A Systematic Review over the Effect of Early Infant Diet on Neurodevelopment: Insights from Neuroimaging
Abstract
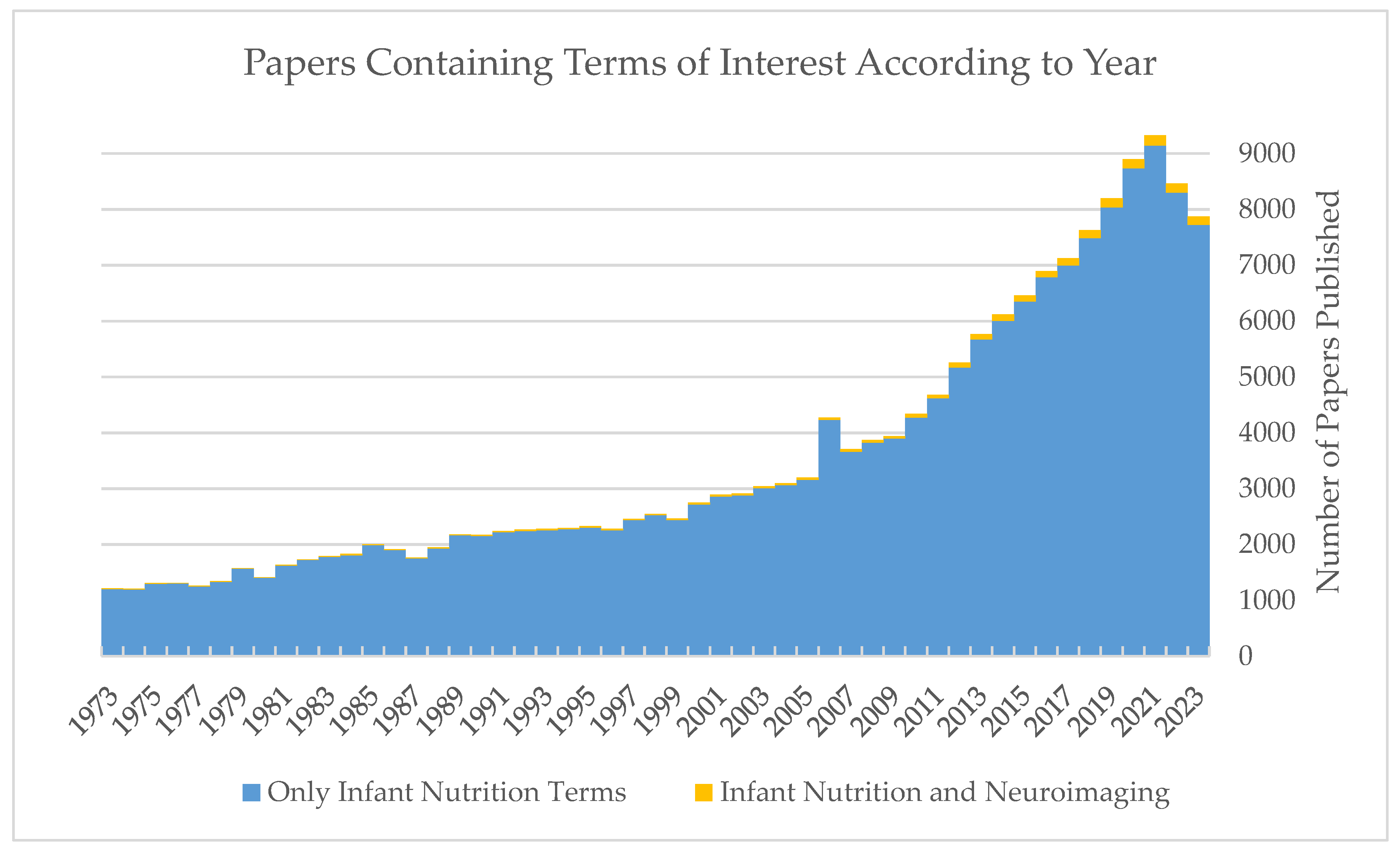
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
3. Results
3.1. Selection of Studies
3.2. Description of Studies
3.3. Study Quality
3.4. Study Participants
4. Discussion
4.1. EEG
4.1.1. Long Chain Fatty Acids’ Effect on Term Infants
4.1.2. Long Chain Fatty Acids’ Effect on Preterm Infants
4.2. MRI
4.2.1. HMOs’ Effect on Term Infants
4.2.2. HMOs’ Effect on Preterm Infants
4.2.3. Supplemented Formula’s Effect on Term Infants
4.2.4. Supplemented Formula’s Effect on Preterm Infants
4.2.5. Effect of Macronutrient Intake on Preterm Infants
5. Limitations and Strengths
6. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Isaacs, E.B. Neuroimaging, a new tool for investigating the effects of early diet on cognitive and brain development. Front. Hum. Neurosci. 2013, 7, 445. [Google Scholar] [CrossRef] [PubMed]
- Meek, J.Y.; Noble, L.; Breastfeeding, S.O. Policy Statement: Breastfeeding and the Use of Human Milk. Pediatrics 2022, 150, e2022057988. [Google Scholar] [CrossRef] [PubMed]
- Deoni, S.; Dean, D.; Joelson, S.; O’Regan, J.; Schneider, N. Early nutrition influences developmental myelination and cognition in infants and young children. NeuroImage 2018, 178, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.; Morley, R.; Isaacs, E. Nutrition and mental development. Nutr. Rev. 2001, 59, S24–S33. [Google Scholar] [CrossRef] [PubMed]
- Prado, E.L.; Dewey, K.G. Nutrition and brain development in early life. Nutr. Rev. 2014, 72, 267–284. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Barros, F.C.; Horta, B.L.; Lima, R.C. Breastfeeding and school achievement in Brazilian adolescents. Acta Paediatr. 2005, 94, 1656–1660. [Google Scholar] [CrossRef]
- Brion, M.-J.A.; Lawlor, D.A.; Matijasevich, A.; Horta, B.; Anselmi, L.; Araújo, C.L.; Menezes, A.M.B.; Victora, C.G.; Smith, G.D. What are the causal effects of breastfeeding on IQ, obesity and blood pressure? Evidence from comparing high-income with middle-income cohorts. Int. J. Epidemiol. 2011, 40, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Luby, J.L.; Belden, A.C.; Whalen, D.; Harms, M.P.; Barch, D.M. Breastfeeding and Childhood IQ: The Mediating Role of Gray Matter Volume. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 367–375. [Google Scholar] [CrossRef]
- Daniels, M.C.; Adair, L.S. Breast-feeding influences cognitive development in Filipino children. J. Nutr. 2005, 135, 2589–2595. [Google Scholar] [CrossRef]
- Kafouri, S.; Kramer, M.; Leonard, G.; Perron, M.; Pike, B.; Richer, L.; Toro, R.; Veillette, S.; Pausova, Z.; Paus, T. Breastfeeding and brain structure in adolescence. Int. J. Epidemiol. 2013, 42, 150–159. [Google Scholar] [CrossRef]
- Kadosh, K.C.; Muhardi, L.; Parikh, P.; Basso, M.; Mohamed, H.J.J.; Prawitasari, T.; Samuel, F.; Ma, G.; Geurts, J.M.W. Nutritional Support of Neurodevelopment and Cognitive Function in Infants and Young Children—An Update and Novel Insights. Nutrients 2021, 13, 199. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cui, Q.; Yan, C. The Effect of Supplementation of Long-Chain Polyunsaturated Fatty Acids during Lactation on Neurodevelopmental Outcomes of Preterm Infant from Infancy to School Age: A Systematic Review and Meta-analysis. Pediatr. Neurol. 2016, 59, 54–61.e1. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, I.S.; Tonato, A.M.C.; Puentes, A.d.l.G.; Nieto, A.; Herrmann, F.; Dieguez, E.; Castellote, A.I.; López-Sabater, M.C.; Rodríguez-Palmero, M.; Campoy, C. The effect of an infant formula supplemented with AA and DHA on fatty acid levels of infants with different FADS genotypes: The COGNIS study. Nutrients 2019, 11, 602. [Google Scholar] [CrossRef] [PubMed]
- Pawlosky, R.J.; Lin, Y.H.; Llanos, A.; Mena, P.; Uauy, R.; Salem, N. Compartmental analyses of plasma 13C- and 2H-labeled n-6 fatty acids arising from oral administrations of 13C-U-18:2n-6 and 2H5-20:3n-6 in newborn infants. Pediatr. Res. 2006, 60, 327–333. [Google Scholar] [CrossRef]
- Bertrand, P.C.; O’Kusky, J.R.; Innis, S.M. Maternal dietary (n-3) fatty acid deficiency alters neurogenesis in the embryonic rat brain. J. Nutr. 2006, 136, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K. DHA deficiency and prefrontal cortex neuropathology in recurrent affective disorders. J. Nutr. 2010, 140, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.; McCandliss, B.D.; Carlson, S.E.; Colombo, J.; Shaddy, D.J.; Kerling, E.H.; Lepping, R.J.; Sittiprapaporn, W.; Cheatham, C.L.; Gustafson, K.M. Event Related Potential Differences in Childrenn Supplemented with Long-chain Polyunsaturated Fatty Acids During Infancy. Dev. Sci. 2017, 20, e12455. [Google Scholar] [CrossRef]
- Deoni, S.C.L. Neuroimaging of the developing brain and impact of nutrition. Nestle Nutr. Inst. Workshop Ser. 2018, 89, 155–174. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.; Shaddy, D.J.; Gustafson, K.; Gajewski, B.J.; Thodosoff, J.M.; Kerling, E.; Carlson, S.E. The Kansas University DHA Outcomes Study (KUDOS) clinical trial: Long-term behavioral follow-up of the effects of prenatal DHA supplementation. Am. J. Clin. Nutr. 2019, 109, 1380–1392. [Google Scholar] [CrossRef]
- van der Merwe, L.F.; Moore, S.E.; Fulford, A.J.; Halliday, K.E.; Drammeh, S.; Young, S.; Prentice, A.M. Long-chain PUFA supplementation in rural African infants: A randomized controlled trial of effects on gut integrity, growth and cognitive development. World Rev. Nutr. Diet. 2014, 109, 43–45. [Google Scholar] [CrossRef]
- Meldrum, S.J.; D’Vaz, N.; Simmer, K.; Dunstan, J.A.; Hird, K.; Prescott, S.L. Effects of high-dose fish oil supplementation during early infancy on neurodevelopment and language: A randomised controlled trial. Br. J. Nutr. 2012, 108, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Birch, E.E.; Garfield, S.; Hoffman, D.R.; Uauy, R.; Birch, D.G. A randomized controlled trial of early dietary supply of long-chain polyunsaturated fatty acids and mental development in term infants. Dev. Med. Child Neurol. 2000, 42, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Natu, V.S.; Rosenke, M.; Wu, H.; Querdasi, F.R.; Kular, H.; Lopez-Alvarez, N.; Grotheer, M.; Berman, S.; Mezer, A.A.; Grill-Spector, K. Infants’ cortex undergoes microstructural growth coupled with myelination during development. Commun. Biol. 2021, 4, 1191. [Google Scholar] [CrossRef] [PubMed]
- Holland, D.; Chang, L.; Ernst, T.M.; Curran, M.; Buchthal, S.D.; Alicata, D.; Skranes, J.; Johansen, H.; Hernandez, A.; Yamakawa, R.; et al. Structural growth trajectories and rates of change in the first 3 months of infant brain development. JAMA Neurol. 2014, 71, 1266–1274. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Shen, D.; Lin, W. Resting-state functional MRI studies on infant brains: A decade of gap-filling efforts. NeuroImage 2019, 185, 664–684. [Google Scholar] [CrossRef] [PubMed]
- Glover, G.H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef]
- Gevins, A.; Smith, M.E.; McEvoy, L.K.; Leong, H.; Le, J. Electroencephalographic imaging of higher brain function. Philos. Trans. R. Soc. B Biol. Sci. 1999, 354, 1125–1134. [Google Scholar] [CrossRef]
- Bosch-Bayard, J.; Biscay, R.J.; Fernandez, T.; Otero, G.A.; Ricardo-Garcell, J.; Aubert-Vazquez, E.; Evans, A.C.; Harmony, T. EEG effective connectivity during the first year of life mirrors brain synaptogenesis, myelination, and early right hemisphere predominance. NeuroImage 2022, 252, 119035. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, 332–336. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Green, S.; Ben Van Den, A. Cochrane handbook for systematic reviews of interventions. Int. Coach. Psychol. Rev. 2020, 15, 123–125. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Saby, J.; Kuschner, E.; Gaetz, W.; Edgar, J.C.; Roberts, T.P. Magnetoencephalography and the infant brain. NeuroImage 2019, 189, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Bouglé, D.; Denise, P.; Vimard, F.; Nouvelot, A.; Penniello, M.-J.; Guillois, B. Early neurological and neurophysiological development of the preterm infant and polyunsaturated fatty acids supply. Clin. Neurophysiol. 1999, 110, 1363–1370. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, C.; Haugholt, K.; Lindgren, M.; Aurvåg, A.K.; Rønnestad, A.; Grønn, M.; Solberg, R.; Moen, A.; Nakstad, B.; Berge, R.K.; et al. Improved cognitive development among preterm infants attributable to early supplementation of human milk with docosahexaenoic acid and arachidonic acid. Pediatrics 2008, 121, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Pivik, R.T.; Dykman, R.A.; Jing, H.; Gilchrist, J.M.; Badger, T.M. The influence of infant diet on early developmental changes in processing human voice speech stimuli: ERP variations in breast and milk formula-fed infants at 3 and 6 months after birth. Dev. Neuropsychol. 2007, 31, 279–335. [Google Scholar] [CrossRef]
- Jing, H.; Pivik, R.T.; Dykman, R.A.; Gilchrist, J.M.; Badger, T.M. Effects of breast milk and milk formula diets on synthesized speech sound-induced event-related potentials in 3- and 6-month-old infants. Dev. Neuropsychol. 2007, 31, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dykman, R.A.; Jing, H.; Gilchrist, J.M.; Badger, T.M.; Pivik, R.T. Cortical responses to speech sounds in 3- and 6-month-old infants fed breast milk, milk formula, or soy formula. Dev. Neuropsychol. 2010, 35, 762–784. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Gilchrist, J.M.; Badger, T.M.; Pivik, R. A longitudinal study of differences in electroencephalographic activity among breastfed, milk formula-fed, and soy formula-fed infants during the first year of life. Early Hum. Dev. 2010, 86, 119–125. [Google Scholar] [CrossRef]
- Pivik, R.; Andres, A.; Badger, T.M. Diet and gender influences on processing and discrimination of speech sounds in 3- and 6-month-old infants: A developmental ERP study. Dev. Sci. 2011, 14, 700–712. [Google Scholar] [CrossRef] [PubMed]
- Pivik, R.T.; Andres, A.; Bai, S.; Cleves, M.A.; Tennal, K.B.; Gu, Y.; Badger, T.M. Infant Diet-Related Changes in Syllable Processing Between 4 and 5 Months: Implications for Developing Native Language Sensitivity. Dev. Neuropsychol. 2016, 41, 215–230. [Google Scholar] [CrossRef]
- Pivik, R.; Andres, A.; Tennal, K.B.; Gu, Y.; Downs, H.; Bellando, B.J.; Jarratt, K.; Cleves, M.A.; Badger, T.M. Resting gamma power during the postnatal critical period for GABAergic system development is modulated by infant diet and sex. Int. J. Psychophysiol. 2018, 135, 73–94. [Google Scholar] [CrossRef]
- Alatorre-Cruz, G.C.; Andres, A.; Gu, Y.; Downs, H.; Hagood, D.; Sorensen, S.T.; Williams, D.K.; Larson-Prior, L.J. Impact of feeding habits on the development of language-specific processing of phonemes in brain: An event-related potentials study. Front. Nutr. 2023, 10, 1032413. [Google Scholar] [CrossRef] [PubMed]
- Gilbreath, D.; Hagood, D.; Alatorre-Cruz, G.C.; Andres, A.; Downs, H.; Larson-Prior, L.J. Effects of Early Nutrition Factors on Baseline Neurodevelopment during the First 6 Months of Life: An EEG Study. Nutrients 2023, 15, 1535. [Google Scholar] [CrossRef]
- Niu, W.; Xu, X.; Zhang, H.; Zhang, Y.; Ni, S.; Liu, T.; Li, Q.; Lai, C.; Sun, Y. Breastfeeding improves dynamic reorganization of functional connectivity in preterm infants: A temporal brain network study. Med. Biol. Eng. Comput. 2020, 58, 2805–2819. [Google Scholar] [CrossRef] [PubMed]
- Strømmen, K.; Blakstad, E.W.; Moltu, S.J.; Almaas, A.N.; Westerberg, A.C.; Amlien, I.K.; Rønnestad, A.E.; Nakstad, B.; Drevon, C.A.; Bjørnerud, A.; et al. Enhanced nutrient supply to very low birth weight infants is associated with improved white matter maturation and head growth. Neonatology 2014, 107, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Vasu, V.; Durighel, G.; Thomas, L.; Malamateniou, C.; Bell, J.D.; Rutherford, M.A.; Modi, N. Preterm nutritional intake and MRI phenotype at term age: A prospective observational study. BMJ Open 2014, 4, e005390. [Google Scholar] [CrossRef] [PubMed]
- Beauport, L.; Schneider, J.; Faouzi, M.; Hagmann, P.; Hüppi, P.S.; Tolsa, J.-F.; Truttmann, A.C.; Fumeaux, C.J.F. Impact of Early Nutritional Intake on Preterm Brain: A Magnetic Resonance Imaging Study. J. Pediatr. 2016, 181, 29–36.e1. [Google Scholar] [CrossRef] [PubMed]
- Coviello, C.; Keunen, K.; Kersbergen, K.J.; Groenendaal, F.; Leemans, A.; Peels, B.; Isgum, I.; Viergever, M.A.; De Vries, L.S.; Buonocore, G.; et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatr. Res. 2017, 83, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Power, V.A.; Spittle, A.J.; Lee, K.J.; Anderson, P.J.; Thompson, D.K.; Doyle, L.W.; Cheong, J.L. Nutrition, Growth, Brain Volume, and Neurodevelopment in Very Preterm Children. J. Pediatr. 2019, 215, 50–55.e3. [Google Scholar] [CrossRef] [PubMed]
- Blesa, M.; Sullivan, G.; Anblagan, D.; Telford, E.J.; Quigley, A.J.; Sparrow, S.A.; Serag, A.; Semple, S.I.; Bastin, M.E.; Boardman, J.P. Early breast milk exposure modifies brain connectivity in preterm infants. NeuroImage 2019, 184, 431–439. [Google Scholar] [CrossRef]
- Hortensius, L.M.; Janson, E.; van Beek, P.E.; Groenendaal, F.; Claessens, N.H.P.; de Veye, H.F.N.S.; Eijsermans, M.J.C.; Koopman-Esseboom, C.; Dudink, J.; van Elburg, R.M.; et al. Nutritional intake, white matter integrity, and neurodevelopment in extremely preterm born infants. Nutrients 2021, 13, 3409. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, Q.; Wang, J.; Wang, H.; Li, Q.; Zhu, B.; Ji, C.; Xu, X.; Johnston, L. The impact of breast milk feeding on early brain development in preterm infants in China: An observational study. PLoS ONE 2022, 17, e0272125. [Google Scholar] [CrossRef]
- Sullivan, G.; Vaher, K.; Blesa, M.; Galdi, P.; Stoye, D.Q.; Quigley, A.J.; Thrippleton, M.J.; Norrie, J.; Bastin, M.E.; Boardman, J.P. Breast Milk Exposure is Associated with Cortical Maturation in Preterm Infants. Ann. Neurol. 2023, 93, 591–603. [Google Scholar] [CrossRef]
- Deoni, S.C.; Dean, D.C., 3rd; Piryatinsky, I.; O’Muircheartaigh, J.; Waskiewicz, N.; Lehman, K.; Han, M.; Dirks, H. Breastfeeding and early white matter development: A cross-sectional study. NeuroImage 2013, 82, 77–86. [Google Scholar] [CrossRef]
- Schneider, N.; Hauser, J.; Oliveira, M.; Cazaubon, E.; Mottaz, S.C.; O’Neill, B.; Steiner, P.; Deoni, S.C.L. Sphingomyelin in brain and cognitive development: Preliminary data. Eneuro 2019, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.K.; Bansal, R.; Sawardekar, S.; Yonemitsu, C.; Furst, A.; Hampson, H.E.; Schmidt, K.A.; Alderete, T.L.; Bode, L.; Goran, M.I.; et al. Associations of Human Milk Oligosaccharides with Infant Brain Tissue Organization and Regional Blood Flow at 1 Month of Age. Nutrients 2022, 14, 3820. [Google Scholar] [CrossRef]
- Schneider, N.; Hartweg, M.; O’regan, J.; Beauchemin, J.; Redman, L.; Hsia, D.S.; Steiner, P.; Carmichael, O.; D’sa, V.; Deoni, S. Impact of a Nutrient Formulation on Longitudinal Myelination, Cognition, and Behavior from Birth to 2 Years: A Randomized Clinical Trial. Nutrients 2023, 15, 4439. [Google Scholar] [CrossRef] [PubMed]
- Ottolini, K.M.; Andescavage, N.; Kapse, K.; Jacobs, M.; Limperopoulos, C. Improved brain growth and microstructural development in breast milk-fed very low birth weight premature infants. Acta Paediatr. 2020, 109, 1580–1587. [Google Scholar] [CrossRef]
- Handu, D.; Moloney, L.; Wolfram, T.; Ziegler, P.; Acosta, A.; Steiber, A. Academy of Nutrition and Dietetics Methodology for Conducting Systematic Reviews for the Evidence Analysis Library. J. Acad. Nutr. Diet. 2016, 116, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Birnholz, J.C.; Benacerraf, B.R. The Development of Human Fetal Hearing. Science 1983, 222, 516–518. [Google Scholar] [CrossRef]
- Benasich, A.A.; Thomas, J.J.; Choudhury, N.; Leppänen, P.H.T. The importance of rapid auditory processing abilities to early language development: Evidence from converging methodologies. Dev. Psychobiol. 2002, 40, 278–292. [Google Scholar] [CrossRef]
- Hall, J.W. Development of the Auditory System. Ear Hear. 1999, 20, 87. [Google Scholar] [CrossRef]
- Choudhury, N.; Benasich, A.A. Maturation of auditory evoked potentials from 6 to 48 months: Prediction to 3 and 4 year language and cognitive abilities. Clin. Neurophysiol. 2011, 122, 320–338. [Google Scholar] [CrossRef] [PubMed]
- Čeponienė, R.; Torki, M.; Alku, P.; Koyama, A.; Townsend, J. Event-related potentials reflect spectral differences in speech and non-speech stimuli in children and adults. Clin. Neurophysiol. 2008, 119, 1560–1577. [Google Scholar] [CrossRef]
- Stekić, K.; Ilić, O.; Ković, V.; Savić, A.M. ERP Indicators of Phonological Awareness Development in Children: A Systematic Review. Brain Sci. 2023, 13, 290. [Google Scholar] [CrossRef]
- Chauncey, K.; Grainger, J.; Holcomb, P.J. The role of subjective frequency in language switching: An ERP investigation using masked priming. Mem. Cogn. 2010, 39, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Khazipov, R.; Milh, M. Early patterns of activity in the developing cortex: Focus on the sensorimotor system. Semin. Cell Dev. Biol. 2018, 76, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Egorov, A.V.; Draguhn, A. Development of coherent neuronal activity patterns in mammalian cortical networks: Common principles and local hetereogeneity. Mech. Dev. 2013, 130, 412–423. [Google Scholar] [CrossRef]
- Saby, J.N.; Marshall, P.J. The Utility of EEG Band Power Analysis in the Study of Infancy and Early Childhood. Dev. Neuropsychol. 2012, 37, 253–273. [Google Scholar] [CrossRef] [PubMed]
- Gasser, T.; Verleger, R.; Bächer, P.; Sroka, L. Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalogr. Clin. Neurophysiol. 1988, 69, 91–99. [Google Scholar] [CrossRef]
- van der Knaap, M.S.; Valk, J.; Bakker, C.J.; Schooneveld, M.; Faber, J.A.J.; Willemse, J.; Gooskens, R.H.J.M. Myelination As an Expression of the Functional Maturity of the Brain. Dev. Med. Child Neurol. 1991, 33, 849–857. [Google Scholar] [CrossRef]
- Shimamura, A.P. The role of the prefrontal cortex in dynamic filtering. Psychobiology 2000, 28, 207–218. [Google Scholar] [CrossRef]
- Gao, W.; Zhu, H.; Giovanello, K.S.; Smith, J.K.; Shen, D.; Gilmore, J.H.; Lin, W. Evidence on the emergence of the brain’s default network from 2-week-old to 2-year-old healthy pediatric subjects. Proc. Natl. Acad. Sci. USA 2009, 106, 6790–6795. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Wang, B. Sialic acid is an essential nutrient for brain development and cognition. Annu. Rev. Nutr. 2009, 29, 177–222. [Google Scholar] [CrossRef] [PubMed]
- Docq, S.; Spoelder, M.; Wang, W.; Homberg, J.R. The protective and long-lasting effects of human milk oligosaccharides on cognition in Mammals. Nutrients 2020, 12, 3572. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Poulsen, M.; Ryoo, J.H.; Peterson, B.S.; Bode, L.; Goran, M.I. Human milk oligosaccharide 2′-fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS ONE 2020, 15, e0228323. [Google Scholar] [CrossRef] [PubMed]
- Nickel, M.; Gu, C. Regulation of central nervous system myelination in higher brain functions. Neural Plast. 2018, 2018, 6436453. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, E.B.; Fischl, B.R.; Quinn, B.T.; Chong, W.K.; Gadian, D.G.; Lucas, A. Impact of Breast Milk on Intelligence Quotient, Brain Size, and White Matter Development. Pediatr. Res. 2010, 67, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Andres, A.; Pivik, R.; Cleves, M.; Snow, J.; Ding, Z.; Badger, T. Voxel-based morphometry and fMRI revealed differences in brain gray matter in breastfed and milk formula-fed children. Am. J. Neuroradiol. 2016, 37, 713–719. [Google Scholar] [CrossRef]
- Latora, V.; Marchiori, M. Economic small-world behavior in weighted networks. Eur. Phys. J. B 2003, 32, 249–263. [Google Scholar] [CrossRef]
- Cas, M.D.; Paroni, R.; Signorelli, P.; Mirarchi, A.; Cerquiglini, L.; Troiani, S.; Cataldi, S.; Codini, M.; Beccari, T.; Ghidoni, R.; et al. Human breast milk as source of sphingolipids for newborns: Comparison with infant formulas and commercial cow’s milk. J. Transl. Med. 2020, 18, 481. [Google Scholar] [CrossRef] [PubMed]
- Decsi, T.; Marosvölgyi, T.; Szabó, É. Docosahexaenoic Acid in Formulas for Term Infants: The Way from Pioneer Idea to Mandatory Dietary Recommendation. Life 2023, 13, 1326. [Google Scholar] [CrossRef] [PubMed]
- Moltu, S.J.; Strømmen, K.; Blakstad, E.W.; Almaas, A.N.; Westerberg, A.C.; Brække, K.; Rønnestad, A.; Nakstad, B.; Berg, J.P.; Veierød, M.B.; et al. Enhanced feeding in very-low-birth-weight infants may cause electrolyte disturbances and septicemia—A randomized, controlled trial. Clin. Nutr. 2013, 32, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, V.; Capozza, M.; Panza, R.; Laforgia, N.; Baldassarre, M.E. Macronutrients and Micronutrients in Parenteral Nutrition for Preterm Newborns: A Narrative Review. Nutrients 2022, 14, 1530. [Google Scholar] [CrossRef] [PubMed]
- Ben, X.-M. Nutritional management of newborn infants: Practical guidelines. World J. Gastroenterol. 2008, 14, 6133–6139. [Google Scholar] [CrossRef]

| Inclusion | Exclusions | Comments | |
|---|---|---|---|
| Population | Healthy infants and young children up to 2 years of age | Developmental disorders, childhood diseases, nutritional deficiencies, animal studies | Neuroimaging in older populations (2+ years of age) with a retrospective on infant diet were excluded |
| Intervention | Various diets including supplementation | Diets specifically combating a nutritional deficit inherent in the study population | Studies that supplemented either human milk or formula with compounds identified to be essential for neurodevelopment were included as well |
| Comparator | Any | No comparisons made | Any comparisons between different healthy diets were included |
| Outcome | Any outcome that can be quantitatively measured by any neuroimaging technique | Purely psychological or behavioral results | Psychological and behavioral outcomes have been assessed previously [1] |
| Studies | Original quantitative studies | Reviews, abstracts, expert opinions, letters to the editor |
| Study | Publication Year | Modality | Age Group | Number of Subjects per Diet | Major Reported Finding | Comments |
|---|---|---|---|---|---|---|
| Bouglé D [32] | 1999 | EEG, Auditory and visual ERPs | Preterm, EEG at TEA | 15 BF, 14 Formula with LCPUFAs, 11 Formula with short-chain PUFAs | No main effects of diet for either age. | N/A |
| Pivik RT [34] | 2007 | EEG, Language ERP | 3 and 6 months | 15 BF, 18 MF | No main effects of diet for either age. | Beginnings Study |
| Jing H [35] | 2007 | EEG, Language ERP | 3 and 6 months | 20 BF, 21 MF | No main effects of diet for either age. | Beginnings Study |
| Henriksen C [33] | 2008 | EEG, ERP related to memory | Preterm, EEG at 6 months | 68 Intervention (DHA and AA), 73 Controls | Infants in the intervention cohort had more negative amplitudes to repetitions of a standard image. | Both cohorts received 0.5 mL of study oil per 100 mL of human milk |
| Li J [36] | 2010 | EEG, Language ERP | 3 and 6 months | 40 BF, 51 MF, 39 SF | P350 amplitude: BF > FF at 3 months. N250 and P350 latencies: BF > SF | Beginnings Study |
| Jing H [37] | 2010 | EEG, Resting State | 3, 6, 9, and 12 months | 40 each BF, MF, SF | 0–3 Hz: FF > BF at 6 months, BF > FF at 9 months, 3–6 Hz: FF > BF at 6 months, 6–9 Hz: MF > BF at 3 months, MF > SF at 6 months; 12–30 Hz: BF > SF and MF > SF. | Beginnings Study |
| Pivik RT [38] | 2011 | EEG, Language ERP | 3 and 6 months | 75 BF, 88 MF, 76 SF | P350 amplitude: BF < SF to the standard syllable across sites at 6 months. | Beginnings Study |
| Pivik RT [39] | 2016 | EEG, Language ERP | 4 and 5 months | 36 BF, 31 MF, 35 SF | P170 Amplitude at 5 months: BF > SF for deviant stimulus; P350 Amplitude: SF > BF for deviant syllable, BF > SF for standard syllable at 4 months, SF < BF deviant and BF < SF standard at 5 months; P600 Amplitude: MF>SF for standard syllable at 4 months. | Beginnings Study |
| Pivik RT [40] | 2019 | EEG, Resting State | 6 months | 170 BF, 186 MF, 162 SF | Differences in gamma power (BF > SF and BF > MF) in two left-sided regions of the brain. | Beginnings Study |
| Alatorre-Cruz C [41] | 2023 | EEG, Language ERP | 3, 6, 9, 12, 24 months | 127 BF, 121 MF, 116 SF | Differences in P2 latency but not amplitude at 12 months (BF, MF > SF) at frontal left ROI and (SF > MF) at temporal right ROI. | Beginnings Study |
| Gilbreath D [42] | 2023 | EEG, Resting State | 2–6 months | ~100 BF, MF, and SF | Global beta and gamma were increased in BF vs. SF at 2 and 6 months, reflected in source modeling of frontal lobe. | Beginnings Study |
| Niu W [43] | 2020 | fMRI, Global Efficiency | Preterm, 40 weeks | 30 BF, 20 FF | BF infants exhibited greater global efficiency in comparison to FF. | N/A |
| Deoni SC [53] | 2013 | MRI (mcDESPOT) VFm | 10 months to 4 years | 85 BF, 38 FF, 51 combined BF and FF | Exclusively BF infants had greater VFm in the frontal regions of the brain, formula-fed groups had increased VFm in right optic radiation and occipital lobe. | N/A |
| Strømmen K [44] | 2015 | MRI, DTI | Preterm, MRI at TEA | 14 Enhanced Nutrition (more calories, amino acids, lipids, fatty acids, and vitamin A), 11 Controls | Enhanced nutrition groups had lower MD values in the cingulum, corticospinal tract, superior longitudinal fasciculi, and uncinate fasciculi. | A significantly higher occurrence of late-onset septicemia was observed in the intervention group |
| Vasu V [45] | 2014 | MRI (Volumes), CAVT | Preterm, MRI at TEA | MRI: 19 preterm, 19 term; CAVT 20 preterm, 13 term | Total human milk intake did not influence brain volumes, but did have a positive correlation with CAVT score. | Macronutrient and human milk intake were calculated through medical records |
| Beauport L [46] | 2017 | MRI (Lesions) | Preterm, MRI at TEA | 42 infants, diets assessed by specific nutrient contents | Increased calories and lipids during the first 2 weeks of life resulted in a reduced risk of a severely abnormal MRI. | Macronutrient and human milk intake were calculated through medical records |
| Coviello C [47] | 2018 | MRI (Volumes), DTI (Microstructure) | Preterm, MRI at TEA | 103, grouped by protein, fat, and caloric intake | Protein, fat, and calorie intake were positively correlated with cerebellar volume. Calorie, protein, and fat intakes were positively associated with FA in the PLIC. | Macronutrient and human milk intake were calculated through medical records |
| Deoni S [3] | 2018 | MRI, mcDESPOT (Myelination) | 3 months to 5 years | 62 BF, 88 FF (21 A, 28 B, and 39 C) | FF groups had an increased MWF before 1 year, a slower MWF between 1 and 2 years compared to BF. | BAMBAM study. Formulas B and C had higher DHA, ARA, choline, and sphingolipids than Formula A. |
| Power V [48] | 2019 | MRI (Volumes) | Preterm, MRI at TEA | 81, diets were assessed by protein, fat, and carbohydrate intake | No relationship between nutrition intake and brain volumes. | 90% of infants were in line with carbohydrate and fat recommendations; only 3.4% were for protein |
| Blesa M [49] | 2019 | sMRI/dMRI; ACT | Preterm, MRI at TEA | 27 BF > 75% of time pre-study, 20 BF < 75% of time pre-study | Infants who were BF for longer had increased FA-weighted connectivity and FA in white matter tracts. No differences in global networks or brain volumes. | N/A |
| Schneider N [54] | 2019 | MRI (Volumes), mcDESPOT (Myelination) | Birth to 2 years | 39 Product A, 28 Product B, 21 Product C | At 1–12 Months: No significant differences. At 12–24 months: Higher SM is associated with more myelin content in the bilateral cerebellum, occipital lobe, visual cortex, internal capsule, parietal lobe, and motor cortices. | BAMBAM study. Minimum time on diet is 3 months, no maximum or total time on diet provided |
| Ottolini KM [57] | 2020 | MRI (Volumes), DTI (Microstructure) | Preterm, MRI at TEA | 44 BF, 24 FF | BF infants had larger total brain volumes, regional brain volumes (amygdala-hippocampus and cerebellum), and greater regional white matter microstructure organization in the corpus callosum, internal capsule, and cerebellum. | N/A |
| Hortensius LM [50] | 2021 | MRI, DTI (Microstructure) | Preterm, DTI at TEA | 62 cohort A, 61 cohort B (higher protein and calories) | Cohort B had higher FA multiple white matter tracts; this effect is most associated with the increase in protein (FA was not associated with lipids or calories). | N/A |
| Berger P [55] | 2022 | MRI, ASL (rCBF), DTI (Microstructure) | MRI at 1 month | 20 mother-infant dyads | Differences in HMO exposure resulted in differences in FA, MD, and rCBF. Certain HMOs are more associated with optimal white matter development. | Human milk from each mother was analyzed for concentrations of candidate HMOs |
| Zhang Y [51] | 2022 | MRI (Volumes) and fMRI | Preterm, MRI at TEA | 34 BF, 22 FF | BF infants had increased regional gray matter development and function compared with FF infants. | N/A |
| Sullivan G [52] | 2023 | MRI (Volumes), DTI (Microstructure) | Preterm, MRI at TEA | 67 BF > 75% of time, 68 BF < 75% of time, 77 term-born infants (controls) | Infants who were BF for longer had lower relative cortical gray matter volume and higher mean cortical FA than infants who were BF a shorter time, and had similar FA to controls. | N/A |
| Schneider N [56] | 2023 | MRI (Volumes), mcDESPOT (Myelination), DTI (Microstructure) | Birth to 2 years | 108 BF, 42 Investigational (increased DHA, AA, B12, folic acid, iron, and SM), 39 Control | Higher myelination was observed in the investigational compared to the control group at 6, 12, 18, and 24 months, higher gray matter volume at 24 months, no differences at any age for WM volumes. | Full comparisons to the BF reference group were not reported |
| First Author, Year of Publication | Primary Research QCC | 1. Was the Research Question Clearly Stated? | 2. Was the Selection of Study Subjects Bias-Free? | 3. Were the Study Groups Comparable? | 4. Was Method of Handling Withdrawals Described? | 5. Was Blinding Used to Prevent Introduction of Bias? | 6. Were Intervention Factors and Any Comparison(s) Described? | 7. Were Outcomes Clearly Defined and the Measurements Valid? | 8. Was the Statistical Analysis Appropriate? | 9. Were Conclusions Supported by Results Considering Biases and Limitations? | 10. Is Bias Due to Study’s Funding Unlikely? | Overall Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bouglé D, 1999 [32] | Y | Y | Y | Y | Y | Y | Y | Y | Y | NA | (+) | |
| Pivik RT, 2007 [34] | Y | Y | Y | Y | Y | Y | Y | N | N | Y | (Ø) | |
| Jing H, 2007 [35] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | (+) | |
| Henriksen C, 2008 [33] | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | (+) | |
| Li J, 2010 [36] | Y | Y | Y | N | Y | Y | N | Y | Y | Y | (Ø) | |
| Jing H, 2010 [37] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | (+) | |
| Pivik RT, 2011 [38] | Y | Y | Y | N | Y | Y | Y | N | Y | Y | (+) | |
| Pivik RT, 2016 [39] | Y | Y | Y | N | Y | Y | Y | N | Y | Y | (+) | |
| Pivik RT, 2019 [40] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | (+) | |
| Alatorre-Cruz C, 2023 [41] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | (+) | |
| Gilbreath D, 2023 [42] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | (+) | |
| Niu W, 2020 [43] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | (+) | |
| Deoni SC, 2013 [53] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | (+) | |
| StØmmen K, 2015 [44] | Y | Y | Y | Y | Y | Y | N | Y | N | NA | (Ø) | |
| Vasu V, 2014 [45] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | (+) | |
| Beauport L, 2017 [46] | Y | Y | Y | Y | Y | Y | Y | Y | Y | NA | (+) | |
| Coviello C, 2018 [47] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | (+) | |
| Deoni S, 2018 [3] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | (+) | |
| Power V, 2019 [48] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | (+) | |
| Blesa M, 2019 [49] | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | (+) | |
| Schneider N, 2019 [54] | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | (+) | |
| Ottolini KM, 2020 [57] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | (+) | |
| Hortensius LM, 2021 [50] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | (+) | |
| Berger P, 2022 [55] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | (+) | |
| Zhang Y, 2022 [51] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | (+) | |
| Sullivan G, 2023 [52] | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | (+) | |
| Schneider N, 2023 [56] | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | (+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilbreath, D.; Hagood, D.; Larson-Prior, L. A Systematic Review over the Effect of Early Infant Diet on Neurodevelopment: Insights from Neuroimaging. Nutrients 2024, 16, 1703. https://doi.org/10.3390/nu16111703
Gilbreath D, Hagood D, Larson-Prior L. A Systematic Review over the Effect of Early Infant Diet on Neurodevelopment: Insights from Neuroimaging. Nutrients. 2024; 16(11):1703. https://doi.org/10.3390/nu16111703
Chicago/Turabian StyleGilbreath, Dylan, Darcy Hagood, and Linda Larson-Prior. 2024. "A Systematic Review over the Effect of Early Infant Diet on Neurodevelopment: Insights from Neuroimaging" Nutrients 16, no. 11: 1703. https://doi.org/10.3390/nu16111703
APA StyleGilbreath, D., Hagood, D., & Larson-Prior, L. (2024). A Systematic Review over the Effect of Early Infant Diet on Neurodevelopment: Insights from Neuroimaging. Nutrients, 16(11), 1703. https://doi.org/10.3390/nu16111703






