What Can the Gut Microbiota of Animals Teach Us about the Relationship between Nutrition and Burden of Lifestyle Diseases?
Abstract
:1. Introduction
2. The Emergence of an Industrialized Microbiota
3. What Does the Natural World Tell Us about an Industrialized Microbiota?
4. Insights Gleaned from Studying the Gut Microbiota of Specific Animals
5. Gut Microbiota in Captive versus Wild Animals
6. Gut Microbiota Changes Due to Changes in the Environment
7. Impact of the SARS-CoV-2 Pandemia on Gut Microbiota
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- O’Toole, P.W.; Shiels, P.G. The Role of the Microbiota in Sedentary Lifestyle Disorders and Ageing: Lessons from the Animal Kingdom. J. Intern. Med. 2020, 287, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Shiels, P.G.; Painer, J.; Natterson-Horowitz, B.; Johnson, R.J.; Miranda, J.J.; Stenvinkel, P. Manipulating the Exposome to Enable Better Ageing. Biochem. J. 2021, 478, 2889–2898. [Google Scholar] [CrossRef] [PubMed]
- Mafra, D.; Borges, N.A.; Lindholm, B.; Shiels, P.G.; Evenepoel, P.; Stenvinkel, P. Food as Medicine: Targeting the Uraemic Phenotype in Chronic Kidney disease. Nat. Rev. Nephrol. 2021, 17, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Painer, J.; Kuro-O, M.; Lanaspa, M.; Arnold, W.; Ruf, T.; Shiels, P.G.; Johnson, R.J. Novel Treatment Strategies for Chronic Kidney Disease: Insights from the Animal Kingdom. Nat. Rev. Nephrol. 2018, 14, 265–284. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.F.V.; Bogatyreva, O.A.; Bogatyrev, N.R.; Bowyer, A.; Pahl, A.-K. Biomimetics: Its Practice and Theory. J. R. Soc. Interface 2006, 3, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P. The One Health concept—The Health of Humans Is Intimately Linked with the Health of Animals and a Sustainable Environment. J. Intern. Med. 2020, 287, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Shiels, P.G.; McGuinness, D.; Eriksson, M.; Kooman, J.P.; Stenvinkel, P. The Role of Epigenetics in Renal Ageing. Nat. Rev. Nephrol. 2017, 13, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Meyer, C.J.; Block, G.A.; Chertow, G.M.; Shiels, P.G. Understanding the Role of the Cytoprotective Transcription Factor Nuclear Factor Erythroid 2-Related Factor 2—Lessons from Evolution, the Animal Kingdom and Rare Progeroid Syndromes. Nephrol. Dial. Transplant. 2020, 35, 2036–2045. [Google Scholar] [CrossRef]
- Chiu, K.; Warner, G.; Nowak, R.A.; A Flaws, J.A.; Mei, W. The Impact of Environmental Chemicals on the Gut Microbiome. Toxicol. Sci. 2020, 176, 253–284. [Google Scholar] [CrossRef]
- Vanhaecke, T.; Bretin, O.; Poirel, M.; Tap, J. Drinking Water Source and Intake Are Associated with Distinct Gut Microbiota Signatures in US and UK Populations. J. Nutr. 2022, 152, 171–182. [Google Scholar] [CrossRef]
- Avesani, C.M.; Cardozo, L.F.; Wang, A.Y.-M.; Shiels, P.G.; Lambert, K.; Lindholm, B.; Stenvinkel, P.; Mafra, D. Planetary Health, Nutrition, and Chronic Kidney Disease: Connecting the Dots for a Sustainable Future. J. Ren. Nutr. 2023, 33, S40–S48. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Sonnenburg, E.D. Vulnerability of the Industrialized Microbiota. Science 2019, 366, eaaw9255. [Google Scholar] [CrossRef] [PubMed]
- Schnorr, S.L.; Candela, M.; Rampelli, S.; Centanni, M.; Consolandi, C.; Basaglia, G.; Turroni, S.; Biagi, E.; Peano, C.; Severgnini, M.; et al. Gut Microbiome of the Hadza Hunter-Gatherers. Nat. Commun. 2014, 5, 3654. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.S.; Shanahan, F.; O’Toole, P.W. The Gut Microbiome as a Modulator of Healthy Ageing. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 565–584. [Google Scholar] [CrossRef] [PubMed]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut-microbiota-targeted diets modulate human immune status. Cell 2021, 184, 4137–4153.e14. [Google Scholar] [CrossRef] [PubMed]
- Lamas, B.; Evariste, L.; Houdeau, E. Dysregulation Along the Gut Microbiota-Immune System Axis after Oral Exposure to Titanium Dioxide Nanoparticles: A Possible Environmental Factor Promoting Obesity-Related Metabolic Disorders. Environ. Pollut. 2023, 330, 121795. [Google Scholar] [CrossRef] [PubMed]
- Fragiadakis, G.K.; Smits, S.A.; Sonnenburg, E.D.; Van Treuren, W.; Reid, G.; Knight, R.; Manjurano, A.; Changalucha, J.; Dominguez-Bello, M.G.; Leach, J.; et al. Links between environment, diet, and the hunter-gatherer microbiome. Gut Microbes 2019, 10, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Rook, G.A.W.; Lowry, C.A.; Raison, C.L. Microbial ‘Old Friends’, immunoregulation and stress resilience. Evol. Med. Public Health 2013, 2013, 46–64. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Zuo, T.; Kamm, M.A.; Colombel, J.-F.; Ng, S.C. Urbanization and the gut microbiota in health and inflammatory bowel disease. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Pehrsson, E.C.; Blaser, M.J.; Sandhu, K.; Gao, Z.; Wang, B.; Magris, M.; Hidalgo, G.; Contreras, M.; Noya-Alarcón, Ó.; et al. The microbiome of uncontacted Amerindians. Sci. Adv. 2015, 1, e1500183. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, N.; Carlsen, B.; Christensen, M.H.; Pertoldi, C.; Nielsen, J.L. The Gut Microbiome of 54 Mammalian Species. Front. Microbiol. 2022, 13, 886252. [Google Scholar] [CrossRef] [PubMed]
- Przybyło, M.; Hummel, J.; Ortmann, S.; Codron, D.; Kohlschein, G.-M.; Kilga, D.; Smithyman, J.; Przybyło, U.; Świerk, S.; Hammer, S.; et al. Digesta passage in nondomestic ruminants: Separation mechanisms in ‘moose-type’ and ‘cattle-type’ species, and seemingly atypical browsers. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 235, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.H.; Sanders, J.G. Roles of the gut microbiota in the adaptive evolution of mammalian species. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190597. [Google Scholar] [CrossRef] [PubMed]
- Moeller, A.H.; Li, Y.; Ngole, E.M.; Ahuka-Mundeke, S.; Lonsdorf, E.V.; Pusey, A.E.; Peeters, M.; Hahn, B.H.; Ochman, H. Rapid changes in the gut microbiome during human evolution. Proc. Natl. Acad. Sci. USA 2014, 111, 16431–16435. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.B.; Vangay, P.; Huang, H.; Ward, T.; Hillmann, B.M.; Al-Ghalith, G.A.; Travis, D.A.; Long, H.T.; Van Tuan, B.; Van Minh, V.; et al. Captivity humanizes the primate microbiome. Proc. Natl. Acad. Sci. USA 2016, 113, 10376–10381. [Google Scholar] [CrossRef] [PubMed]
- Hale, L.; Feng, W.; Yin, H.; Guo, X.; Zhou, X.; Bracho, R.; Pegoraro, E.; Penton, C.R.; Wu, L.; Cole, J.; et al. Tundra microbial community taxa and traits predict decomposition parameters of stable, old soil organic carbon. ISME J. 2019, 13, 2901–2915. [Google Scholar] [CrossRef] [PubMed]
- Kohl, K.D.; Oakeson, K.F.; Orr, T.J.; Miller, A.W.; Forbey, J.S.; Phillips, C.D.; Dale, C.; Weiss, R.B.; Dearing, M.D. Metagenomic sequencing provides insights into microbial detoxification in the guts of small mammalian herbivores (Neotoma spp.). FEMS Microbiol. Ecol. 2018, 94, fiy184. [Google Scholar] [CrossRef]
- Amato, K.R.; Leigh, S.R.; Kent, A.; Mackie, R.I.; Yeoman, C.J.; Stumpf, R.M.; Wilson, B.A.; Nelson, K.E.; White, B.A.; Garber, P.A. The Gut Microbiota Appears to Compensate for Seasonal Diet Variation in the Wild Black Howler Monkey (Alouatta pigra). Microb. Ecol. 2015, 69, 434–443. [Google Scholar] [CrossRef]
- Groussin, M.; Mazel, F.; Sanders, J.G.; Smillie, C.S.; Lavergne, S.; Thuiller, W.; Alm, E.J. Unraveling the processes shaping mammalian gut microbiomes over evolutionary time. Nat. Commun. 2017, 8, 14319. [Google Scholar] [CrossRef]
- Sommer, F.; Ståhlman, M.; Ilkayeva, O.; Arnemo, J.M.; Kindberg, J.; Josefsson, J.; Newgard, C.B.; Fröbert, O.; Bäckhed, F. The Gut Microbiota Modulates Energy Metabolism in the Hibernating Brown Bear Ursus arctos. Cell Rep. 2016, 14, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Debebe, T.; Biagi, E.; Soverini, M.; Holtze, S.; Hildebrandt, T.B.; Birkemeyer, C.; Wyohannis, D.; Lemma, A.; Brigidi, P.; Savkovic, V.; et al. Unraveling the gut microbiome of the long-lived naked mole-rat. Sci. Rep. 2017, 7, 9590. [Google Scholar] [CrossRef]
- Xiao, G.; Liu, S.; Xiao, Y.; Zhu, Y.; Zhao, H.; Li, A.; Li, Z.; Feng, J. Seasonal Changes in Gut Microbiota Diversity and Composition in the Greater Horseshoe Bat. Front. Microbiol. 2019, 10, 2247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, W.; Hou, R.; Zhang, L.; Schmitz-Esser, S.; Sun, H.; Xie, J.; Zhang, Y.; Wang, C.; Li, L.; et al. Age-associated microbiome shows the giant panda lives on hemicelluloses, not on cellulose. ISME J. 2018, 12, 1319–1328. [Google Scholar] [CrossRef]
- Deng, F.; Wang, C.; Li, D.; Peng, Y.; Deng, L.; Zhao, Y.; Zhang, Z.; Wei, M.; Wu, K.; Zhao, J.; et al. The unique gut microbiome of giant pandas involved in protein metabolism contributes to the host’s dietary adaption to bamboo. Microbiome 2023, 11, 180. [Google Scholar] [CrossRef]
- Ilmberger, N.; Güllert, S.; Dannenberg, J.; Rabausch, U.; Torres, J.; Wemheuer, B.; Alawi, M.; Poehlein, A.; Chow, J.; Turaev, D.; et al. A Comparative Metagenome Survey of the Fecal Microbiota of a Breast- and a Plant-Fed Asian Elephant Reveals an Unexpectedly High Diversity of Glycoside Hydrolase Family Enzymes. PLoS ONE 2014, 9, e106707. [Google Scholar] [CrossRef]
- Klinhom, S.; Sriwichaiin, S.; Kerdphoo, S.; Khonmee, J.; Chattipakorn, N.; Chattipakorn, S.C.; Thitaram, C. Characteristics of gut microbiota in captive Asian elephants (Elephas maximus) from infant to elderly. Sci. Rep. 2023, 13, 23027. [Google Scholar] [CrossRef]
- Ning, Y.; Qi, J.; Dobbins, M.T.; Liang, X.; Wang, J.; Chen, S.; Ma, J.; Jiang, G. Comparative Analysis of Microbial Community Structure and Function in the Gut of Wild and Captive Amur Tiger. Front. Microbiol. 2020, 11, 1665. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, W.; Su, L.; Huang, M.; Lin, L.; Su, Q.; Li, G.; Ahmad, H.I.; Li, L.; Zhang, X.; et al. Impact of host intraspecies genetic variation, diet, and age on bacterial and fungal intestinal microbiota in tigers. Microbiologyopen 2020, 9, e1050. [Google Scholar] [CrossRef]
- Schmidt, J.M.; Henken, S.; Dowd, S.E.; McLaughlin, R.W. Analysis of the Microbial Diversity in the Fecal Material of Giraffes. Curr. Microbiol. 2018, 75, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Wang, X.; Bernstein, S.; Huffman, M.A.; Xia, D.-P.; Gu, Z.; Chen, R.; Sheeran, L.K.; Wagner, R.S.; Li, J. Marked variation between winter and spring gut microbiota in free-ranging Tibetan Macaques (Macaca thibetana). Sci. Rep. 2016, 6, 26035. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yao, Y.; Dong, M.; Xia, T.; Li, D.; Xie, M.; Wu, J.; Wen, A.; Wang, Q.; Zhu, G.; et al. Characterisation of the gut microbial community of rhesus macaques in high-altitude environments. BMC Microbiol. 2020, 20, 68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, D.; Wang, L.; Hao, J.; Wang, J.; Zhou, X.; Wang, W.; Qiu, Q.; Huang, X.; Zhou, J.; et al. Convergent Evolution of Rumen Microbiomes in High-Altitude Mammals. Curr. Biol. 2016, 26, 1873–1879. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qu, J.; Li, T.; Wirth, S.; Zhang, Y.; Zhao, X.; Li, X. Diet simplification selects for high gut microbial diversity and strong fermenting ability in high-altitude pikas. Appl. Microbiol. Biotechnol. 2018, 102, 6739–6751. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, R.; Yu, Q.; Feng, T.; Li, H. Gut microbiome adaptation to extreme cold winter in wild plateau pika (Ochotona curzoniae) on the Qinghai-Tibet Plateau. FEMS Microbiol. Lett. 2020, 367, fnaa134. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.M.; Nguyen, B.N.; Neumann, L.M.; Miller, M.; Buss, P.; Daniels, S.; Ahn, M.J.; Crandall, K.A.; Pukazhenthi, B. Gut microbiome differences between wild and captive black rhinoceros—Implications for rhino health. Sci. Rep. 2019, 9, 7570. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.-S.; Liang, X.-X.; Yang, M.-Y.; Wang, T.-T.; Chen, J.-P.; Du, W.-G.; Li, H.; Sun, B.-J. Captivity Influences Gut Microbiota in Crocodile Lizards (Shinisaurus crocodilurus). Front. Microbiol. 2020, 11, 550. [Google Scholar] [CrossRef]
- Song, X.; Zhang, J.; Song, J.; Zhai, Y. Decisive Effects of Life Stage on the Gut Microbiota Discrepancy Between Two Wild Populations of Hibernating Asiatic Toads (Bufo gargarizans). Front. Microbiol. 2021, 12, 665849. [Google Scholar] [CrossRef]
- Chua, K.O.; Fatima, I.; Lau, Y.Y.; Hong, K.W.; Yin, W.F.; Mardaryev, A.; Chan, K.G.; Chang, C.Y. Bacterial Microbiome of Faecal Samples of Naked Mole-Rat Collected from the Toilet Chamber. BMC Res. Notes 2022, 15, 107. [Google Scholar] [CrossRef]
- Debebe, T.; Holtze, S.; Morhart, M.; Hildebrandt, T.B.; Rodewald, S.; Huse, K.; Platzer, M.; Wyohannes, D.; Yirga, S.; Lemma, A.; et al. Analysis of cultivable microbiota and diet intake pattern of the long-lived naked mole-rat. Gut Pathog. 2016, 8, 25. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.-S.; Soundrarajan, N.; Le Van Chanh, Q.; Jeon, H.; Cha, S.-Y.; Kang, M.; Ahn, B.Y.; Hong, K.; Song, H.; Kim, J.-H.; et al. The novel cathelicidin of naked mole rats, Hg-CATH, showed potent antimicrobial activity and low cytotoxicity. Gene 2018, 676, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Shebzukhov, Y.; Holtze, S.; Hirseland, H.; Schäfer, H.; Radbruch, A.; Hildebrandt, T.; Grützkau, A. Identification of cross-reactive antibodies for the detection of lymphocytes, myeloid cells and haematopoietic precursors in the naked mole rat. Eur. J. Immunol. 2019, 49, 2103–2110. [Google Scholar] [CrossRef] [PubMed]
- Braude, S.; Holtze, S.; Begall, S.; Brenmoehl, J.; Burda, H.; Dammann, P.; del Marmol, D.; Gorshkova, E.; Henning, Y.; Hoeflich, A.; et al. Surprisingly long survival of premature conclusions about naked mole-rat biology. Biol. Rev. Camb. Philos. Soc. 2021, 96, 376–393. [Google Scholar] [CrossRef] [PubMed]
- Abegglen, L.M.; Caulin, A.F.; Chan, A.; Lee, K.; Robinson, R.; Campbell, M.S.; Kiso, W.K.; Schmitt, D.L.; Waddell, P.J.; Bhaskara, S.; et al. Potential Mechanisms for Cancer Resistance in Elephants and Comparative Cellular Response to DNA Damage in Humans. JAMA 2015, 314, 1850–1860. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.M.; Olm, M.R.; Merrill, B.D.; Dahan, D.; Tripathi, S.; Spencer, S.P.; Yu, F.B.; Jain, S.; Neff, N.; Jha, A.R.; et al. Ultra-deep sequencing of Hadza hunter-gatherers recovers vanishing gut microbes. Cell 2023, 186, 3111–3124.e13. [Google Scholar] [CrossRef] [PubMed]
- Lobel, L.; Cao, Y.G.; Fenn, K.; Glickman, J.N.; Garrett, W.S. Diet posttranslationally modifies the mouse gut microbial proteome to modulate renal function. Science 2020, 369, 1518–1524. [Google Scholar] [CrossRef]
- Viltard, M.; Durand, S.; Pérez-Lanzón, M.; Aprahamian, F.; Lefevre, D.; Leroy, C.; Madeo, F.; Kroemer, G.; Friedlander, G. The metabolomic signature of extreme longevity: Naked mole rats versus mice. Aging 2019, 11, 4783–4800. [Google Scholar] [CrossRef] [PubMed]
- Cong, W.; Xing, J.; Feng, Y.; Wang, J.; Fu, R.; Yue, B.; He, Z.; Lin, L.; Yang, W.; Cheng, J.; et al. The microbiota in the intestinal and respiratory tracts of naked mole-rats revealed by high-throughput sequencing. BMC Microbiol. 2018, 18, 89. [Google Scholar] [CrossRef]
- Zhao, S.; Lin, L.; Kan, G.; Xu, C.; Tang, Q.; Yu, C.; Sun, W.; Cai, L.; Xu, C.; Cui, S. High Autophagy in the Naked Mole Rat may Play a Significant Role in Maintaining Good Health. Cell. Physiol. Biochem. 2014, 33, 321–332. [Google Scholar] [CrossRef]
- Triplett, J.C.; Tramutola, A.; Swomley, A.; Kirk, J.; Grimes, K.; Lewis, K.; Orr, M.; Rodriguez, K.; Cai, J.; Klein, J.B.; et al. Age-related changes in the proteostasis network in the brain of the naked mole-rat: Implications promoting healthy longevity. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 2213–2224. [Google Scholar] [CrossRef]
- Park, T.J.; Reznick, J.; Peterson, B.L.; Blass, G.; Omerbašić, D.; Bennett, N.C.; Kuich, P.H.J.L.; Zasada, C.; Browe, B.M.; Hamann, W.; et al. Fructose-driven glycolysis supports anoxia resistance in the naked mole-rat. Science 2017, 356, 307–311. [Google Scholar] [CrossRef]
- Singhal, R.; Shah, Y.M. Oxygen battle in the gut: Hypoxia and hypoxia-inducible factors in metabolic and inflammatory responses in the intestine. J. Biol. Chem. 2020, 295, 10493–10505. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, H.; Xu, T.; Hu, Y.; Jiang, Y. Acute exposure to simulated high-altitude hypoxia alters gut microbiota in mice. Arch. Microbiol. 2022, 204, 412. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Zhang, H.; Wei, Q.; Zhao, C.; Yang, X.; Wu, X.; Xia, T.; Liu, G.; Zhang, L.; Gao, Y.; et al. Comparative Analyses of Fecal Microbiota in European Mouflon (Ovis orientalis musimon) and Blue Sheep (Pseudois nayaur) Living at Low or High Altitudes. Front. Microbiol. 2019, 10, 1735. [Google Scholar] [CrossRef]
- Li, L.; Zhao, X. Comparative analyses of fecal microbiota in Tibetan and Chinese Han living at low or high altitude by barcoded 454 pyrosequencing. Sci. Rep. 2015, 5, 14682. [Google Scholar] [CrossRef]
- De Cuyper, A.; Meloro, C.; Abraham, A.J.; Müller, D.W.H.; Codron, D.; Janssens, G.P.J.; Clauss, M. The uneven weight distribution between predators and prey: Comparing gut fill between terrestrial herbivores and carnivores. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2020, 243, 110683. [Google Scholar] [CrossRef] [PubMed]
- Goodrich, J.K.; Davenport, E.R.; Beaumont, M.; Jackson, M.A.; Knight, R.; Ober, C.; Spector, T.D.; Bell, J.T.; Clark, A.G.; Ley, R.E. Genetic Determinants of the Gut Microbiome in UK Twins. Cell Host Microbe 2016, 19, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, R.; Zhong, Y.; Liu, X.; Deng, W.; Huang, X.; Price, M.; Li, J. Age-related alterations in metabolome and microbiome provide insights in dietary transition in giant pandas. mSystems 2023, 8, e0025223. [Google Scholar] [CrossRef]
- Cao, H.; Xu, M.; Dong, W.; Deng, B.; Wang, S.; Zhang, Y.; Wang, S.; Luo, S.; Wang, W.; Qi, Y.; et al. Secondary bile acid-induced dysbiosis promotes intestinal carcinogenesis. Int. J. Cancer 2017, 140, 2545–2556. [Google Scholar] [CrossRef]
- Barrasa, J.I.; Olmo, N.; Pérez-Ramos, P.; Santiago-Gómez, A.; Lecona, E.; Turnay, J.; Lizarbe, M.A. Deoxycholic and chenodeoxycholic bile acids induce apoptosis via oxidative stress in human colon adenocarcinoma cells. Apoptosis 2011, 16, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xu, B.; Huang, Z.; Lu, T. Metagenomic Analysis of the Fecal Microbiomes of Wild Asian Elephants Reveals Microflora and Enzymes that Mainly Digest Hemicellulose. J. Microbiol. Biotechnol. 2019, 29, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Millette, J.B.; Sauther, M.L.; Cuozzo, F.P.; Ness, J.L. The impact of dental impairment on ring-tailed lemur food processing performance. Am. J. Phys. Anthr. 2012, 148, 238–248. [Google Scholar] [CrossRef]
- Nagpal, R.; Mainali, R.; Ahmadi, S.; Wang, S.; Singh, R.; Kavanagh, K.; Kitzman, D.W.; Kushugulova, A.; Marotta, F.; Yadav, H. Gut microbiome and aging: Physiological and mechanistic insights. Nutr. Healthy Aging 2018, 4, 267–285. [Google Scholar] [CrossRef]
- Gani, M.; Mohd-Ridwan, A.R.; Sitam, F.T.; Kamarudin, Z.; Selamat, S.S.; Awang, N.M.Z.; Karuppannan, K.V.; Md-Zain, B.M. Habitat shapes the gut microbiome diversity of Malayan tigers (Panthera tigris jacksoni) as revealed through metabarcoding 16S rRNA profiling. World J. Microbiol. Biotechnol. 2024, 40, 111. [Google Scholar] [CrossRef]
- Zepeda-Rivera, M.; Minot, S.S.; Bouzek, H.; Wu, H.; Blanco-Míguez, A.; Manghi, P.; Jones, D.S.; LaCourse, K.D.; Wu, Y.; McMahon, E.F.; et al. A distinct Fusobacterium nucleatum clade dominates the colorectal cancer niche. Nature 2024, 628, 424–432. [Google Scholar] [CrossRef]
- He, F.; Liu, D.; Zhai, J.; Zhang, L.; Ma, Y.; Xu, Y.; Rong, K.; Ma, J. Metagenomic analysis revealed the effects of goat milk feeding and breast feeding on the gut microbiome of Amur tiger cubs. Biochem. Biophys. Res. Commun. 2018, 503, 2590–2596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liao, Y.; Qin, T.; Ma, J.; Liu, J.; Zou, J.; Huang, H.; Zhong, X.; Yang, M. Developmental stage variation in the gut microbiome of South China tigers. Front. Microbiol. 2022, 13, 962614. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Han, Z.; Wang, H.; Liu, C.; Si, H.; Xu, C. Adaptation of the Gut Microbiota of Amur Tigers to a Special Diet. Curr. Microbiol. 2021, 78, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Guan, Y.; Dou, H.; Yang, H.; Yao, M.; Ge, J.; Feng, L. Comparison of the fecal microbiota of two free-ranging Chinese subspecies of the leopard (Panthera pardus) using high-throughput sequencing. PeerJ 2019, 7, e6684. [Google Scholar] [CrossRef]
- Brice, K.L.; Trivedi, P.; Jeffries, T.C.; Blyton, M.D.J.; Mitchell, C.; Singh, B.K.; Moore, B.D. The Koala (Phascolarctos cinereus) faecal microbiome differs with diet in a wild population. PeerJ 2019, 7, e6534. [Google Scholar] [CrossRef] [PubMed]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Blyton, M.D.J.; Pascoe, J.; Hynes, E.; Soo, R.M.; Hugenholtz, P.; Moore, B.D. The koala gut microbiome is largely unaffected by host translocation but rather influences host diet. Front. Microbiol. 2023, 14, 1085090. [Google Scholar] [CrossRef]
- Davies, T.J.; Benjamino, J.; Gogarten, J.P.; Graf, J.; Mielke, A.; Mundry, R.; Nelson, M.C.; Wittig, R.M.; Leendertz, F.H.; Calvignac-Spencer, S. Factors influencing bacterial microbiome composition in a wild non-human primate community in Taï National Park, Côte d’Ivoire. ISME J. 2018, 12, 2559–2574. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, V.J.; Song, S.J.; Delsuc, F.; Prest, T.L.; Oliverio, A.M.; Korpita, T.M.; Alexiev, A.; Amato, K.R.; Metcalf, J.L.; Kowalewski, M.; et al. The Effects of Captivity on the Mammalian Gut Microbiome. Integr. Comp. Biol. 2017, 57, 690–704. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, M.A.M.; Chel, H.M.; Thu, M.J.; Bawm, S.; Htun, L.L.; Win, M.M.; Oo, Z.M.; Ohsawa, N.; Lahdenperä, M.; Mohamed, W.M.A.; et al. Anthropogenic interferences lead to gut microbiome dysbiosis in Asian elephants and may alter adaptation processes to surrounding environments. Sci. Rep. 2021, 11, 741. [Google Scholar] [CrossRef]
- Liu, X.; Yu, J.; Huan, Z.; Xu, M.; Song, T.; Yang, R.; Zhu, W.; Jiang, J. Comparing the gut microbiota of Sichuan golden monkeys across multiple captive and wild settings: Roles of anthropogenic activities and host factors. BMC Genom. 2024, 25, 148. [Google Scholar] [CrossRef] [PubMed]
- Huus, K.E.; Ley, R.E. Blowing Hot and Cold: Body Temperature and the Microbiome. mSystems 2021, 6, e0070721. [Google Scholar] [CrossRef]
- Fumagalli, M.R.; Zapperi, S.; La Porta, C.A.M. Role of body temperature variations in bat immune response to viral infections. J. R. Soc. Interface 2021, 18, 20210211. [Google Scholar] [CrossRef]
- Almblad, H.; Randall, T.E.; Liu, F.; Leblanc, K.; Groves, R.A.; Kittichotirat, W.; Winsor, G.L.; Fournier, N.; Au, E.; Groizeleau, J.; et al. Bacterial cyclic diguanylate signaling networks sense temperature. Nat. Commun. 2021, 12, 1986. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Wang, D.-H. Gut Microbial Community and Host Thermoregulation in Small Mammals. Front. Physiol. 2022, 13, 888324. [Google Scholar] [CrossRef]
- Ebert, T.; Painer, J.; Bergman, P.; Qureshi, A.R.; Giroud, S.; Stalder, G.; Kublickiene, K.; Göritz, F.; Vetter, S.; Bieber, C.; et al. Insights in the regulation of trimetylamine N-oxide production using a comparative biomimetic approach suggest a metabolic switch in hibernating bears. Sci. Rep. 2020, 10, 20323. [Google Scholar] [CrossRef] [PubMed]
- Dill-McFarland, K.A.; Suen, G.; Carey, H.V. Bears Arouse Interest in Microbiota’s Role in Health. Trends Microbiol. 2016, 24, 245–246. [Google Scholar] [CrossRef]
- Gunawan, S.P.; Huang, S.-Y.; Wang, C.-C.; Huynh, L.B.P.; Nguyen, N.N.; Hsu, S.-Y.; Chen, Y.-C. Sleep deprivation alters pubertal timing in humans and rats: The role of the gut microbiome. Sleep 2024, 47, zsad308. [Google Scholar] [CrossRef]
- López-Roig, M.; Piera, E.; Serra-Cobo, J. Thinner bats to face hibernation as response to climate warming. Sci. Rep. 2024, 14, 2117. [Google Scholar] [CrossRef]
- Stenvinkel, P.; Painer, J.; Shiels, P.G.; Bansal, A.; Fereidouni, S.; Natterson-Horowitz, B.; Johnson, R.J.; Miranda, J.J. SARS-COV-2 and biomimetics: What saves the planet will save our health. J. Intern. Med. 2021, 289, 244–246. [Google Scholar] [CrossRef]
- Schlottau, K.; Rissmann, M.; Graaf, A.; Schön, J.; Sehl, J.; Wylezich, C.; Höper, D.; Mettenleiter, T.C.; Balkema-Buschmann, A.; Harder, T.; et al. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: An experimental transmission study. Lancet Microbe 2020, 1, e218–e225. [Google Scholar] [CrossRef]
- Missotten, J.A.M.; Michiels, J.; Degroote, J.; De Smet, S. Fermented liquid feed for pigs: An ancient technique for the future. J. Anim. Sci. Biotechnol. 2015, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Olukomaiya, O.; Fernando, C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Solid-state fermented plant protein sources in the diets of broiler chickens: A review. Anim. Nutr. 2019, 5, 319–330. [Google Scholar] [CrossRef]
- Wadman, M. Flawed interferon response spurs severe illness. Science 2020, 369, 1550–1551. [Google Scholar] [CrossRef] [PubMed]
- Elbanna, K.; El Hadad, S.; Assaeedi, A.; Aldahlawi, A.; Khider, M.; Alhebshi, A. In vitro and in vivo evidences for innate immune stimulators lactic acid bacterial starters isolated from fermented camel dairy products. Sci. Rep. 2018, 8, 12553. [Google Scholar] [CrossRef]
- Moran, N.A.; Yun, Y. Experimental replacement of an obligate insect symbiont. Proc. Natl. Acad. Sci. USA 2015, 112, 2093–2096. [Google Scholar] [CrossRef] [PubMed]
- Reshef, L.; Koren, O.; Loya, Y.; Zilber-Rosenberg, I.; Rosenberg, E. The Coral Probiotic Hypothesis. Environ. Microbiol. 2006, 8, 2068–2073. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, M.; Seneca, F.O.; Yum, L.K.; Palumbi, S.R.; Voolstra, C.R. Bacterial community dynamics are linked to patterns of coral heat tolerance. Nat. Commun. 2017, 8, 14213. [Google Scholar] [CrossRef]
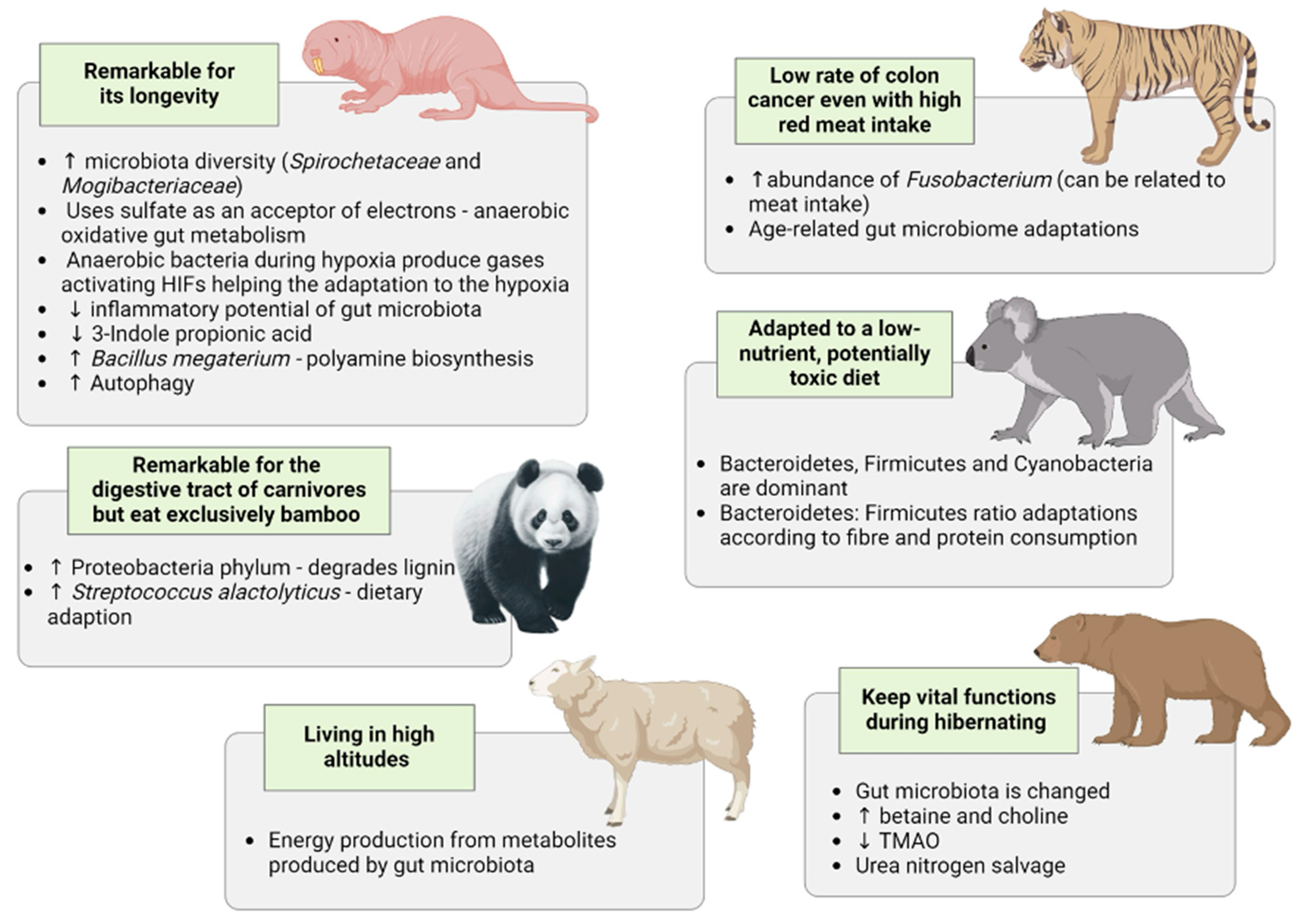
| References | Animals | Findings |
|---|---|---|
| Sommer et al. (2016) [32] |  Bear | fat gain and glucose tolerance compared microbiota from the winter. |
| Debebe et al. (2017) [33] |  Naked Mole rat | capacity to produce SCFA, mono-disaccharide enrichment of SCFA, and carbohydrate degradation products. |
| Xiao et al. (2019) [34] |  Bat | Proteobacteria when compared to humans. |
| Zhang et al. (2018) [35] Deng et al. (2023) [36] |  Panda | Large and diverse gene for hemicellulose hydrolysis. Proteobacteria. Streptococcus alactolyticus, affecting essential amino-acid biosynthesis. Primary to secondary bile acid conversion by gut microbiota in old pandas. |
| Ilmberger et al. (2014) [37] Klinhom et al. (2023) [38] |  Elephant | Bifidobacterium and Akkermansia. Firmicutes, Bacteroidetes, and Actinobacteria. Firmicutes → lignocellulose-degrading enzymes. |
| Ning et al. (2020) [39] Jiang et al. (2020) [40] |  Tiger | Firmicutes and Proteobacteria. Collinsella, dominant genus. Fusobacterium → related to meat intake. Captive and wild, different bacterial community. |
| Schmidt et al. (2018) [41] |  Giraffe | Giraffes from a zoo: Firmicutes and Bacteroidetes were the most abundant phyla, and Firmicutes, Bacteroides, and Spirochaetes represented the main phylum levels. |
| Amato et al. (2015) [30] |  Mexican black howler monkeys | Ruminococcaceae abundance in periods with low energy intake, producing more energy. genus Butyricicoccus with high unripe fruit intake. |
| Sun et al. (2016) [42] |  Tibetan Macaques | Genus Succinivibrio (degradation of cellulose and hemicellulose) in the winter. Genus Prevotella (degradation of carbohydrates) in the spring. |
| Wu et al. (2020) [43] |  Rhesus | In alpha diversity and a high Firmicutes/Bacteroidetes ratio. Abundance Christensenellaceae, Ruminococcaceae (Firmicutes). |
| Zhang et al. (2016) [44] |  Sheep | methane production. |
| Li et al. (2018) [45] Wang et al. (2020) [46] |  Plateau Pika | Prevotella alpha diversity. Cellulase activity in the summer. |
| Gibson et al. (2019) [47] |  Rhino | Proteobacteria dominate the phyla in wild rhinos, followed by Bacteroidetes. |
| Tang et al. (2020) [48] |  Crocodile | Firmicutes and Proteobacteria are the dominant phyla. |
| Song et al. (2021) [49] |  Asiatic Toad | Proteobacteria is the dominant phyla during hibernation (especially Pseudomonas). |
| Moeller and Sanders (2020) [25] |  Koala | Bacteroidetes, Firmicutes, and Cyanobacteria are the dominant phyla.Koala and gut microbiota are co-adapted to a low-nutrient potentially toxic diet. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mafra, D.; Borges, N.A.; Baptista, B.G.; Martins, L.F.; Borland, G.; Shiels, P.G.; Stenvinkel, P. What Can the Gut Microbiota of Animals Teach Us about the Relationship between Nutrition and Burden of Lifestyle Diseases? Nutrients 2024, 16, 1789. https://doi.org/10.3390/nu16111789
Mafra D, Borges NA, Baptista BG, Martins LF, Borland G, Shiels PG, Stenvinkel P. What Can the Gut Microbiota of Animals Teach Us about the Relationship between Nutrition and Burden of Lifestyle Diseases? Nutrients. 2024; 16(11):1789. https://doi.org/10.3390/nu16111789
Chicago/Turabian StyleMafra, Denise, Natália A. Borges, Beatriz G. Baptista, Layla F. Martins, Gillian Borland, Paul G. Shiels, and Peter Stenvinkel. 2024. "What Can the Gut Microbiota of Animals Teach Us about the Relationship between Nutrition and Burden of Lifestyle Diseases?" Nutrients 16, no. 11: 1789. https://doi.org/10.3390/nu16111789







