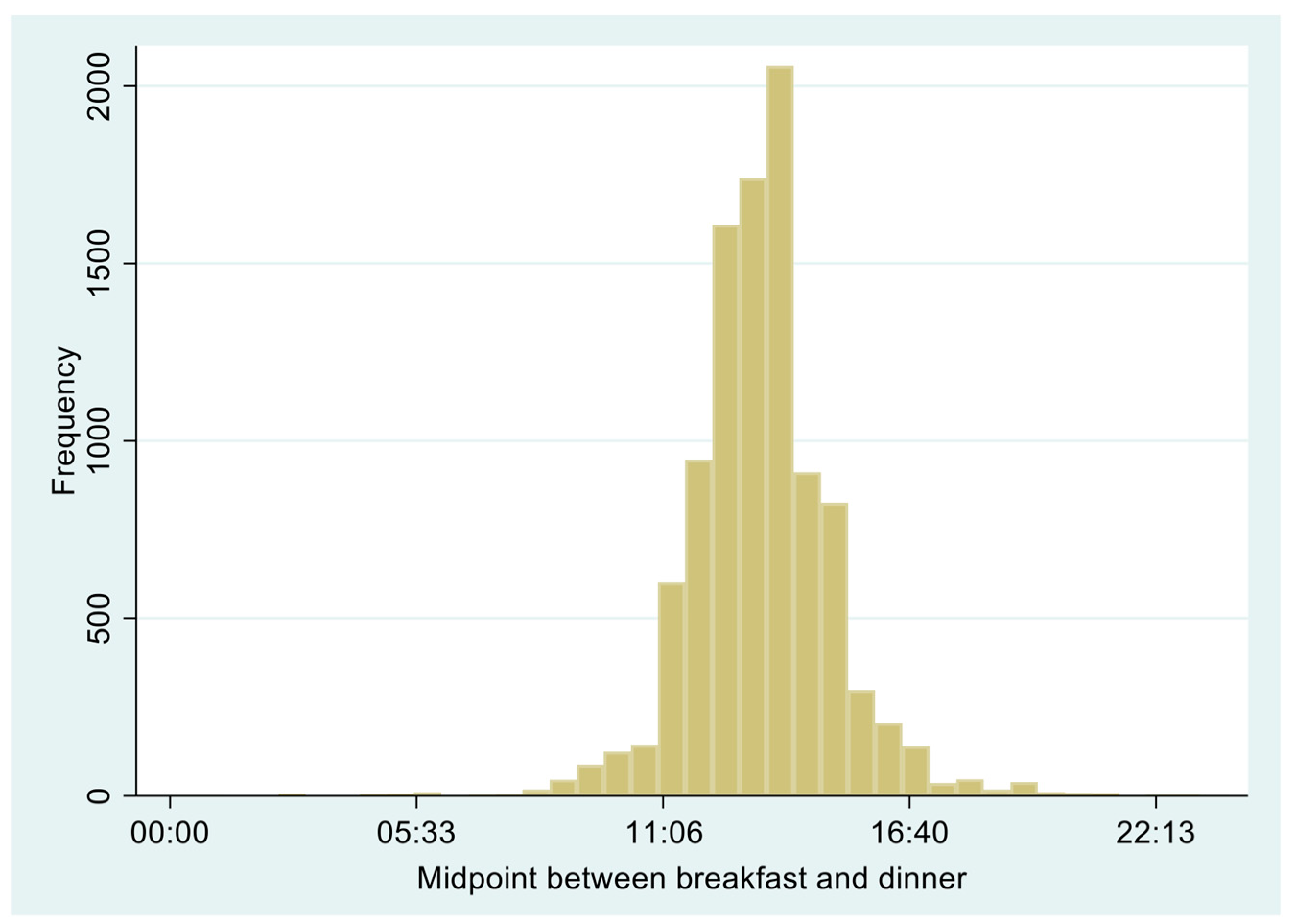
| Total | Favorable (12:30–13:15) | Unfavorable | Meal Skipping | p-Value | |
|---|---|---|---|---|---|
| n = 10,486 | n = 1577 | n = 4537 | n = 4372 | ||
| Energy intake (kcal/day) | 2023.8 (781.8) | 2066.3 (740.9) | 2083.1 (769.2) | 1946.8 (802.5) | <0.001 |
| Protein intake (g/day) | 80.2 (34.0) | 83.5 (33.0) | 83.5 (33.5) | 75.5 (34.3) | <0.001 |
| Fat intake (g/day) | 76.6 (36.3) | 78.6 (35.6) | 79.4 (36.0) | 73.1 (36.4) | <0.001 |
| Carbohydrate intake (g/day) | 246.5 (100.5) | 251.5 (94.0) | 252.9 (99.5) | 238.2 (103.1) | <0.001 |
| Healthy Eating Index | 51.7 (12.0)) | 54.7 (12.2) | 53.2 (12.1) | 49.2 (11.3) | <0.001 |
| Age (years) | 50.3 (17.6) | 54.3 (17.2) | 50.4 (17.2) | 48.7 (17.9) | <0.001 |
| Sex | <0.001 | ||||
| Men | 5147 (49.1%) | 724 (45.9%) | 2128 (46.9%) | 2295 (52.5%) | |
| Women | 5339 (50.9%) | 853 (54.1%) | 2409 (53.1%) | 2077 (47.5%) | |
| Ethnicity | <0.001 | ||||
| Non-Hispanic White | 4973 (47.4%) | 1002 (63.5%) | 2304 (50.8%) | 1667 (38.1%) | |
| Non-Hispanic Black | 2002 (19.1%) | 176 (11.2%) | 745 (16.4%) | 1081 (24.7%) | |
| Mexican American | 1587 (15.1%) | 180 (11.4%) | 678 (14.9%) | 729 (16.7%) | |
| Other | 1924 (18.3%) | 219 (13.9%) | 810 (17.9%) | 895 (20.5%) | |
| Education | <0.001 | ||||
| <11 grade | 2494 (23.8%) | 270 (17.1%) | 898 (19.8%) | 1326 (30.4%) | |
| High school | 2400 (22.9%) | 326 (20.7%) | 964 (21.3%) | 1110 (25.4%) | |
| Some college | 3043 (29.0%) | 467 (29.6%) | 1353 (29.8%) | 1223 (28.0%) | |
| Higher than college | 2541 (24.3%) | 513 (32.6%) | 1318 (29.1%) | 710 (16.3%) | |
| Smoking | <0.001 | ||||
| Never | 5694 (54.3%) | 884 (56.1%) | 2572 (56.7%) | 2238 (51.2%) | |
| Former | 2698 (25.7%) | 450 (28.5%) | 1214 (26.8%) | 1034 (23.7%) | |
| Current smoker | 2090 (19.9%) | 243 (15.4%) | 749 (16.5%) | 1098 (25.1%) | |
| Alcohol intake (past 12 months) | 0.004 | ||||
| No | 1938 (18.5%) | 264 (16.7%) | 797 (17.6%) | 877 (20.1%) | |
| Yes | 7143 (68.1%) | 1078 (68.4%) | 3141 (69.2%) | 2924 (66.9%) | |
| Missing | 1405 (13.4%) | 235 (14.9%) | 599 (13.2%) | 571 (13.1%) | |
| BMI (kg/m2) | 29.1 (6.7) | 28.9 (6.6) | 28.9 (6.7) | 29.3 (6.8) | 0.012 |
| Leisure time physical activity (MET min/week) | <0.001 | ||||
| <600 | 4153 (39.6%) | 620 (39.3%) | 1711 (37.7%) | 1822 (41.7%) | |
| 600–1200 | 1218 (11.6%) | 211 (13.4%) | 557 (12.3%) | 450 (10.3%) | |
| ≥1200 | 5114 (48.8%) | 746 (47.3%) | 2268 (50.0%) | 2100 (48.0%) | |
| Ratio of family income to poverty | <0.001 | ||||
| <1.30 | 2904 (29.9%) | 309 (21.1%) | 1073 (25.4%) | 1522 (38.0%) | |
| 1.3–3.5 | 3717 (38.3%) | 538 (36.7%) | 1585 (37.5%) | 1594 (39.8%) | |
| >3.5 | 3084 (31.8%) | 619 (42.2%) | 1571 (37.1%) | 894 (22.3%) | |
| Hypertension | 3871 (37.0%) | 653 (41.4%) | 1657 (36.6%) | 1561 (35.8%) | <0.001 |
| Central obesity | 6056 (57.8%) | 937 (59.4%) | 2599 (57.3%) | 2520 (57.6%) | 0.33 |
| Elevated glucose | 5567 (53.1%) | 824 (52.3%) | 2377 (52.4%) | 2366 (54.1%) | 0.20 |
| Elevated triglycerides | 4495 (42.9%) | 676 (42.9%) | 1905 (42.0%) | 1914 (43.8%) | 0.23 |
| Reduced HDL-C | 4724 (45.1%) | 702 (44.5%) | 2024 (44.6%) | 1998 (45.7%) | 0.53 |
| Elevated blood pressure | 5137 (49.0%) | 831 (52.7%) | 2196 (48.4%) | 2110 (48.3%) | 0.006 |
| Depression symptoms | 2421 (23.1%) | 288 (18.3%) | 1006 (22.2%) | 1127 (25.8%) | <0.001 |
| Short sleep | 3657 (34.9%) | 443 (28.1%) | 1531 (33.7%) | 1683 (38.5%) | <0.001 |
| Metabolic Syndrome | 5124 (48.9%) | 796 (50.5%) | 2190 (48.3%) | 2138 (48.9%) | 0.32 |
| Circadian Syndrome | 4331 (41.3%) | 632 (40.1%) | 1828 (40.3%) | 1871 (42.8%) | 0.032 |
| Unadjusted | Model 1 | Model 2 | Model 3 | |||||
|---|---|---|---|---|---|---|---|---|
| Coef. (95% CI) | p-Value | Coef. (95% CI) | p-Value | Coef. (95% CI) | p-Value | Coef. (95% CI) | p-Value | |
| Favorable mealtime (12:30–13:15) | ||||||||
| Favorable | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| Unfavorable | 1.08 (0.94–1.25) | 0.270 | 1.28 (1.11–1.48) | 0.001 | 1.26 (1.08–1.46) | 0.003 | 1.24 (1.07–1.44) | 0.005 |
| Meal skipping | 1.25 (1.06–1.48) | 0.010 | 1.70 (1.43–2.02) | <0.001 | 1.47 (1.22–1.76) | <0.001 | 1.39 (1.16–1.67) | <0.001 |
| Favorable Mealtime a | Unfavorable Mealtime | Meal Skipping b | |||
|---|---|---|---|---|---|
| OR (95%CI) | p-Value | OR (95%CI) | p-Value | ||
| Central obesity | 1 | 1.08 (0.93–1.27) | 0.313 | 1.23 (1.03–1.47) | 0.026 |
| Elevated glucose | 1 | 1.20 (1.03–1.40) | 0.019 | 1.29 (1.10–1.50) | 0.002 |
| Elevated triglycerides | 1 | 1.08 (0.91–1.29) | 0.385 | 1.19 (1.00–1.43) | 0.051 |
| Low HDL-C | 1 | 1.13 (0.98–1.30) | 0.083 | 1.22 (1.04–1.44) | 0.015 |
| Elevated blood pressure | 1 | 1.04 (0.85–1.26) | 0.71 | 1.01 (0.83–1.22) | 0.937 |
| Depressive symptom | 1 | 1.38 (1.14–1.68) | 0.001 | 1.57 (1.31–1.89) | <0.001 |
| Short sleep | 1 | 1.20 (1.02–1.42) | 0.031 | 1.37 (1.17–1.61) | <0.001 |
| Shiftwork | |||
|---|---|---|---|
| No | Yes | p-Value | |
| Unadjusted | 1.00 | 1.23 (0.89–1.70) | 0.198 |
| Model 1 | 1.00 | 1.53 (1.12–2.10) | 0.009 |
| Model 2 | 1.00 | 1.40 (1.02–1.93) | 0.038 |
| Model 3 | 1.00 | 1.37 (1.01–1.87) | 0.044 |
| Favorable Mealtime a (12:30−13:15) | |||||
|---|---|---|---|---|---|
| Favorable | Unfavorable | Meal Skipping b | p for Trend c | p for Interaction | |
| Ethnicity | 0.095 | ||||
| Non-Hispanic White | 1.00 | 1.33 (1.12−1.58) | 1.61 (1.27−2.03) | <0.001 | |
| Non-Hispanic Black | 1.00 | 0.80 (0.49−1.30) | 0.84 (0.54−1.31) | 0.665 | |
| Mexican American | 1.00 | 1.05 (0.64−1.73) | 1.07 (0.71−1.59) | 0.748 | |
| Others | 1.00 | 0.99 (0.60−1.65) | 1.18 (0.71−1.97) | 0.353 | |
| Sex | 0.192 | ||||
| Men | 1.00 | 1.09 (0.85−1.40) | 1.41 (1.07−1.84) | 0.005 | |
| Women | 1.00 | 1.43 (1.17−1.74) | 1.51 (1.19−1.92) | 0.002 | |
| Age | 0.549 | ||||
| 20−39 | 1.00 | 1.08 (0.73−1.59) | 1.49 (1.01−2.20) | 0.010 | |
| 40−59 | 1.00 | 1.41 (1.08−1.85) | 1.68 (1.24−2.27) | 0.001 | |
| 60+ | 1.00 | 1.14 (0.90−1.43) | 1.23 (0.93−1.63) | 0.150 | |
| Ratio of family income to poverty | 0.933 | ||||
| <1.30 | 1.00 | 1.47 (0.97−2.25) | 1.58 (1.11−2.27) | 0.011 | |
| 1.3−3.5 | 1.00 | 1.20 (0.90−1.62) | 1.36 (0.98−1.87) | 0.062 | |
| >3.5 | 1.00 | 1.30 (1.00−1.70) | 1.57 (1.13−2.20) | 0.008 | |
| Education | 0.259 | ||||
| <11 grade | 1.00 | 1.60 (1.11−2.32) | 1.62 (1.13−2.33) | 0.033 | |
| High school | 1.00 | 1.36 (0.96−1.92) | 1.68 (1.17−2.42) | 0.005 | |
| Some college | 1.00 | 1.30 (0.99−1.72) | 1.31 (0.94−1.84) | 0.171 | |
| >college | 1.00 | 1.09 (0.83−1.44) | 1.50 (1.08−2.08) | 0.017 | |
| Leisure time physical activity (MET min/week) | 0.126 | ||||
| <600 | 1.00 | 1.24 (0.93−1.66) | 1.41 (1.02−1.93) | 0.031 | |
| 600−1200 | 1.00 | 1.78 (1.03−3.07) | 1.49 (0.85−2.62) | 0.227 | |
| ≥1200 | 1.00 | 1.18 (0.93−1.50) | 1.50 (1.17−1.92) | <0.001 | |
| Smoking | 0.659 | ||||
| Never | 1.00 | 1.27 (1.01−1.62) | 1.55 (1.17−2.04) | 0.002 | |
| Former | 1.00 | 1.16 (0.86−1.57) | 1.39 (1.01−1.90) | 0.036 | |
| Current smoker | 1.00 | 1.40 (0.95−2.07) | 1.45 (0.97−2.16) | 0.124 | |
| Healthy Eating Index | 0.374 | ||||
| Q1 | 1.00 | 1.54 (1.08−2.20) | 1.68 (1.15−2.47) | 0.022 | |
| Q2 | 1.00 | 1.08 (0.77−1.53) | 1.27 (0.87−1.87) | 0.182 | |
| Q3 | 1.00 | 1.11 (0.80−1.53) | 1.22 (0.87−1.69) | 0.229 | |
| Q4 | 1.00 | 1.37 (1.00−1.89) | 1.67 (1.16−2.41) | 0.007 | |
| Shiftwork | ||||
|---|---|---|---|---|
| No | Yes | p for Trend | p for Interaction | |
| Ethnicity | 0.510 | |||
| Non-Hispanic White | 1.00 | 1.38 (0.89–2.14) | 0.141 | |
| Non-Hispanic Black | 1.00 | 1.20 (0.69–2.09) | 0.509 | |
| Mexican American | 1.00 | 1.05 (0.50–2.18) | 0.899 | |
| Others | 1.00 | 1.95 (1.01–3.76) | 0.047 | |
| Sex | 0.065 | |||
| Men | 1.00 | 1.86 (1.22–2.84) | 0.005 | |
| Women | 1.00 | 0.87 (0.49–1.55) | 0.636 | |
| Age group | 0.912 | |||
| 20–39 | 1.00 | 1.47 (0.92–2.35) | 0.109 | |
| 40–59 | 1.00 | 1.41 (0.89–2.22) | 0.139 | |
| 60+ | 1.00 | 1.35 (0.55–3.34) | 0.507 | |
| Ratio of family income to poverty | 0.686 | |||
| <1.30 | 1.00 | 1.51 (0.72–3.18) | 0.269 | |
| 1.3–3.5 | 1.00 | 1.30 (0.84–2.01) | 0.228 | |
| >3.5 | 1.00 | 1.65 (1.06–2.57) | 0.026 | |
| Education | 0.814 | |||
| <11 grade | 1.00 | 1.43 (0.65–3.14) | 0.370 | |
| High school | 1.00 | 1.15 (0.58–2.26) | 0.688 | |
| Some college | 1.00 | 1.35 (0.89–2.05) | 0.153 | |
| Higher than college | 1.00 | 1.78 (0.97–3.27) | 0.063 | |
| Leisure time physical activity (MET min/week) | 0.300 | |||
| <600 | 1.00 | 1.12 (0.61–2.06) | 0.702 | |
| 600–1200 | 1.00 | 1.69 (0.81–3.55) | 0.161 | |
| ≥1200 | 1.00 | 1.50 (1.07–2.12) | 0.021 | |
| Smoking | 0.485 | |||
| Never | 1.00 | 1.42 (0.97–2.08) | 0.073 | |
| Former | 1.00 | 1.05 (0.63–1.75) | 0.846 | |
| Current smoker | 1.00 | 1.70 (0.94–3.09) | 0.081 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbar, Z.; Shi, Z. Unfavorable Mealtime, Meal Skipping, and Shiftwork Are Associated with Circadian Syndrome in Adults Participating in NHANES 2005–2016. Nutrients 2024, 16, 1581. https://doi.org/10.3390/nu16111581
Akbar Z, Shi Z. Unfavorable Mealtime, Meal Skipping, and Shiftwork Are Associated with Circadian Syndrome in Adults Participating in NHANES 2005–2016. Nutrients. 2024; 16(11):1581. https://doi.org/10.3390/nu16111581
Chicago/Turabian StyleAkbar, Zoha, and Zumin Shi. 2024. "Unfavorable Mealtime, Meal Skipping, and Shiftwork Are Associated with Circadian Syndrome in Adults Participating in NHANES 2005–2016" Nutrients 16, no. 11: 1581. https://doi.org/10.3390/nu16111581
APA StyleAkbar, Z., & Shi, Z. (2024). Unfavorable Mealtime, Meal Skipping, and Shiftwork Are Associated with Circadian Syndrome in Adults Participating in NHANES 2005–2016. Nutrients, 16(11), 1581. https://doi.org/10.3390/nu16111581







