A Lombard Variety of Sweet Pepper Regulating Senescence and Proliferation: The Voghera Pepper
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Cultures and Treatments
2.2. Clonal Cell Survival Assay
2.3. Phase Contrast Microscopy
2.4. Flow Cytometry, Cell Cycle and Cell Death Analyses
2.5. Immunofluorescence Reactions
2.6. Fluorescence Microscopy
2.7. Immunofluorescence Analyses
2.8. Transmission Electron Microscopy (TEM)
2.9. Statistical Analysis
3. Results
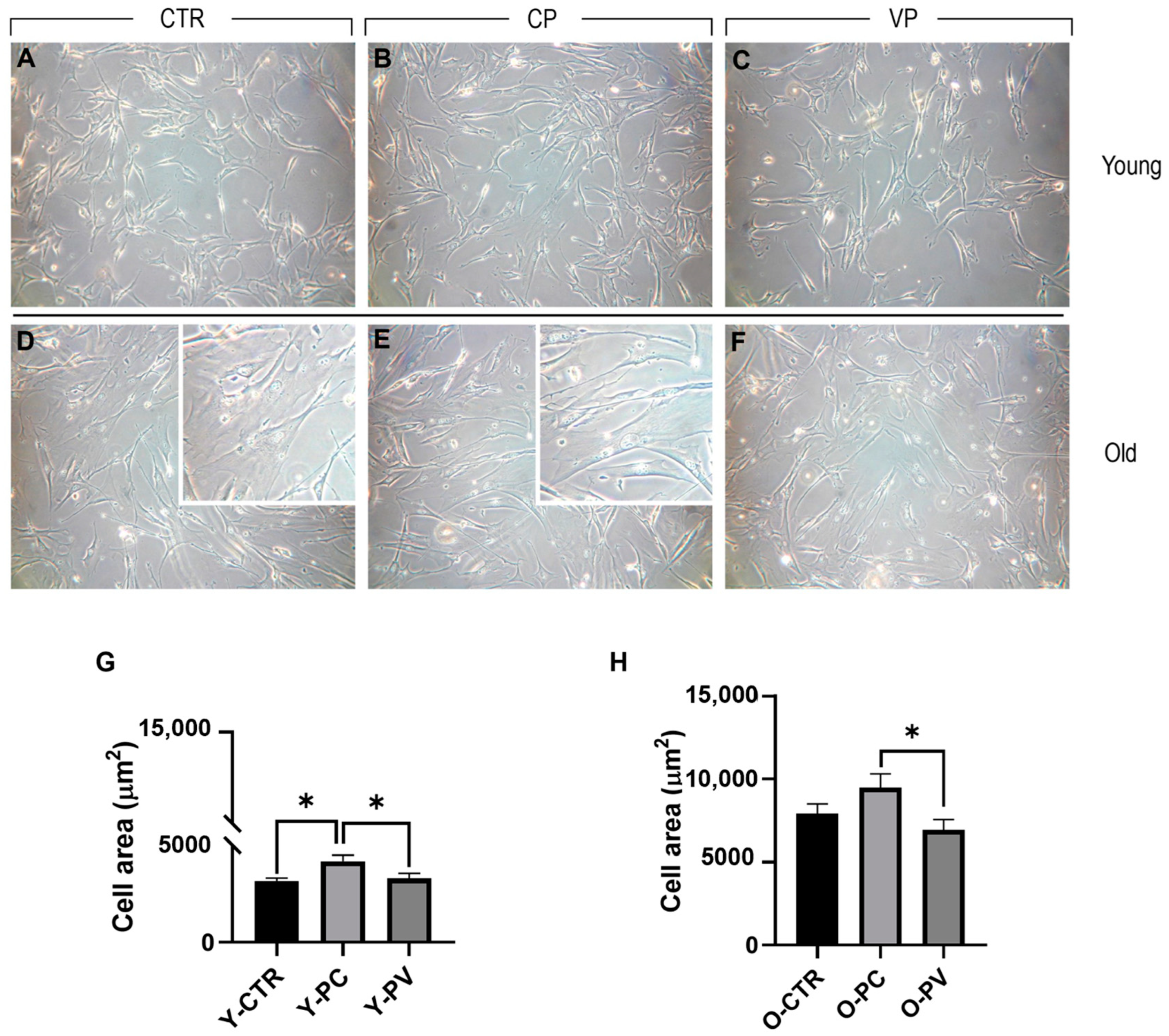
3.1. Effects on Cell Morphology
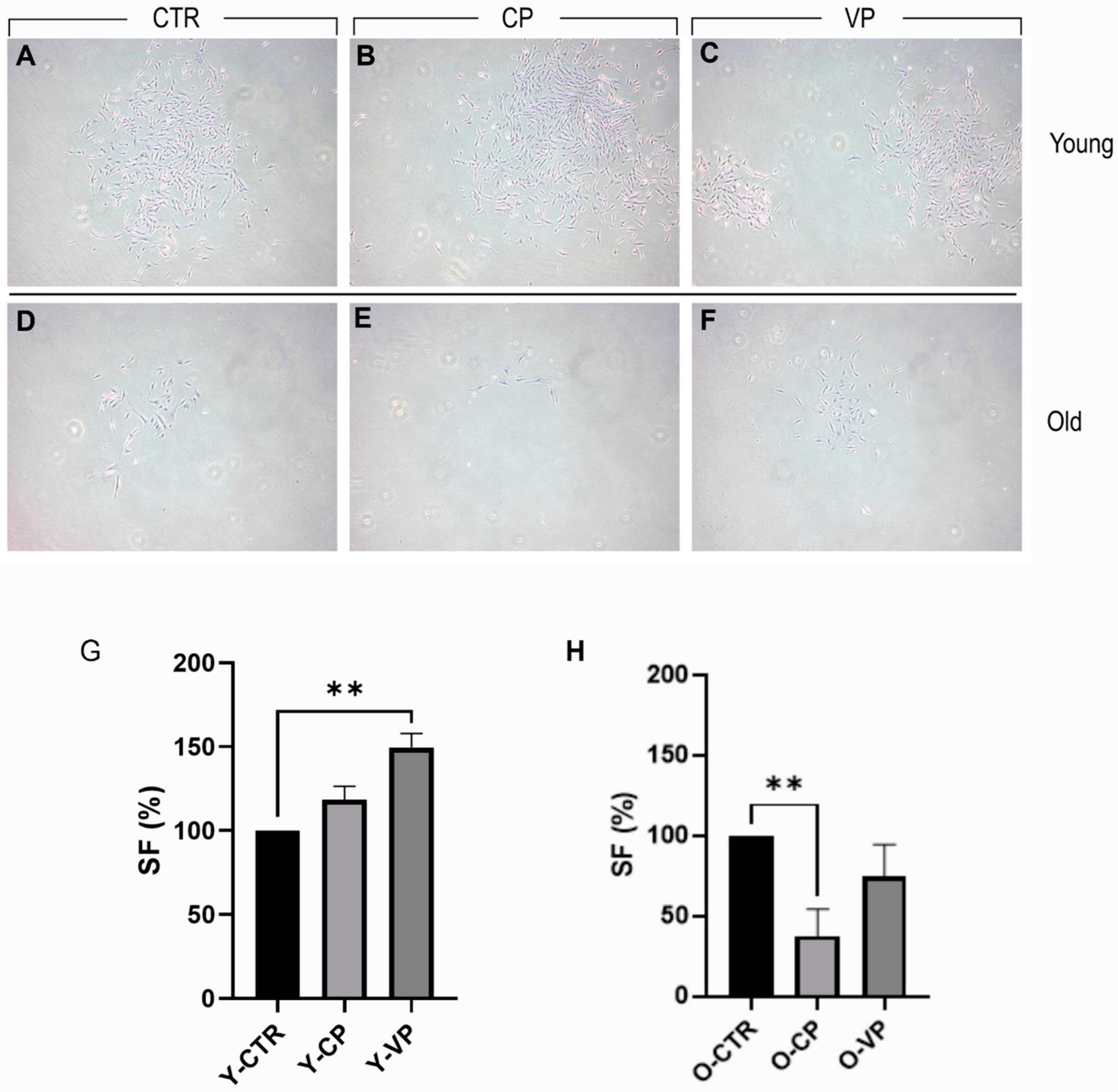
3.2. Pepper Extracts Differently Affect Young and Old NHDF Clonogenic Capability
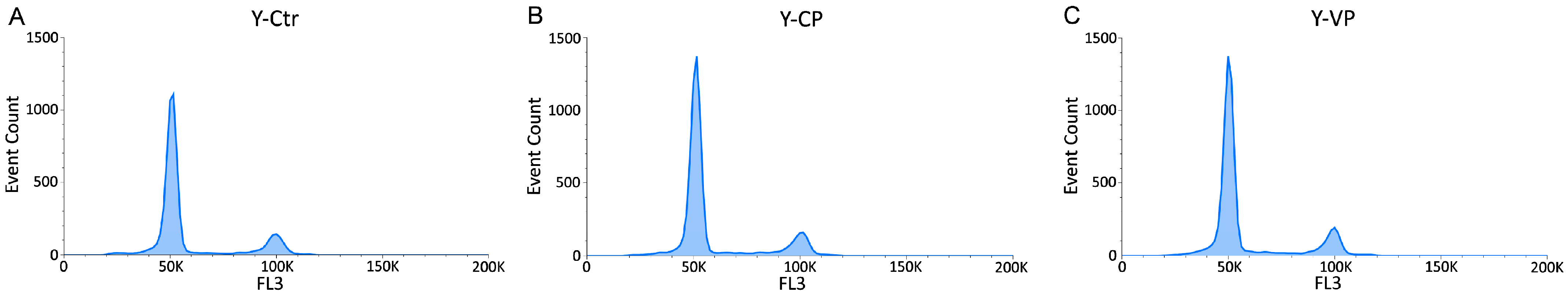
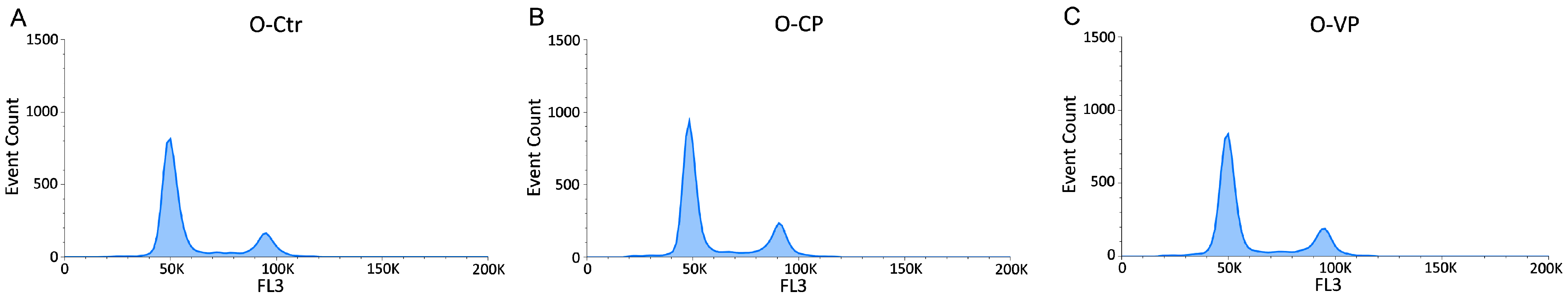
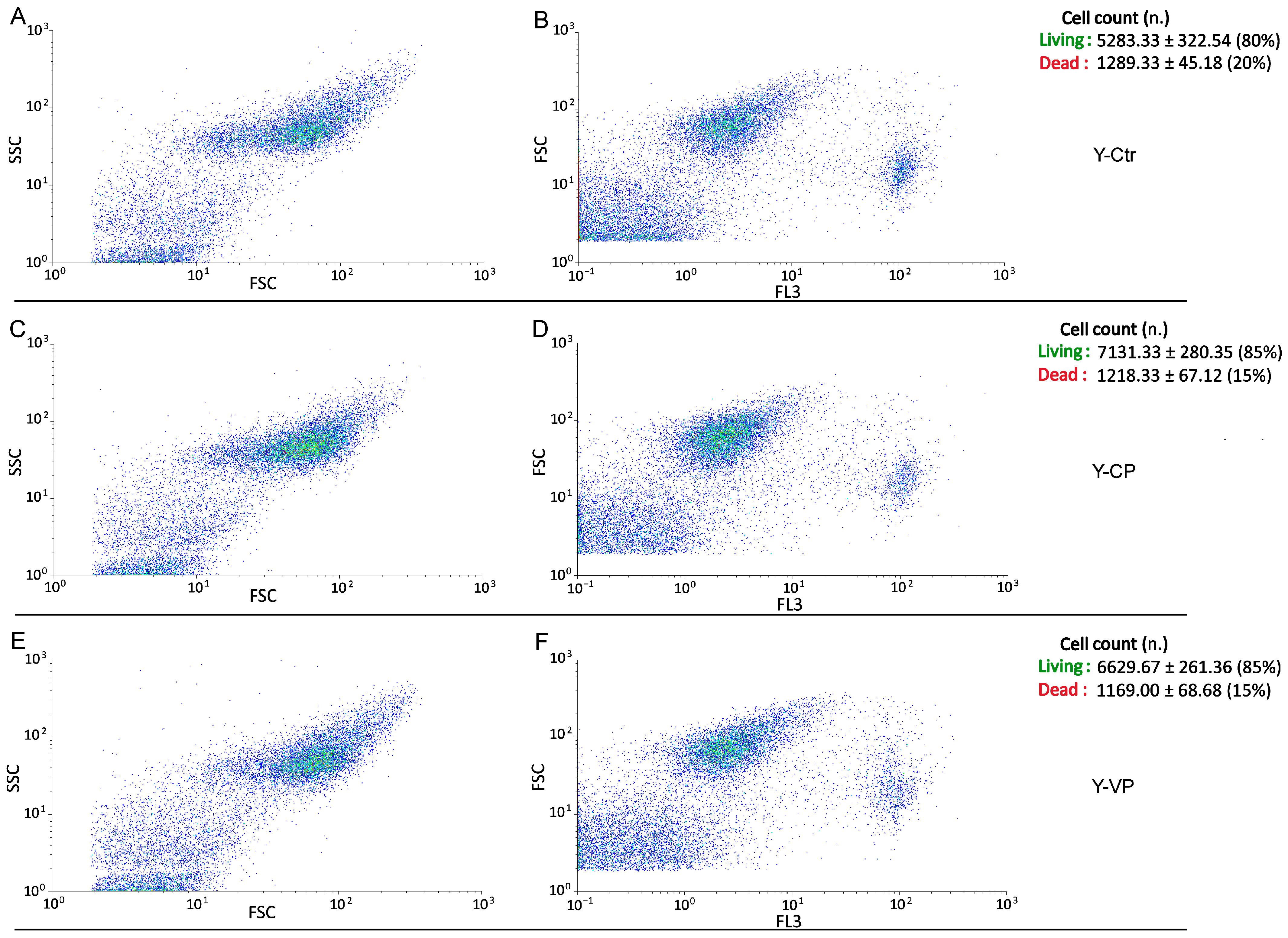
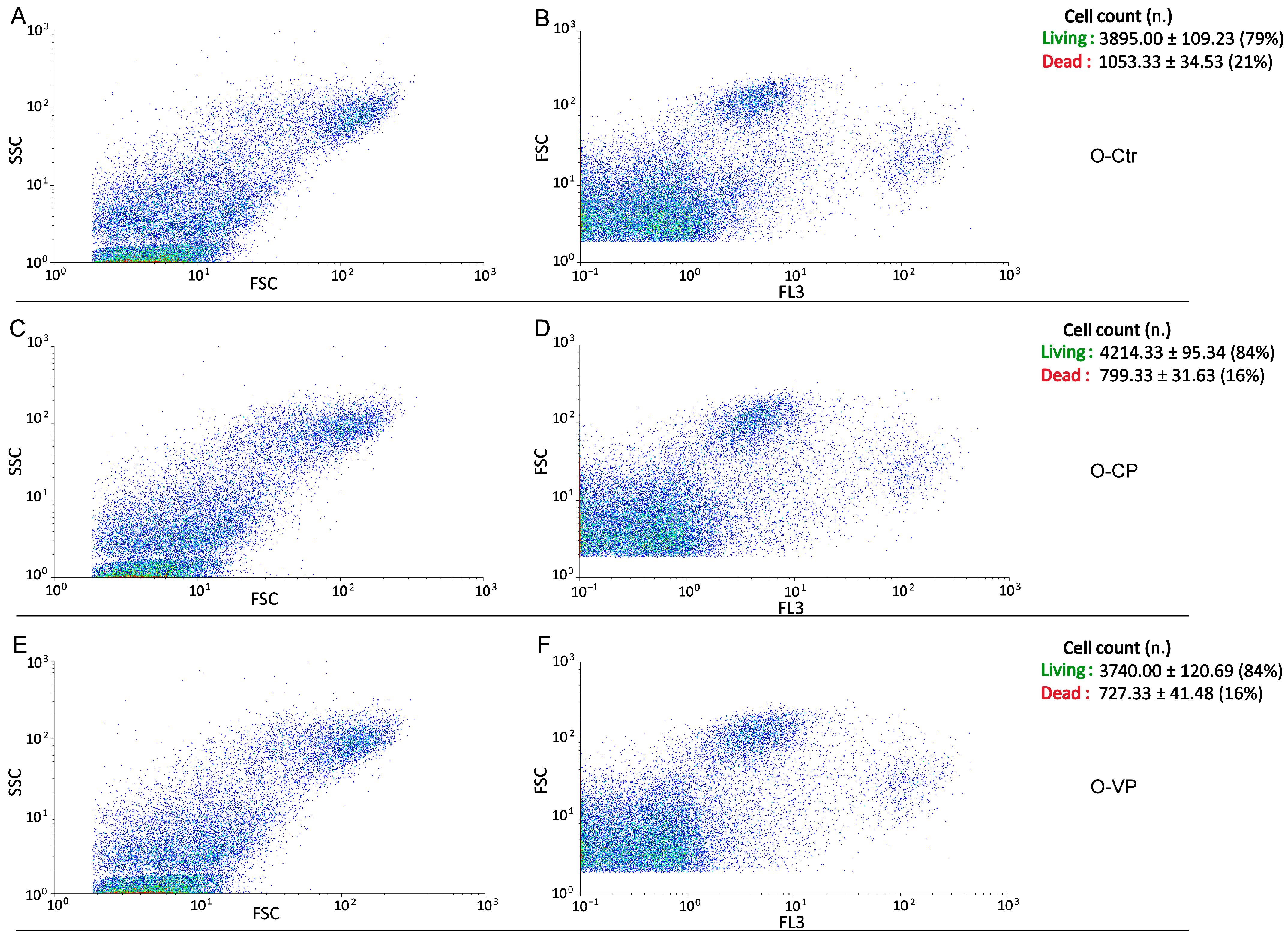
3.3. Cell Cycle Distribution and Cell Death
3.4. Cellular Proliferation
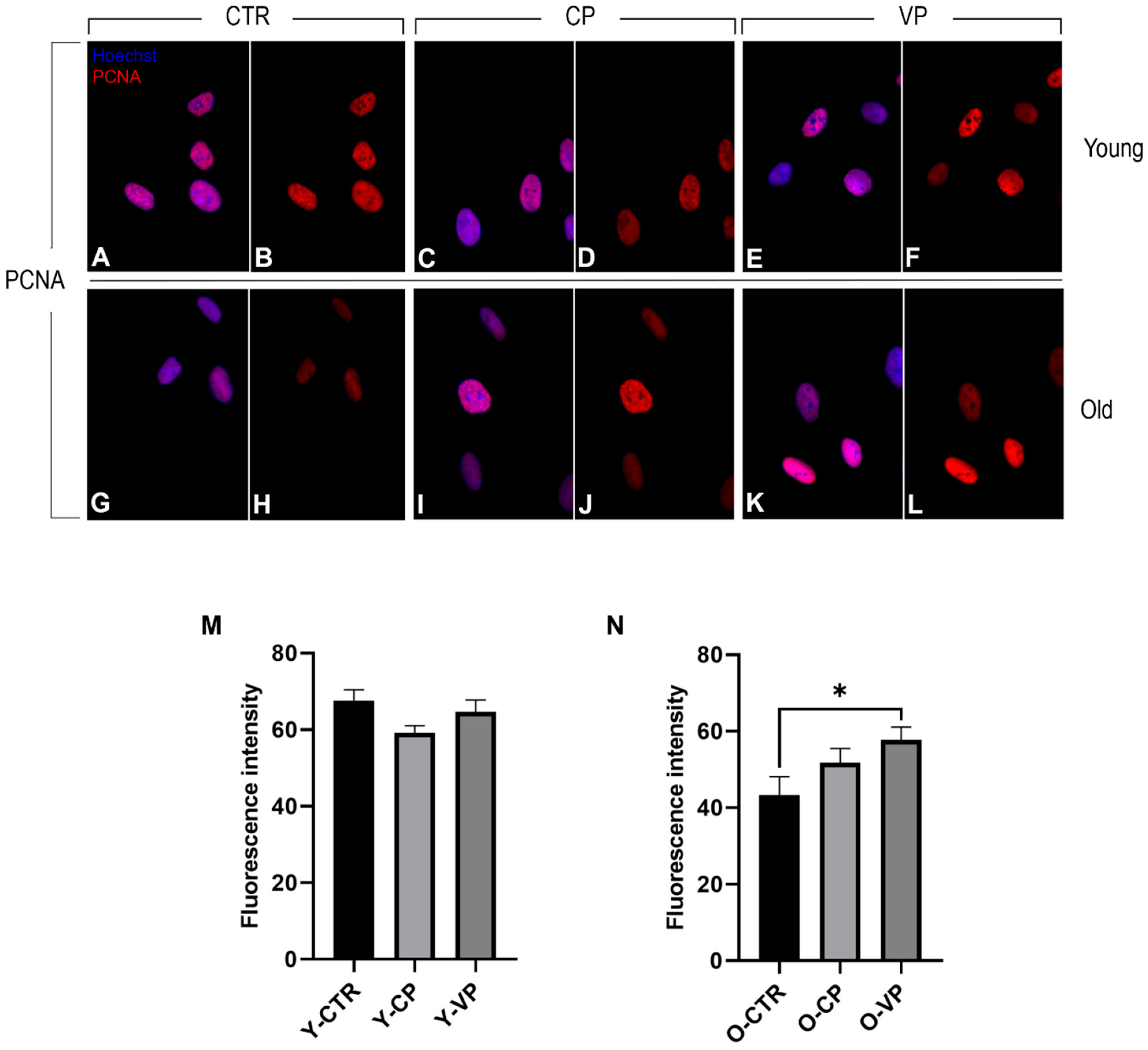
3.5. Anti-Aging Effects
3.6. TEM
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Misra, B.B. The Chemical Exposome of Human Aging. Front. Genet. 2020, 11, 574936. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, C.; Marchi, S.; Simoes, I.C.M.; Ren, Z.; Morciano, G.; Perrone, M.; Patalas-Krawczyk, P.; Borchard, S.; Jędrak, P.; Pierzynowska, K.; et al. Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 2018, 340, 209–344. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Ali, S.; Tahir, H.M.; Kazmi, S.A.R.; Shakir, H.A.; Mughal, T.A.; Mumtaz, S.; Summer, M.; Farooq, M.A. Aging and its treatment with vitamin C: A comprehensive mechanistic review. Mol. Biol. Rep. 2021, 48, 8141–8153. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Herranz, N.; Gil, J. Mechanisms and functions of cellular senescence. J. Clin. Investig. 2018, 128, 1238–1246. [Google Scholar] [CrossRef] [PubMed]
- Stewart, C.A.; Dell’Orco, R.T. Age related decline in the expression of proliferating cell nuclear antigen in human diploid fibroblasts. Mech. Ageing Dev. 1992, 66, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Burton, D.G.A.; Krizhanovsky, V. Physiological and pathological consequences of cellular senescence. Cell. Mol. Life Sci. 2014, 71, 4373–4386. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Quan, J.; Chen, S.; Yang, X.; Huang, S.; Yang, G.; Zhang, Y. A protocol for rapid construction of senescent cells. Front. Integr. Neurosci. 2022, 16, 929788. [Google Scholar] [CrossRef]
- Bakac, E.R.; Percin, E.; Gunes-Bayir, A.; Dadak, A. A Narrative Review: The Effect and Importance of Carotenoids on Aging and Aging-Related Diseases. Int. J. Mol. Sci. 2023, 24, 15199. [Google Scholar] [CrossRef]
- Song, L.; Zhang, S. Anti-Aging Activity and Modes of Action of Compounds from Natural Food Sources. Biomolecules 2023, 13, 1600. [Google Scholar] [CrossRef] [PubMed]
- Combsjr, G.F.; Mcclung James, P. The Vitamins: Fundamental Aspects in Nutrition and Health; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Drouin, G.; Godin, J.-R.; Page, B. The Genetics of Vitamin C Loss in Vertebrates. Curr. Genom. 2011, 12, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Bereswill, S.; Heimesaat, M.M. Immunomodulatory and Antimicrobial Effects of Vitamin C. Eur. J. Microbiol. Immunol. 2019, 9, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Activity of Ascorbic Acid. Antioxidants 2022, 11, 1993. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Ahmad, I.; Basit, A.; Shehata, W.F.; Hassan, U.; Shah, S.T.; Haleema, B.; Jalal, A.; Amin, R.; Khalid, M.A.; et al. Ascorbic Acid Enhances Growth and Yield of Sweet Peppers (Capsicum annum) by Mitigating Salinity Stress. Gesunde Pflanz. 2022, 74, 423–433. [Google Scholar] [CrossRef]
- Fratianni, F.; D’acierno, A.; Cozzolino, A.; Spigno, P.; Riccardi, R.; Raimo, F.; Pane, C.; Zaccardelli, M.; Lombardo, V.T.; Tucci, M.; et al. Biochemical Characterization of Traditional Varieties of Sweet Pepper (Capsicum annuum L.) of the Campania Region, Southern Italy. Antioxidants 2020, 9, 556. [Google Scholar] [CrossRef] [PubMed]
- Mennella, G.; D’Alessandro, A.; Francese, G.; Fontanella, D.; Parisi, M.; Tripodi, P. Occurrence of variable levels of health-promoting fruit compounds in horn-shaped Italian sweet pepper varieties assessed by a comprehensive approach. J. Sci. Food Agric. 2018, 98, 3280–3289. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Guzzon, F.; Canella, M.; Tazzari, E.R.; Cauzzi, P.; Bodino, S.; Ardenghi, N.M.G. Le varietà agronomiche lombarde tradizionali a rischio di estinzione o di erosione genetica. In Ortive e Cerealicole: Uno Sguardo D’insieme; Pavia University Press: Lombardia, Italy, 2019. [Google Scholar]
- Gola, F.; Gaiaschi, L.; Roda, E.; De Luca, F.; Ferulli, F.; Vicini, R.; Rossi, P.; Bottone, M.G. Voghera Sweet Pepper: A Potential Ally against Oxidative Stress and Aging. Int. J. Mol. Sci. 2023, 24, 3782. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; An, C.; Hwang, S.D.; Kim, Y.S. Ceriporia lacerata mycelium culture medium as a novel anti-aging microbial material for cosmeceutical application. Cosmetics 2021, 8, 101. [Google Scholar] [CrossRef]
- Amatori, S.; Mazzoni, L.; Alvarez-Suarez, J.M.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Provenzano, A.E.; Persico, G.; Mezzetti, B.; et al. Polyphenol-rich strawberry extract (PRSE) shows in vitro and in vivo biological activity against invasive breast cancer cells. Sci. Rep. 2016, 6, 30917. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; Van Bree, C. Clonogenic assay of cells in vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Roda, E.; De Luca, F.; Priori, E.C.; Ratto, D.; Pinelli, S.; Corradini, E.; Mozzoni, P.; Poli, D.; Mazzini, G.; Bottone, M.G.; et al. The Designer Drug αPHP Affected Cell Proliferation and Triggered Deathly Mechanisms in Murine Neural Stem/Progenitor Cells. Biology 2023, 12, 1225. [Google Scholar] [CrossRef]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 2021, 22, 13173. [Google Scholar] [CrossRef]
- Passos, J.F.; Simillion, C.; Hallinan, J.; Wipat, A.; von Zglinicki, T. Cellular senescence: Unravelling complexity. AGE 2009, 31, 353–363. [Google Scholar] [CrossRef]
- Khalil, R.; Diab-Assaf, M.; Lemaitre, J.-M. Emerging Therapeutic Approaches to Target the Dark Side of Senescent Cells: New Hopes to Treat Aging as a Disease and to Delay Age-Related Pathologies. Cells 2023, 12, 915. [Google Scholar] [CrossRef]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Butnariu, M.; Peana, M.; Sarac, I.; Strus, O.; Smetanina, K.; Chirumbolo, S. Natural Compounds and Products from an Anti-Aging Perspective. Molecules 2022, 27, 7084. [Google Scholar] [CrossRef]
- Woo, J.; Shin, S.; Cho, E.; Ryu, D.; Garandeau, D.; Chajra, H.; Fréchet, M.; Park, D.; Jung, E. Senotherapeutic-like effect of Silybum marianum flower extract revealed on human skin cells. PLoS ONE 2021, 16, e0260545. [Google Scholar] [CrossRef]
- Telang, P. Vitamin C in dermatology. Indian Dermatol. Online J. 2013, 4, 143–146. [Google Scholar] [CrossRef]
- Boo, Y.C. Ascorbic Acid (Vitamin C) as a Cosmeceutical to Increase Dermal Collagen for Skin Antiaging Purposes: Emerging Combination Therapies. Antioxidants 2022, 11, 1663. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef]
- Farriol, M.; Mourelle, M.; Schwartz, S. Effect of ascorbic acid and vitamin E analog on aged fibroblasts. Rev. Esp. Fisiol. 1994, 50, 253–257. [Google Scholar] [PubMed]
- Kashino, G.; Kodama, S.; Nakayama, Y.; Suzuki, K.; Fukase, K.; Goto, M.; Watanabe, M. Relief of oxidative stress by ascorbic acid delays cellular senescence of normal human and Werner syndrome fibroblast cells. Free Radic. Biol. Med. 2003, 35, 438–443. [Google Scholar] [CrossRef]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Lekic, P.; Pender, N.; McCulloch, C. Is Fibroblast Heterogeneity Relevant to the Health, Diseases, and Treatments of Periodontal Tissues? Crit. Rev. Oral Biol. Med. 1997, 8, 253–268. [Google Scholar] [CrossRef]
- Ravikanth, M.; Soujanya, P.; Manjunath, K.; Saraswathi, T.; Ramachandran, C. Heterogenecity of fibroblasts. J. Oral Maxillofac. Pathol. 2011, 15, 247–250. [Google Scholar] [CrossRef]
- Campbell, J.D.; Cole, M.; Bunditrutavorn, B.; Vella, A.T. Ascorbic Acid Is a Potent Inhibitor of Various Forms of T Cell Apoptosis. Cell Immunol. 1999, 194, 1–5. [Google Scholar] [CrossRef]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front. Cell Dev. Biol. 2021, 9, 645593. [Google Scholar] [CrossRef]
- Mijit, M.; Caracciolo, V.; Melillo, A.; Amicarelli, F.; Giordano, A. Role of p53 in the Regulation of Cellular Senescence. Biomolecules 2020, 10, 420. [Google Scholar] [CrossRef]
- Lago, J.C.; Puzzi, M.B. The effect of aging in primary human dermal fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef]
- Roda, E.; De Luca, F.; Locatelli, C.A.; Ratto, D.; Di Iorio, C.; Savino, E.; Bottone, M.G.; Rossi, P. From a Medicinal Mushroom Blend a Direct Anticancer Effect on Triple-Negative Breast Cancer: A Preclinical Study on Lung Metastases. Molecules 2020, 25, 5400. [Google Scholar] [CrossRef]
- Green, D.R.; Kroemer, G. Cytoplasmic functions of the tumour suppressor p53. Nature 2009, 458, 1127–1130. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chien, C.-Y.; Huang, T.-L.; Chiu, T.-J.; Wang, Y.-M.; Fang, F.-M.; Li, S.-H. Low p16 Cytoplasmic Staining Predicts Poor Treatment Outcome in Patients with p16-Negative Locally Advanced Head and Neck Squamous Cell Carcinoma Receiving TPF Induction Chemotherapy. Biomedicines 2023, 11, 339. [Google Scholar] [CrossRef]
- Ratto, D.; Ferrari, B.; Roda, E.; Brandalise, F.; Siciliani, S.; De Luca, F.; Priori, E.C.; Di Iorio, C.; Cobelli, F.; Veneroni, P.; et al. Squaring the Circle: A New Study of Inward and Outward-Rectifying Potassium Currents in U251 GBM Cells. Cell Mol. Neurobiol. 2020, 40, 813–828. [Google Scholar] [CrossRef]
- Gunin, A.G.; Kornilova, N.K.; Petrov, V.V.; Vasil’Eva, O.V. Age-related changes in the number and proliferation of fibroblasts in the human skin. Adv. Gerontol. 2011, 24, 43–47. [Google Scholar] [PubMed]
- Brix, N.; Samaga, D.; Hennel, R.; Gehr, K.; Zitzelsberger, H.; Lauber, K. The clonogenic assay: Robustness of plating efficiency-based analysis is strongly compromised by cellular cooperation. Radiat. Oncol. 2020, 15, 248. [Google Scholar] [CrossRef]
- Di Fonso, A.; Pietrangelo, L.; D’onofrio, L.; Michelucci, A.; Boncompagni, S.; Protasi, F. Ageing Causes Ultrastructural Modification to Calcium Release Units and Mitochondria in Cardiomyocytes. Int. J. Mol. Sci. 2021, 22, 8364. [Google Scholar] [CrossRef]
- Boak, A.; Bittles, A.; Quinn, P. Age-related ultrastructural changes in human embryonic lung fibroblasts. Exp. Gerontol. 1983, 18, 139–146. [Google Scholar] [CrossRef]
- Huang, H.-M.; Fowler, C.; Xu, H.; Zhang, H.; Gibson, G.E. Mitochondrial function in fibroblasts with aging in culture and/or Alzheimer’s disease. Neurobiol. Aging 2005, 26, 839–848. [Google Scholar] [CrossRef]
- Goldstein, S.; Korczack, L.B. Status of mitochondria in living human fibroblasts during growth and senescence in vitro: Use of the laser dye rhodamine 123. J. Cell Biol. 1981, 91 Pt 1, 392–398. [Google Scholar] [CrossRef]
- Sainz-Urruela, C.; Vera-López, S.; San Andrés, M.P.; Díez-Pascual, A.M. Graphene-Based Sensors for the Detection of Bioactive Compounds: A Review. Int. J. Mol. Sci. 2021, 22, 3316. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, J.; Shin, D.W. The Molecular Mechanism of Polyphenols with Anti-Aging Activity in Aged Human Dermal Fibroblasts. Molecules 2022, 27, 4351. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Martens, U.M.; Chavez, E.A.; Poon, S.S.; Schmoor, C.; Lansdorp, P.M. Accumulation of Short Telomeres in Human Fibroblasts Prior to Replicative Senescence. Exp. Cell Res. 2000, 256, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Walen, K.H. Human diploid fibroblast cells in senescence cycling through polyploidy to mitotic cells. Vitr. Cell. Dev. Biol.-Anim. 2006, 42, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Ohshima, S. Abnormal mitosis in hypertetraploid cells causes aberrant nuclear morphology in association with H2O2-induced premature senescence. Cytom. Part A 2008, 73A, 808–815. [Google Scholar] [CrossRef]
- Holt, D.J.; Grainger, D.W. Multinucleated giant cells from fibroblast cultures. Biomaterials 2011, 32, 3977–3987. [Google Scholar] [CrossRef]



| Composition | Fruit Body (i.e., Endocarp, Mesocarp and Pericarp) |
|---|---|
| Initial biomass | 15 g |
| Extraction medium | water/1,2-propanediol (45–55%) |
| Stock solution concentration | 150 mg/mL |
| Work solution concentration | 1 mg/mL |
| Purification method | Membrane filtration |
| Storage | Dark environment and controlled temperature (−20 °C) |
| Ascorbic Acid (µg/g) | 724.2 ± 0.37 (VP) 378.5 ± 0.25 (CP) |
| Total carotenoids (µg/g) | 55.4 (VP) 112.5 (CP) |
| Antigen | Immunogen | Manufacturer, Species, Mono-Polyclonal, Cat./Lot. | Dilution | |
|---|---|---|---|---|
| Primary Antibodies | Anti-Proliferating Cell Nuclear Antigen (Ab-1) | Purified antibody raised against the ~37 kDa PCNA protein of mouse origin | Sigma-Aldrich (St. Louis, MO, USA), Mouse monoclonal IgG2a, Cat# NA03-200UG | 1:500 |
| Anti-p53 (Ab-5) | Purified antibody raised against the ~53 kDa wild type p53 protein of mouse origin | Sigma-Aldrich (St. Louis, MO, USA), Mouse monoclonal IgG2a, Cat# OP33-100UG | 1:100 | |
| P16INK4a/CDKN2A | A synthetic peptide corresponding to a sequence within amino acids 20 to the C-terminus of human CDKN2A/p16INK4a | GeneTex (Irvine, CA, USA), Mouse monoclonal IgG, Cat# GTX03119 | 1:250 | |
| Secondary Antibodies | Alexa Fluor™ 594 goat anti-mouse IgG (H + L) Highly Cross-Adsorbed Secondary Antibody | Gamma immunoglobins Heavy and light chains | Thermo Fisher Scientific (Monza, Italy) | 1:200 |
| Y-CTR | Y-CP | Y-VP | |
|---|---|---|---|
| Y-CTR (3240.21 ± 137.26) | -- | * | ns |
| Y-CP (4281.35 ± 304.99) | * | -- | * |
| Y-VP (3364.10 ± 258.55) | ns | * | -- |
| O-CTR | O-CP | O-VP | |
| O-CTR (7911.64 ± 532.97) | -- | ns | ns |
| O-CP (9487 ± 809.23) | ns | -- | * |
| O-VP (6917.25 ± 631.65) | ns | * | -- |
| Y-CTR | Y-CP | Y-VP | |
|---|---|---|---|
| Y-CTR (100) | -- | ns | ** |
| Y-CP (118.37± 1.81) | ns | -- | ns |
| Y-VP (149.66 ± 1.84) | ** | ns | -- |
| O-CTR | O-CP | O-VP | |
| O-CTR (100) | -- | ** | ns |
| O-CP (37.5 ± 2.62) | ** | -- | ns |
| O-VP (47.43 ± 2.81) | ns | ns | -- |
| Y-CTR | Y-CP | Y-VP | |
|---|---|---|---|
| Y-CTR (67.65 ± 2.78) | -- | ns | ns |
| Y-CP (59.16 ± 1.89) | Ns | -- | ns |
| Y-VP (64.68 ± 3.08) | Ns | ns | -- |
| O-CTR | O-CP | O-VP | |
| O-CTR (43.36 ± 4.67) | -- | ns | * |
| O-CP (51.74 ± 3.77) | -- | -- | -- |
| O-VP (57.69 ± 3.40) | * | -- | -- |
| O-CTR | O-CP | O-VP | |
|---|---|---|---|
| O-CTR (50.16 ± 3.41) | -- | * | * |
| O-CP (18.10 ± 3.55) | * | -- | ns |
| O-VP (8.40 ± 2.60) | * | ns | -- |
| Y-CTR | Y-CP | Y-VP | |
| Y-CTR (13.56 ± 1.57) | -- | *** | ns |
| Y-CP (1.37 ± 0.32) | *** | -- | * |
| Y-VP (0.46 ± 0.25) | ns | * | -- |
| Y-CTR | Y-CP | Y-VP | |
|---|---|---|---|
| Y-CTR (43.44 ± 2.68) | -- | *** | *** |
| Y-CP (28.59 ± 2.45) | *** | -- | * |
| Y-VP (38.63 ± 2.93) | *** | * | -- |
| O-CTR | O-CP | O-VP | |
| O-CTR (61.87 ± 4.25) | -- | *** | *** |
| O-CP (48.58 ± 4.05) | *** | -- | ns |
| O-VP (46.70 ± 3.79) | *** | ns | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, F.; Gola, F.; Azzalin, A.; Casali, C.; Gaiaschi, L.; Milanesi, G.; Vicini, R.; Rossi, P.; Bottone, M.G. A Lombard Variety of Sweet Pepper Regulating Senescence and Proliferation: The Voghera Pepper. Nutrients 2024, 16, 1681. https://doi.org/10.3390/nu16111681
De Luca F, Gola F, Azzalin A, Casali C, Gaiaschi L, Milanesi G, Vicini R, Rossi P, Bottone MG. A Lombard Variety of Sweet Pepper Regulating Senescence and Proliferation: The Voghera Pepper. Nutrients. 2024; 16(11):1681. https://doi.org/10.3390/nu16111681
Chicago/Turabian StyleDe Luca, Fabrizio, Federica Gola, Alberto Azzalin, Claudio Casali, Ludovica Gaiaschi, Gloria Milanesi, Riccardo Vicini, Paola Rossi, and Maria Grazia Bottone. 2024. "A Lombard Variety of Sweet Pepper Regulating Senescence and Proliferation: The Voghera Pepper" Nutrients 16, no. 11: 1681. https://doi.org/10.3390/nu16111681
APA StyleDe Luca, F., Gola, F., Azzalin, A., Casali, C., Gaiaschi, L., Milanesi, G., Vicini, R., Rossi, P., & Bottone, M. G. (2024). A Lombard Variety of Sweet Pepper Regulating Senescence and Proliferation: The Voghera Pepper. Nutrients, 16(11), 1681. https://doi.org/10.3390/nu16111681










