Assessment of the Diuretic Properties of Rice Bean Accessions Using a Mouse Model and Identification of Active Polyphenolic Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Gastric Infusions
2.3. Gavage and Data Collection
2.4. Analysis of Polyphenols in Legume Seeds
2.5. Data Analysis
3. Results
3.1. Effect of Bean Extracts on Mouse Urine Output
3.2. Effect of Bean Extracts on Mouse Weight
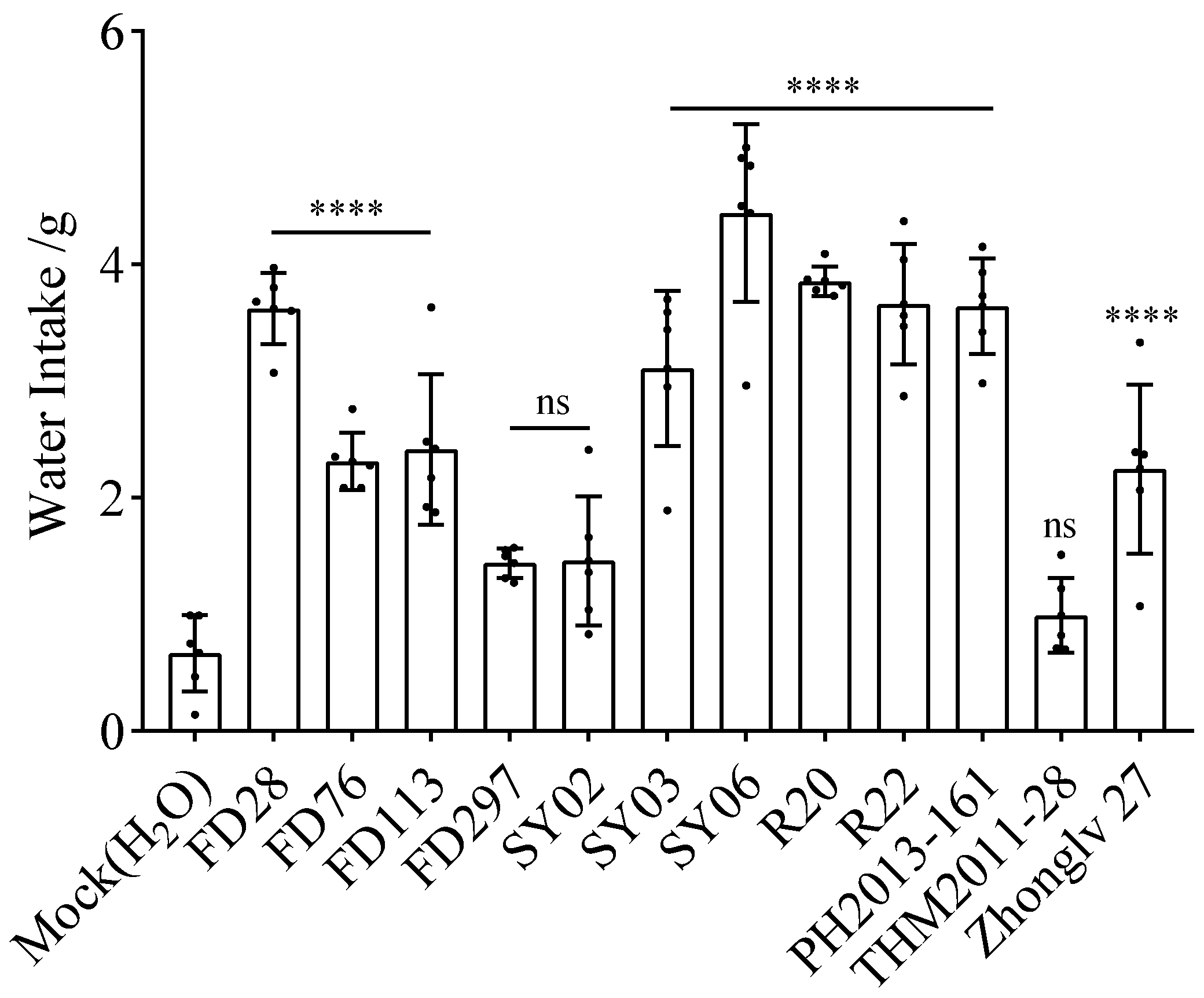
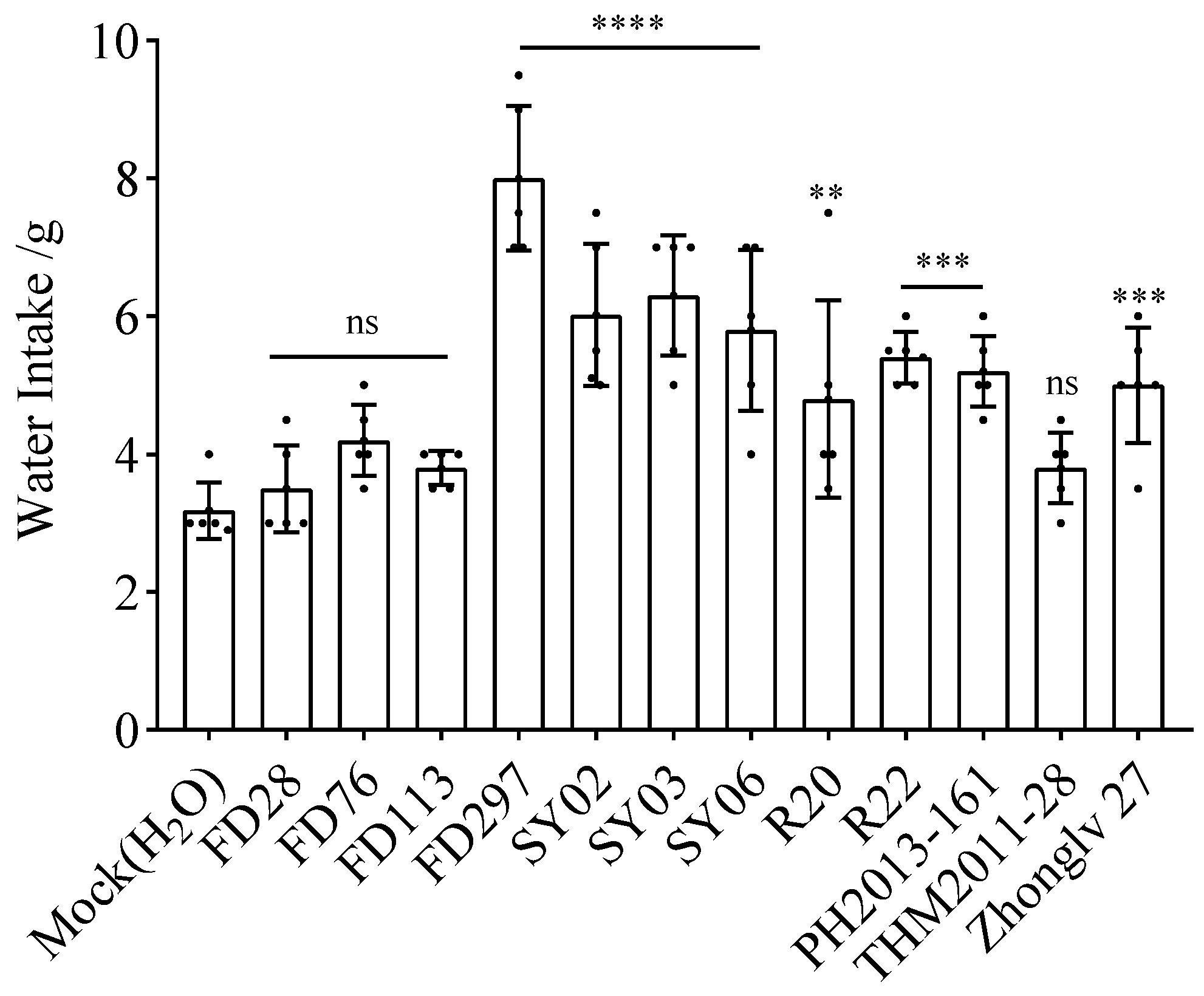
3.3. Water Intake after Gavage with Bean Extracts
3.4. Correlation between Seed Traits and Rice Bean Diuretic Effects
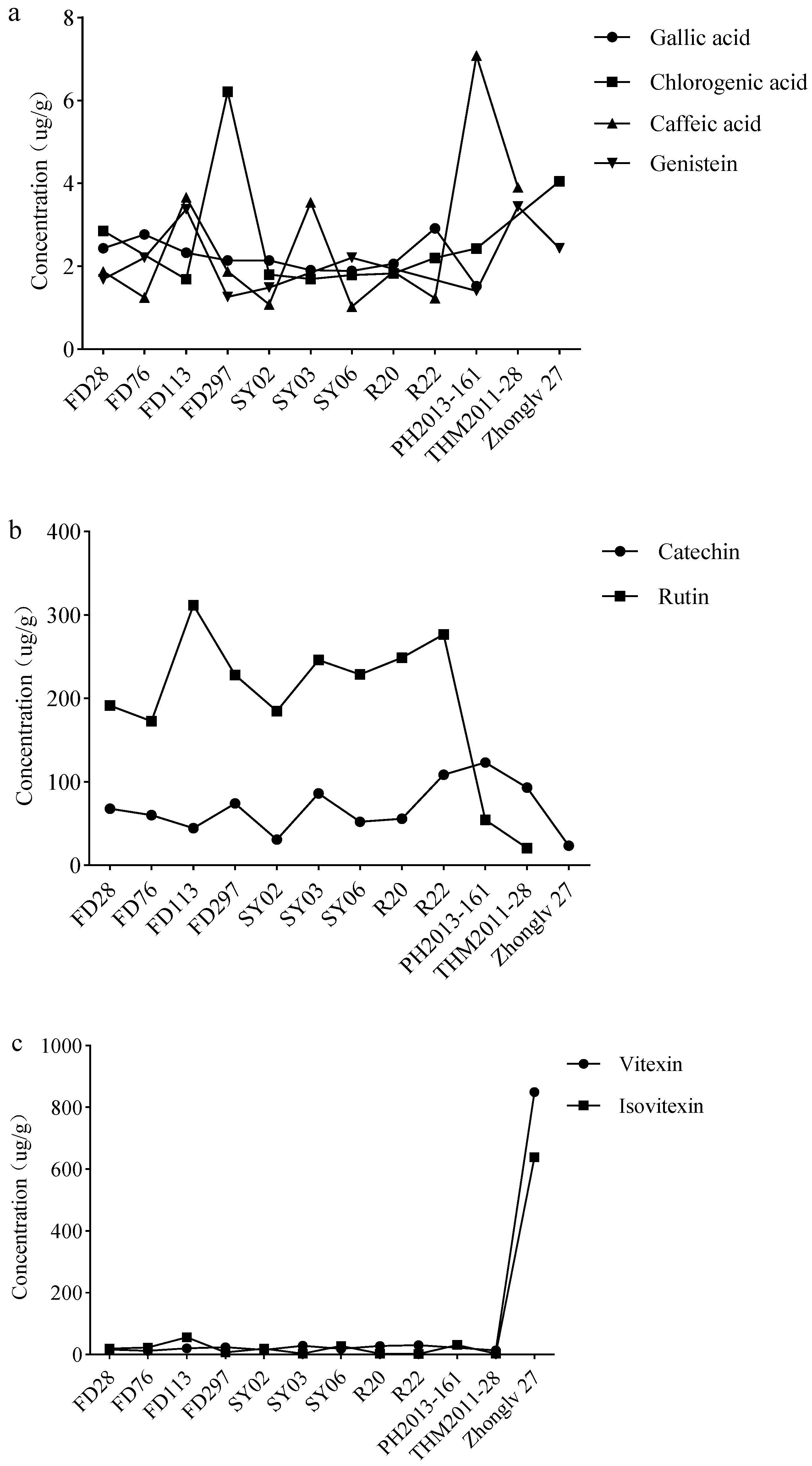
3.5. Polyphenol Contents of Dry Seeds
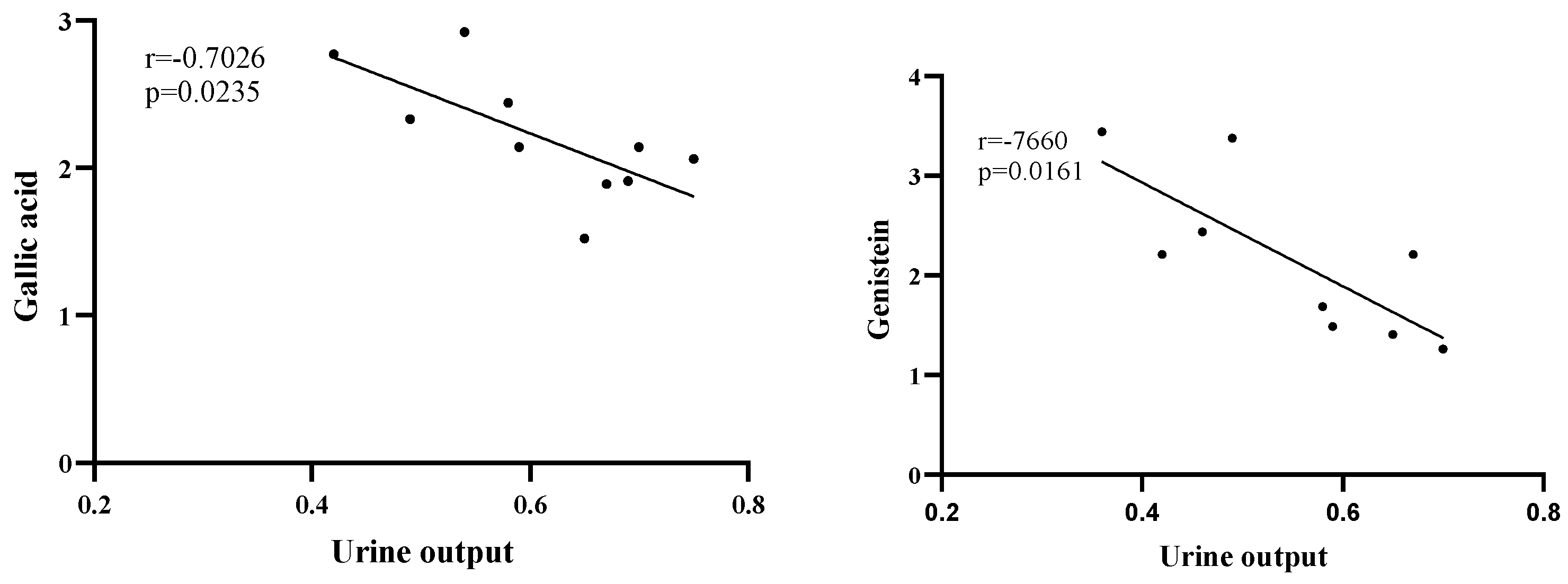
3.6. Correlation between Polyphenol Concentrations and the Diuresis-Related Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guan, J.; Zhang, J.; Gong, D.; Zhang, Z.; Yu, Y.; Luo, G.; Somta, P.; Hu, Z.; Wang, S.; Yuan, X.; et al. Genomic Analyses of Rice Bean Landraces Reveal Adaptation and Yield Related Loci to Accelerate Breeding. Nat. Commun. 2022, 13, 5707. [Google Scholar] [CrossRef]
- Doi, K.; Kaga, A.; Tomooka, N.; Vaughan, D.A. Molecular Phylogeny of Genus Vigna Subgenus Ceratotropis Based on rDNA ITS and atpB-rbcL Intergenic Spacer of cpDNA Sequences. Genetica 2002, 114, 129–145. [Google Scholar] [CrossRef]
- Seehalak, W.; Tomooka, N.; Waranyuwat, A.; Thipyapong, P.; Laosuwan, P.; Kaga, A.; Vaughan, D.A. Genetic Diversity of the Vigna Germplasm from Thailand and Neighboring Regions Revealed by AFLP Analysis. Genet. Resour. Crop Evol. 2006, 53, 1043–1059. [Google Scholar] [CrossRef]
- Pattanayak, A.; Roy, S.; Sood, S.; Iangrai, B.; Banerjee, A.; Gupta, S.; Joshi, D.C. Rice Bean: A Lesser Known Pulse with Well-Recognized Potential. Planta 2019, 250, 873–890. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kaur, J.; Anjali; Sharma, P. Ricebean. In The Beans and the Peas; Elsevier: Amsterdam, The Netherlands, 2021; pp. 55–66. ISBN 978-0-12-821450-3. [Google Scholar]
- Asha, R.; Koundinya, A.; Das, A.; Chattopadhyay, S. A Review on an Underutilised Multipurpose Legume: Rice Bean. Acta Hortic. 2019, 1241, 57–64. [Google Scholar] [CrossRef]
- Joshi, K.D.; Bhandari, B.; Gautam, R.; Bajracharya, J.; Hollington, P. Ricebean: A Multipurpose Underutilised Legume. In New Crops and Uses: Their Role in a Rapidly Changing World; CUC: London, UK, 2008; pp. 234–248. [Google Scholar]
- Bhanu, A.N.; Singh, M.N.; Srivastava, K. Efficient Hybridization Procedure for Better Pod Setting in Inter-Specific Crosses Involving Vigna Species. Adv. Plants Agric. Res. 2018, 8, 101–105. [Google Scholar] [CrossRef]
- Kashiwaba, K.; Tomooka, N.; Kaga, A.; Han, O.K.; Vaughan, D.A. Characterization of Resistance to Three Bruchid Species (Callosobruchus Spp., Coleoptera, Bruchidae) in Cultivated Rice Bean (Vigna umbellata). J. Econ. Entomol. 2003, 96, 207–213. [Google Scholar] [CrossRef]
- Kaur, H.; Gill, R.S.; Kaur, R. Correlation between Biophysical Seed Characteristics of Rice Bean, Vigna Umbellata (Fabaceae: Faboideae: Phaseoleae) and the Development of Callosobruchus Maculatus (Coleoptera: Chrysomelidae: Bruchinae). J. Stored Prod. Res. 2019, 83, 9–13. [Google Scholar] [CrossRef]
- Pavithravani, B.V.; Gowda, R.; Bhanuprakash, K.; Ramesh, S.; Rao, M.A.; Subramanya, S.; Gireesh, C. Biochemical Components: An Index of Bruchid Resistance in Rice Bean [Vigna umbellata (Thunb.) Ohwi and Ohashi]. Legume Res. 2013, 36, 582–588. [Google Scholar]
- Sudha, M.; Anusuya, P.; Mahadev, N.G.; Karthikeyan, A.; Nagarajan, P.; Raveendran, M.; Senthil, N.; Pandiyan, M.; Angappan, K.; Balasubramanian, P. Molecular Studies on Mungbean (Vigna radiata (L.) Wilczek) and Ricebean (Vigna umbellata (Thunb.)) Interspecific Hybridisation for Mungbean Yellow Mosaic Virus Resistance and Development of Species-Specific SCAR Marker for Ricebean. Arch. Phytopathol. Plant Prot. 2013, 46, 503–517. [Google Scholar] [CrossRef]
- Atta, K.; Pal, A.K.; Jana, K. Effects of Salinity, Drought and Heavy Metal Stress during Seed Germination Stage in Ricebean [Vigna umbellata (Thunb.) Ohwi and Ohashi]. Plant Physiol. Rep. 2021, 26, 109–115. [Google Scholar] [CrossRef]
- Wanek, W.; Richter, A. Biosynthesis and Accumulation of D-Ononitol in Vigna umbellata in Response to Drought Stress. Physiol. Plant. 2010, 101, 416–424. [Google Scholar] [CrossRef]
- Dhillon, P.K.; Tanwar, B. Rice Bean: A Healthy and Cost-Effective Alternative for Crop and Food Diversity. Food Secur. 2018, 10, 525–535. [Google Scholar] [CrossRef]
- Wang, L.X.; Cheng, X.Z.; Wang, S.H. Genetic Diversity Analysis and a Core Collection Construction of Rice Bean (Vigna umbellata) in China. J. Plant Genet. Resour. 2014, 15, 242–247. (In Chinese) [Google Scholar]
- Arora, R.K.; Chandel, K.P.S.; Joshi, B.S.; Pant, K.C. Rice Bean: Tribal Pulse of Eastern India. Econ. Bot. 1980, 34, 260–263. [Google Scholar] [CrossRef]
- Mohan, V.; Janardhanan, K. Chemical Composition and Nutritional Evaluation of Raw Seeds of Six Ricebean Varieties. J. Indian Bot. Soc. 1994, 73, 259–263. [Google Scholar]
- Katoch, R. Nutritional Potential of Rice Bean (Vigna umbellata): An Underutilized Legume. J. Food Sci. 2013, 78, C8–C16. [Google Scholar] [CrossRef]
- Bepary, R.H.; Wadikar, D.D.; Neog, S.B.; Patki, E.P. Studies on Physico-Chemical and Cooking Characteristics of Rice Bean Varieties Grown in NE Region of India. J. Food Sci. Technol. 2017, 54, 973–986. [Google Scholar] [CrossRef]
- Rodriguez, M.S.; Mendoza, E.M.T. Nutritional Assessment of Seed Proteins in Rice Bean [Vigna umbellata (Thumb.) Ohwi and Ohashi. Plant Foods Hum. Nutr. 1991, 41, 1–9. [Google Scholar] [CrossRef]
- Cui, G.; Chang, Y. Red bean and mung bean with homologous medicinal and food sources. Cap. Food Med. 2014, 21, 49. (In Chinese) [Google Scholar]
- Chau, C.F.; Cheung, P.C. Effects of the Physico-Chemical Properties of Three Legume Fibers on Cholesterol Absorption in Hamsters. Nutr. Res. 1999, 19, 257–265. [Google Scholar] [CrossRef]
- Yao, Y.; Cheng, X.Z.; Wang, L.X.; Wang, S.H.; Ren, G.X. Biological Potential of Sixteen Legumes in China. Int. J. Mol. Sci. 2011, 12, 7048–7058. [Google Scholar] [CrossRef]
- Hong, J.; Bai, B.; Bi, Y. Study on Antioxidant Activity of Total Polyphenols of Phaseolus Calcaratus Roxb. Guangdong Chem. Ind. 2020, 47, 280–281. (In Chinese) [Google Scholar]
- Yu, Y.; Hu, D.; Liu, J.; Wu, C.; Sun, Y.; Lang, M.; Han, X.; Kang, D.; Min, J.Z.; Cui, H.; et al. Constituents of Chimaphila Japonica and Their Diuretic Activity. Mol. Basel Switz. 2024, 29, 1092. [Google Scholar] [CrossRef]
- Ngamlai, E.V.; Pradhan, R.B.; Lalbiaknii, P.C.; Ralte, V.; Lalnunmawia, F.; Vanlalhluna, P.C.; Mehta, S.K. Diuretic Activity Evaluation and Chemical Composition Analysis of Hedyotis Scandens Extract from Mizoram, India, in Rat Models. J. Ethnopharmacol. 2024, 319, 117079. [Google Scholar] [CrossRef]
- Boockvar, K.S.; Song, W.; Lee, S.; Intrator, O. Comparing Outcomes Between Thiazide Diuretics and Other First-Line Antihypertensive Drugs in Long-Term Nursing Home Residents. Clin. Ther. 2020, 42, 583–591. [Google Scholar] [CrossRef]
- Cox, Z.L.; Sarrell, B.A.; Cella, M.K.; Tucker, B.; Arroyo, J.P.; Umanath, K.; Tidwell, W.; Guide, A.; Testani, J.M.; Lewis, J.B.; et al. Multinephron Segment Diuretic Therapy to Overcome Diuretic Resistance in Acute Heart Failure: A Single-Center Experience. J. Card. Fail. 2022, 28, 21–31. [Google Scholar] [CrossRef]
- Gupta, S.; Neyses, L. Diuretic Usage in Heart Failure: A Continuing Conundrum in 2005. Eur. Heart J. 2005, 26, 644–649. [Google Scholar] [CrossRef]
- He, H.; Sui, Y.; Yu, X.; Luo, G.; Xue, J.; Yang, W.; Long, Y. Potential Low Toxic Alternative for Na-Cl Cotransporter Inhibition: A Diuretic Effect and Mechanism Study of Pyrrosia Petiolosa. Ann. Pharm. Françaises 2024, 82, 44–52. [Google Scholar] [CrossRef]
- Titko, T.; Perekhoda, L.; Drapak, I.; Tsapko, Y. Modern Trends in Diuretics Development. Eur. J. Med. Chem. 2020, 208, 112855. [Google Scholar] [CrossRef]
- Gupta, S.; Kozak, M.; Sahay, G.; Durrai, A.A.; Mitra, J.; Verma, M.R.; Pattanayak, A.; Thongbam, P.D.; Das, A. Genetic Parameters of Selection and Stability and Identification of Divergent Parents for Hybridization in Rice Bean (Vigna umbellata Thunb. (Ohwi and Ohashi) in India. J. Agric. Sci.-Camb. 2009, 147, 581–588. [Google Scholar] [CrossRef]
- Singh, G.; Singh, M.; Dhiman, K.R. Correlation and Path Analysis in Ricebean under Mid Altitude Conditions. Crop Improv. 1992, 9, 152–154. [Google Scholar]
- Singh, M.R.K.; Chakravarti, D.; Singh, N.B. Genetic Variability, Correlation and Path Analysis in Rice Bean (Vigna umbellata (Thunb.) Ohwi and Ohashi) Cultivars of Manipur. Indian J. Hill Farming 1997, 10, 23–28. [Google Scholar]
- Isemura, T.; Kaga, A.; Tomooka, N.; Shimizu, T.; Vaughan, D.A. The Genetics of Domestication of Rice Bean, Vigna umbellata. Ann. Bot. 2010, 106, 927–944. [Google Scholar] [CrossRef]
- Chen, H.; Chen, X.; Tian, J.; Yang, Y.; Liu, Z.; Hao, X.; Wang, L.; Wang, S.; Liang, J.; Zhang, L. Development of Gene-Based SSR Markers in Rice Bean (Vigna umbellata L.) Based on Transcriptome Data. PLoS ONE 2016, 11, e0151040. [Google Scholar] [CrossRef]
- Muthusamy, S.; Kanagarajan, S.; Ponnusamy, S. Efficiency of RAPD and ISSR Markers System in Accessing Genetic Variation of Rice Bean (Vigna umbellata) Landraces. Electron. J. Biotechnol. 2008, 11, 32–41. [Google Scholar] [CrossRef]
- Wang, L.; Kim, K.D.; Gao, D.; Chen, H.; Wang, S.; Lee, S.H.; Jackson, S.A.; Cheng, X. Analysis of Simple Sequence Repeats in Rice Bean (Vigna umbellata) Using an SSR-Enriched Library. Crop J. 2016, 4, 40–47. [Google Scholar] [CrossRef]
- Kurkin, V.A.; Zaitseva, E.N.; Kurkina, A.V.; Dubishchev, A.V.; Pravdivtseva, O.E. Comparative Study of Diuretic Activity of Hydroalcoholic Extracts from Medicinal Plants Containing Flavonoids. Bull. Exp. Biol. Med. 2015, 159, 368–371. [Google Scholar] [CrossRef]
- Schlickmann, F.; Boeing, T.; Mariano, L.N.B.; da Silva, R.D.C.M.V.D.A.F.; da Silva, L.M.; de Andrade, S.F.; de Souza, P.; Cechinel-Filho, V. Gallic Acid, a Phenolic Compound Isolated from Mimosa Bimucronata (DC.) Kuntze Leaves, Induces Diuresis and Saluresis in Rats. Naunyn. Schmiedebergs Arch. Pharmacol. 2018, 391, 649–655. [Google Scholar] [CrossRef]
- Liu, C.; Chen, Y.; Hu, Y. Study on the Content and Antioxidant Effect of Catechin and Rutin in Different Tea. Chin. J. Public Health Eng. 2022, 21, 453–455+458. (In Chinese) [Google Scholar] [CrossRef]
- Mu, Z.; Tian, X.; Zhou, J.; Shi, Y. The Determination of Rutin Content in Buckwheat by HPLC. J. Shanxi Agric. Sci. 2015, 43, 1408–1410. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, H.; Ma, R.; Liu, Y.; Wei, Y.; Dai, L. Simultaneous determination of 4 components in Fagopyrum dibotrys by HPLC. Cent. South Pharm. 2018, 16, 1612–1615. (In Chinese) [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural Products for Drug Discovery in the 21st Century: Innovations for Novel Drug Discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [PubMed]
- Tong, C.; Liu, X. Pharmacokinetics of vitexin in rats. J. China Pharm. Univ. 2007, 38, 65–68. (In Chinese) [Google Scholar]
- Huang, Y.; Zhang, Z.; Zheng, L.; He, F.; Shi, L.; Lan, Y. UPLC—MS /MS tissue distribution of four bioactive flavonoids in Polygonum orientale L. extract. Chin. J. Pharm. Anal. 2012, 32, 2126–2131. (In Chinese) [Google Scholar] [CrossRef]
- Feng, X.; Chen, Y.; Cai, C.; Jiang, J.; Shi, G. Diuretic effect of different extracted parts of grape on mice. J. Shantou Univ. Med. Coll. 2015, 28, 132–133. (In Chinese) [Google Scholar] [CrossRef]
- Abarikwu, S.O.; Njoku, R.C.; Lawrence, C.J.; Charles, I.A.; Ikewuchi, J.C. Rutin Ameliorates Oxidative Stress and Preserves Hepatic and Renal Functions Following Exposure to Cadmium and Ethanol. Pharm. Biol. 2017, 55, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O.E. Rutin as a Potent Antioxidant: Implications for Neurodegenerative Disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, Y.; Wang, L. Protective effect of rutin on kidney damage induced by high sugar and fat diet in mice. J. Bengbu Med. Coll. 2022, 47, 989–993. (In Chinese) [Google Scholar] [CrossRef]
- Fu, W.; Yang, J.; Hong, Z.; Gui, C.; Song, J.; Zhang, L. A Primary Study on General Pharmacologic Action of Tea Polyphenols. Acta Acad. Med. Wannan 1999, 18, 257–258+264. (In Chinese) [Google Scholar]
- Khan, N.; Mukhtar, H. Tea Polyphenols in Promotion of Human Health. Nutrients 2018, 11, 39. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer Prevention by Tea: Animal Studies, Molecular Mechanisms and Human Relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, C.; Wang, X. Research progress in the biological activity and pharmacology of tea polyphenols. J. Anhui Tcm Coll. 2002, 21, 57–60. (In Chinese) [Google Scholar]
- Tian, L.; Liu, Z.; Song, L.; Yao, Y. Research progress of catechins in treating kidney disease. Guangdong Tea 2010, 32–35. (In Chinese) [Google Scholar]
- Wongmekiat, O.; Peerapanyasut, W.; Kobroob, A. Catechin Supplementation Prevents Kidney Damage in Rats Repeatedly Exposed to Cadmium through Mitochondrial Protection. Naunyn. Schmiedebergs Arch. Pharmacol. 2018, 391, 385–394. [Google Scholar] [CrossRef]
- Cai, Z.-Y.; Li, X.-M.; Liang, J.-P.; Xiang, L.-P.; Wang, K.-R.; Shi, Y.-L.; Yang, R.; Shi, M.; Ye, J.-H.; Lu, J.-L.; et al. Bioavailability of Tea Catechins and Its Improvement. Mol. Basel Switz. 2018, 23, 2346. [Google Scholar] [CrossRef]



| Species | Code | Color | 100-Grain Weight/g |
|---|---|---|---|
| Rice bean | FD28 | Red | 11.92 |
| FD76 | Red | 4.59 | |
| FD113 | Red | 4.17 | |
| FD297 | Black | 5.44 | |
| SY02 | Red | 8.76 | |
| SY03 | Yellow | 5.06 | |
| SY06 | Red | 10.98 | |
| R20 | Yellow | 8.10 | |
| R22 | Dotted | 9.90 | |
| Adzuki bean | PH2013-161 | Red | 13.9 |
| THM2011-28 | Red | 16.2 | |
| Mung bean | Zhonglv 27 | Green | 4.5 |
| Group | 1th h | 2nd h | 3rd h | 4th h | 5th h | 6th h | 7th h | 8th h |
|---|---|---|---|---|---|---|---|---|
| FD28 | 1.54 ± 0.15 **** | 0.78 ± 0.21 *** | 0.35 ± 0.18 | 0.40 ± 0.23 | 0.32 ± 0.07 | 0.31 ± 0.03 | 0.35 ± 0.13 | 0.45 ± 0.21 |
| FD76 | 0.73 ± 0.16 | 0.50 ± 0.09 | 0.29 ± 0.12 | 0.26 ± 0.12 | 0.29 ± 0.15 | 0.27 ± 0.19 | 0.31 ± 0.18 | 0.38 ± 0.15 |
| FD113 | 1.08 ± 0.16 ** | 0.71 ± 0.23 ** | 0.23 ± 0.06 | 0.35 ± 0.16 | 0.26 ± 0.14 | 0.25 ± 0.13 | 0.29 ± 0.19 | 0.61 ± 0.15 * |
| FD297 | 1.56 ± 0.11 **** | 0.81 ± 0.18 *** | 0.25 ± 0.08 | 0.34 ± 0.13 | 0.35 ± 0.21 | 0.80 ± 0.40 **** | 0.62 ± 0.17 ** | 0.81 ± 0.25 *** |
| SY02 | 1.54 ± 0.32 **** | 0.69 ± 0.08 ** | 0.36 ± 0.16 | 0.22 ± 0.17 | 0.37 ± 0.22 | 0.32 ± 0.10 | 0.45 ± 0.10 | 0.62 ± 0.21 * |
| SY03 | 1.76 ± 0.34 **** | 0.90 ± 0.22 **** | 0.29 ± 0.09 | 0.36 ± 0.29 | 0.27 ± 0.06 | 0.27 ± 0.06 | 0.46 ± 0.21 | 0.67 ± 0.24 * |
| SY06 | 2.02 ± 0.58 **** | 0.70 ± 0.16 ** | 0.42 ± 0.23 * | 0.46 ± 0.12 | 0.37 ± 0.16 | 0.44 ± 0.12 | 0.41 ± 0.23 | 0.46 ± 0.27 |
| R20 | 1.94 ± 0.16 **** | 0.86 ± 0.19 **** | 0.59 ± 0.26 *** | 0.40 ± 0.27 | 0.37 ± 0.16 | 0.41 ± 0.13 | 0.55 ± 0.16 * | 0.51 ± 0.11 |
| R22 | 1.50 ± 0.30 **** | 0.44 ± 0.10 | 0.27 ± 0.26 | 0.35 ± 0.12 | 0.32 ± 0.10 | 0.41 ± 0.11 | 0.46 ± 0.34 | 0.59 ± 0.25 |
| PH2013-161 | 1.92 ± 0.27 **** | 0.65 ± 0.18 * | 0.40 ± 0.14 | 0.39 ± 0.09 | 0.28 ± 0.11 | 0.30 ± 0.12 | 0.66 ± 0.12 ** | 0.62 ± 0.24 * |
| THM2011-28 | 1.23 ± 0.27 *** | 0.30 ± 0.14 | 0.14 ± 0.10 | 0.30 ± 0.18 | 0.09 ± 0.06 | 0.14 ± 0.06 | 0.17 ± 0.05 | 0.45 ± 0.28 |
| Zhonglv 27 | 1.51 ± 0.19 **** | 0.52 ± 0.15 | 0.26 ± 0.08 | 0.18 ± 0.08 | 0.10 ± 0.09 | 0.18 ± 0.07 | 0.19 ± 0.11 | 0.56 ± 0.19 |
| Mock (H2O) | 0.39 ± 0.17 | 0.31 ± 0.11 | 0.13 ± 0.08 | 0.19 ± 0.09 | 0.13 ± 0.09 | 0.19 ± 0.07 | 0.22 ± 0.12 | 0.21 ± 0.11 |
| Urine Output | Weight Loss | Water Intake | |
|---|---|---|---|
| Gallic acid | −0.70 * | −0.15 | −0.35 |
| Chlorogenic acid | 0.02 | −0.52 | 0.51 |
| Catechin | 0.08 | 0.22 | 0.06 |
| Caffeic acid | −0.05 | −0.01 | −0.16 |
| Rutin | 0.37 | 0.32 | 0.22 |
| Vitexin | −0.26 | −0.12 | −0.03 |
| Isovitexin | −0.3 | −0.14 | −0.07 |
| Genistein | −0.75 * | −0.23 | −0.62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, D.; Zhang, B.; Yao, Y.; Wang, S.; Xiong, T.; Wang, L. Assessment of the Diuretic Properties of Rice Bean Accessions Using a Mouse Model and Identification of Active Polyphenolic Compounds. Nutrients 2024, 16, 1603. https://doi.org/10.3390/nu16111603
Gong D, Zhang B, Yao Y, Wang S, Xiong T, Wang L. Assessment of the Diuretic Properties of Rice Bean Accessions Using a Mouse Model and Identification of Active Polyphenolic Compounds. Nutrients. 2024; 16(11):1603. https://doi.org/10.3390/nu16111603
Chicago/Turabian StyleGong, Dan, Bin Zhang, Yang Yao, Suhua Wang, Tao Xiong, and Lixia Wang. 2024. "Assessment of the Diuretic Properties of Rice Bean Accessions Using a Mouse Model and Identification of Active Polyphenolic Compounds" Nutrients 16, no. 11: 1603. https://doi.org/10.3390/nu16111603






