Maternal Diet Associated with Oligosaccharide Abundances in Human Milk from Latina Mothers
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Demographics and Anthropometrics
2.3. Dietary Assessment
2.4. Human Milk Sample Collection
2.5. HMO Measurements
2.6. Statistical Analysis
3. Results
3.1. Description of Participants
3.2. Maternal Dietary Intake at Baseline, 6 and 12 Months
3.3. Cross-Sectional Association of Maternal Macronutrient Intake and HMO Profile
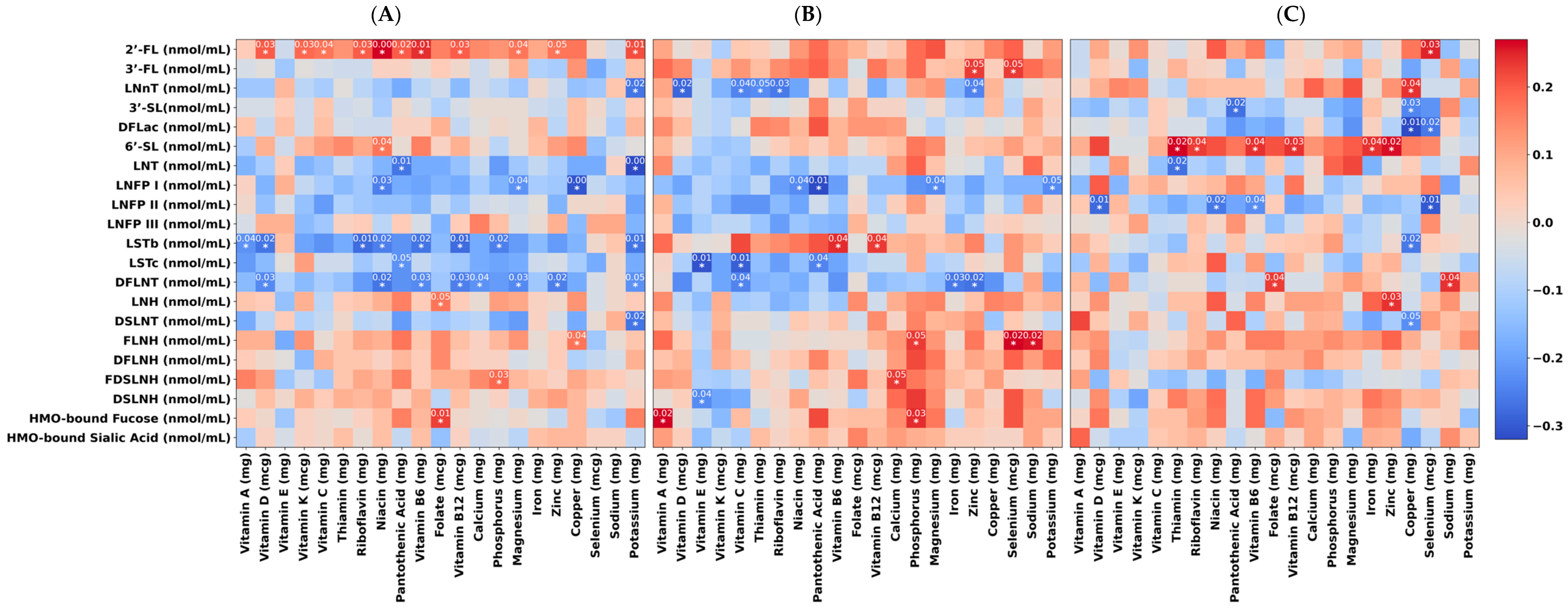
3.4. Cross-Sectional Association of Maternal Micronutrient Intake and HMO Profile
3.5. Longitudinal Associations of Maternal Diet with the HMOs 2′-FL, 3′-FL, and 3′-SL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Guideline: Counselling of Women to Improve Breastfeeding Practices; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- Kelishadi, R.; Farajian, S. The protective effects of breastfeeding on chronic non-communicable diseases in adulthood: A review of evidence. Adv. Biomed. Res. 2014, 3, 3. [Google Scholar] [CrossRef]
- Bider-Canfield, Z.; Martinez, M.; Wang, X.; Yu, W.; Bautista, M.; Brookey, J.; Page, K.; Buchanan, T.; Xiang, A. Maternal obesity, gestational diabetes, breastfeeding and childhood overweight at age 2 years. Pediatr. Obes. 2017, 12, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Belfort, M.B.; Rifas-Shiman, S.L.; Kleinman, K.P.; Guthrie, L.B.; Bellinger, D.C.; Taveras, E.M.; Gillman, M.W.; Oken, E. Infant feeding and childhood cognition at ages 3 and 7 years: Effects of breastfeeding duration and exclusivity. JAMA Pediatr. 2013, 167, 836–844. [Google Scholar] [CrossRef]
- Bode, L. Human milk oligosaccharides: Every baby needs a sugar mama. Glycobiology 2012, 22, 1147–1162. [Google Scholar] [CrossRef]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Ryoo, J.H.; Bode, L.; Goran, M.I. Human milk oligosaccharides and Hispanic infant weight gain in the first 6 months. Obesity 2020, 28, 1519–1525. [Google Scholar] [CrossRef] [PubMed]
- Larsson, M.W.; Lind, M.V.; Laursen, R.P.; Yonemitsu, C.; Larnkjær, A.; Mølgaard, C.; Michaelsen, K.F.; Bode, L. Human milk oligosaccharide composition is associated with excessive weight gain during exclusive breastfeeding—An explorative study. Front. Pediatr. 2019, 7, 297. [Google Scholar] [CrossRef]
- Alderete, T.L.; Autran, C.; Brekke, B.E.; Knight, R.; Bode, L.; Goran, M.I.; Fields, D.A. Associations between human milk oligosaccharides and infant body composition in the first 6 mo of life. Am. J. Clin. Nutr. 2015, 102, 1381–1388. [Google Scholar] [CrossRef]
- Moossavi, S.; Miliku, K.; Sepehri, S.; Khafipour, E.; Azad, M.B. The prebiotic and probiotic properties of human milk: Implications for infant immune development and pediatric asthma. Front. Pediatr. 2018, 6, 197. [Google Scholar] [CrossRef] [PubMed]
- Berger, P.K.; Plows, J.F.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Poulsen, M.; Ryoo, J.H.; Peterson, B.S.; Bode, L.; Goran, M.I. Human milk oligosaccharide 2′-fucosyllactose links feedings at 1 month to cognitive development at 24 months in infants of normal and overweight mothers. PLoS ONE 2020, 15, e0228323. [Google Scholar] [CrossRef]
- Wang, B.; Yu, B.; Karim, M.; Hu, H.; Sun, Y.; McGreevy, P.; Petocz, P.; Held, S.; Brand-Miller, J. Dietary sialic acid supplementation improves learning and memory in piglets. Am. J. Clin. Nutr. 2007, 85, 561–569. [Google Scholar] [CrossRef]
- Jacobi, S.K.; Yatsunenko, T.; Li, D.; Dasgupta, S.; Yu, R.K.; Berg, B.M.; Chichlowski, M.; Odle, J. Dietary isomers of sialyllactose increase ganglioside sialic acid concentrations in the corpus callosum and cerebellum and modulate the colonic microbiota of formula-fed piglets. J. Nutr. 2016, 146, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Dinleyici, M.; Barbieur, J.; Dinleyici, E.C.; Vandenplas, Y. Functional effects of human milk oligosaccharides (HMOs). Gut Microbes 2023, 15, 2186115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bode, L.; Jantscher-Krenn, E. Structure-function relationships of human milk oligosaccharides. Adv. Nutr. 2012, 3, 383S–391S. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Robertson, B.; Atakora, F.; Becker, A.B.; Subbarao, P.; Moraes, T.J.; Mandhane, P.J.; Turvey, S.E.; Lefebvre, D.L.; Sears, M.R. Human milk oligosaccharide concentrations are associated with multiple fixed and modifiable maternal characteristics, environmental factors, and feeding practices. J. Nutr. 2018, 148, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.M.; Binia, A.; de Castro, C.A.; Thakkar, S.K.; Billeaud, C.; Agosti, M.; Al-Jashi, I.; Costeira, M.J.; Marchini, G.; Martínez-Costa, C. Impact of maternal characteristics on human milk oligosaccharide composition over the first 4 months of lactation in a cohort of healthy European mothers. Sci. Rep. 2019, 9, 11767. [Google Scholar] [CrossRef]
- Plows, J.F.; Berger, P.K.; Jones, R.B.; Alderete, T.L.; Yonemitsu, C.; Najera, J.A.; Khwajazada, S.; Bode, L.; Goran, M.I. Longitudinal changes in human milk oligosaccharides (HMOs) over the course of 24 months of lactation. J. Nutr. 2021, 151, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Quin, C.; Vicaretti, S.D.; Mohtarudin, N.A.; Garner, A.M.; Vollman, D.M.; Gibson, D.L.; Zandberg, W.F. Influence of sulfonated and diet-derived human milk oligosaccharides on the infant microbiome and immune markers. J. Biol. Chem. 2020, 295, 4035–4048. [Google Scholar] [CrossRef] [PubMed]
- Seferovic, M.D.; Mohammad, M.; Pace, R.M.; Engevik, M.; Versalovic, J.; Bode, L.; Haymond, M.; Aagaard, K.M. Maternal diet alters human milk oligosaccharide composition with implications for the milk metagenome. Sci. Rep. 2020, 10, 22092. [Google Scholar] [CrossRef]
- Jones, R.B.; Berger, P.K.; Plows, J.F.; Alderete, T.L.; Millstein, J.; Fogel, J.; Iablokov, S.N.; Rodionov, D.A.; Osterman, A.L.; Bode, L. Lactose-reduced infant formula with added corn syrup solids is associated with a distinct gut microbiota in Hispanic infants. Gut Microbes 2020, 12, 1813534. [Google Scholar] [CrossRef]
- Conway, J.M.; Ingwersen, L.A.; Moshfegh, A.J. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: An observational validation study. J. Am. Diet. Assoc. 2004, 104, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Harnack, L. Nutrition data system for research (NDSR). In Encyclopedia of Behavioral Medicine; Gellman, M.D., Turner, J.R., Eds.; Springer: Cham, Switzerland, 2013; pp. 1348–1350. [Google Scholar]
- Schakel, S.F. Maintaining a Nutrient Database in a Changing Marketplace: Keeping Pace with Changing Food Products—A Research Perspective; Elsevier: Amsterdam, The Netherlands, 2001; pp. 315–322. [Google Scholar]
- World Health Organization. Sugars intake for adults and children. In Guideline; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Fields, D.A.; Demerath, E.W. Relationship of insulin, glucose, leptin, IL-6 and TNF-α in human breast milk with infant growth and body composition. Pediatr. Obes. 2012, 7, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Hollingshead, A.B. Four Factor Index of Social Status; Yale University: New Haven, CT, USA, 1975. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2010. [Google Scholar]
- Van Rossum, G.; Drake, F.L. Introduction to Python 3: Python Documentation Manual Part 1; CreateSpace: Scotts Valley, CA, USA, 2009. [Google Scholar]
- Biddulph, C.; Holmes, M.; Tran, T.D.; Kuballa, A.; Davies, P.S.; Koorts, P.; Maher, J. Associations between Maternal Nutrition and the Concentrations of Human Milk Oligosaccharides in a Cohort of Healthy Australian Lactating Women. Nutrients 2023, 15, 2093. [Google Scholar] [CrossRef] [PubMed]
- Selma-Royo, M.; González, S.; Gueimonde, M.; Chang, M.; Fürst, A.; Martínez-Costa, C.; Bode, L.; Collado, M.C. Maternal Diet Is Associated with Human Milk Oligosaccharide Profile. Mol. Nutr. Food Res. 2022, 66, e2200058. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, W.; Vervoort, J.; Pan, J.; Gao, P.; Zhu, H.; Wang, X.; Zhang, Y.; Chen, B.; Liu, Y.; Li, Y. Comparison of twelve human milk oligosaccharides in mature milk from different areas in China in the Chinese Human Milk Project (CHMP) study. Food Chem. 2022, 395, 133554. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mao, Y.; Liu, S.; Wang, J.; Li, X.; Zhao, Y.; Hill, D.R.; Wang, S. Vitamins, Vegetables and Metal Elements Are Positively Associated with Breast Milk Oligosaccharide Composition among Mothers in Tianjin, China. Nutrients 2022, 14, 4131. [Google Scholar] [CrossRef] [PubMed]
- DeRose, V.J.; Yglesias, M.V. Metal Ion Interactions with DNA, RNA, and Nucleic Acid Enzymes. In Comprehensive Coordination Chemistry III; Constable, E.C., Parkin, G., Que, L., Jr., Eds.; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Lin, Y.; Li, Y.; Ren, F.; Guo, H. How far is it from infant formula to human milk? A look at the human milk oligosaccharides. Trends Food Sci. Technol. 2021, 118, 374–387. [Google Scholar] [CrossRef]
- Biddulph, C.; Holmes, M.; Kuballa, A.; Davies, P.S.W.; Koorts, P.; Carter, R.J.; Maher, J. Human Milk Oligosaccharide Profiles and Associations with Maternal Nutritional Factors: A Scoping Review. Nutrients 2021, 13, 965. [Google Scholar] [CrossRef]
- Gelman, A.; Hill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Knief, U.; Forstmeier, W. Violating the normality assumption may be the lesser of two evils. Behav. Res. Methods 2021, 53, 2576–2590. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Walsh, C.; Lane, J.A.; van Sinderen, D.; Hickey, R.M. Human milk oligosaccharides: Shaping the infant gut microbiota and supporting health. J. Funct. Foods 2020, 72, 104074. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| 1 Month n = 210 | 6 Months n = 131 | 12 Months n = 84 | p-Value | |
|---|---|---|---|---|
| Mothers | ||||
| Maternal Age (years) | 28.9 ± 6.1 | 29.5 ± 6.2 | 29.9 ± 6.3 | 0.39 |
| Pre-pregnancy maternal BMI (kg/m2) | 28.6 ± 5.9 | 28.5 ± 5.8 | 27.7 ± 5.2 | 0.46 |
| Maternal BMI (kg/m2) | 30.3 ± 5.2 | 30.4 ± 5.5 | 29.2 ± 5.4 | 0.19 |
| Breastfeeding/day | 8.2 ± 2.7 | 5.4 ± 3.9 | 4.2 ± 3.5 | <0.00 * |
| Secretors, n (%) | 183 (87.56) | 114 (87.02) | 76 (89.41) | <0.00 * |
| Infants | ||||
| Age (days) | 32.6 ± 4.6 | 184.9 ± 9.2 | 367.3.3 ± 8.3 | <0.00 * |
| HMO distribution (nmol/mL) | ||||
| 2′-Fucosyllactose (2′-FL) | 6262 ± 3634.4 | 6515.5 ± 3875.1 | 6322.7 ± 3209.5 | 0.819 |
| 3′-Fucosyllactose (3′-FL) | 941.8 ± 1375.1 | 2281.2 ± 2299.2 | 3623.2 ± 2569.9 | <0.01 * |
| 3′-Sialyllactose (3′-SL) | 547.5 ± 507.9 | 739.6 ± 414.4 | 905 ± 654.7 | <0.01 * |
| 6′-Sialyllactose (6′-SL) | 1015.9 ± 338.6 | 280.2 ± 191.5 | 147.9 ± 126.9 | <0.01 * |
| Difucosyllactose (DFLac) | 423 ± 318.2 | 606.3 ± 424.8 | 662.9 ± 408.8 | <0.01 * |
| Difucosyllacto-N-hexaose (DFLNH) | 148.7 ± 130.6 | 64.3 ± 57 | 62.6 ± 53.2 | <0.01 * |
| Difucosyllacto-N-tetrose (DFLNT) | 1382.7 ± 753.3 | 1155.4 ± 696.1 | 1065.2 ± 586.3 | <0.01 * |
| Disialyllacto-N-hexaose (DSLNH) | 251.4 ± 108.9 | 72.8 ± 62.3 | 36.1 ± 28.4 | <0.01 * |
| Disialyllacto-N-tetraose (DSLNT) | 398.4 ± 191.6 | 292 ± 177 | 257.8 ± 140.7 | <0.01 * |
| Fucodisialyllacto-N-hexaose (FDSLNH) | 171.7 ± 190.6 | 217.9 ± 149.1 | 153.4 ± 89.1 | <0.01 * |
| Fucosyllacto-N-hexaose (FLNH) | 158.7 ± 112.1 | 75.6 ± 73.9 | 68.3 ± 47.4 | <0.01 * |
| Lacto-N-fucopentaose I (LNFP-I) | 1359.6 ± 1189.8 | 500.8 ± 539 | 476.6 ± 569.1 | <0.01 * |
| Lacto-N-fucopentaose II (LNFP-II) | 970.1 ± 608.6 | 1325 ± 683 | 1208.6 ± 581.5 | <0.01 * |
| Lacto-N-fucopentaose III (LNFP-III) | 64.9 ± 39.4 | 69.2 ± 59.4 | 54.4 ± 28.6 | 0.12 |
| Lacto-N-hexaose (LNH) | 102 ± 63.7 | 74.9 ± 55.6 | 71.6 ± 58.7 | <0.01 * |
| Lacto-N-neotetraose (LNnT) | 565.3 ± 337.4 | 339.4 ± 229.1 | 251.4 ± 125.1 | <0.01 * |
| Lacto-N-tetraose (LNT) | 1132.5 ± 692.2 | 734.7 ± 532.9 | 746.3 ± 407.4 | <0.01 * |
| Sialyllacto-N-tetraose b (LSTb) | 91.4 ± 65.5 | 80.7 ± 58.6 | 80.6 ± 63.7 | 0.218 |
| Sialyllacto-N-tetraose c (LSTc) | 312.4 ± 138.1 | 84.9 ± 88.1 | 62 ± 80.5 | <0.01 * |
| HMO-bound Fucose | 13,837.6 ± 3328.7 | 14,637.2 ± 4181.5 | 15,488.5 ±3205 | <0.01 * |
| HMO-bound Sialic Acid | 3610.2 ± 840 | 2350.7 ± 804.3 | 2090.2 ± 721.9 | <0.01 * |
| Dietary Variables | 1 Month n = 210 | 6 Months n = 131 | 12 Months n = 84 | p-Value |
|---|---|---|---|---|
| Energy | 1800 ± 590 | 1770 ± 510 | 1701 ± 460 | 0.39 |
| Macronutrients | ||||
| Protein (g) | 78.7 ± 25.2 | 79.9 ± 24.2 | 76.4 ± 21.2 | 0.58 |
| Fat (g) | 62 ± 26.5 | 66.3 ± 24.3 | 64 ± 23.6 | 0.30 |
| Saturated fat (g) | 20.5 ± 10.2 | 20.9 ± 8.5 | 20.3 ± 7.9 | 0.90 |
| Monounsaturated fat (g) | 21.1 ± 9.6 | 23.3 ± 9 | 22.6 ± 9.1 | 0.09 |
| Polyunsaturated fat (g) | 14.2 ± 7.1 | 15.7 ± 8 | 14.8 ± 6.9 | 0.21 |
| Carbohydrate (g) | 238.8 ± 87.8 | 219.1 ± 71.8 | 210.4 ± 65.9 | 0.04 |
| Total fiber (g) | 18.6 ± 7.2 | 17.9 ± 7 | 18.2 ± 7.6 | 0.68 |
| Insoluble fiber (g) | 12.6 ± 5.1 | 12.4 ± 5.8 | 12.8 ± 6.1 | 0.86 |
| Soluble fiber (g) | 5.9 ± 3.1 | 5.3 ± 2 | 5.2 ± 2.1 | 0.18 |
| Total Sugar (g) | 103.2 ± 51.7 | 88.9 ± 39.8 | 80.6 ± 38.1 | <0.01 * |
| Added sugar (g) | 59.2 ± 40.8 | 54.7 ± 33.7 | 51.4 ± 34 | 0.23 |
| Free sugar (g) | 63.4 ± 41.2 | 57 ± 33.6 | 52.7 ± 33.9 | 0.06 |
| Sugary Sweetened Beverage (SSB) | ||||
| SSB excluding juice (servings/day) | 0.9 ± 1 | 0.9 ± 9.9 | 1 ± 1.3 | 0.76 |
| SSB excluding juice and dairy (servings/day) | 0.8 ± 1 | 0.9 ± 0.9 | 1 ± 1.3 | 0.60 |
| SSB plus juice (servings/day) | 2.4 ± 2.7 | 5.4 ± 3.9 | 4.2 ± 3.5 | <0.01 * |
| Micronutrients | ||||
| Vitamin A (mcg) | 660.5 ± 389.7 | 533.5 ± 268.7 | 496.4 ± 232.1 | <0.01 * |
| Vitamin D (mcg) | 11.2 ± 7.7 | 8.1 ± 8.5 | 8.5 ± 12.2 | <0.01 * |
| Vitamin E (mg) | 6.6 ± 3.3 | 7.3 ± 4.1 | 7 ± 3.9 | 0.21 |
| Vitamin K (mcg) | 76.7 ± 66.1 | 85.6 ± 65.5 | 96.1 ± 120.9 | 0.15 |
| Vitamin C (mg) | 148.8 ± 94.9 | 116.1 ± 78.8 | 121.5 ± 178.2 | 0.01 * |
| Thiamin (mg) | 2.5 ± 1.6 | 2.3 ± 4.5 | 2.4 ± 3.2 | 0.88 |
| Riboflavin (mg) | 2.8 ± 1.8 | 2.6 ± 4.5 | 2.6 ± 3.3 | 0.74 |
| Niacin (mg) | 31.9 ± 13.9 | 27.8 ± 13.3 | 26.4 ± 15.6 | <0.01 * |
| Pantothenic acid (mg) | 5.2 ± 2.5 | 5.9 ± 5.7 | 6.3 ± 6.5 | 0.13 |
| Vitamin B6 (mg) | 3.4 ± 2.4 | 3.1 ± 4.9 | 3 ± 3.7 | 0.55 |
| Folate (mcg) | 368.4 ± 185.4 | 335.4 ± 142.9 | 323.3 ± 138.7 | 0.05 |
| Vitamin B12 (mcg) | 8.7 ± 7.1 | 14.2 ± 87.4 | 6.8 ± 7.7 | 0.48 |
| Calcium (mg) | 1023.9 ± 528.4 | 834.1 ± 430.2 | 842.3 ± 403.5 | <0.01 * |
| Phosphorus (mg) | 1307.7 ± 462.8 | 1229.4 ± 395.7 | 1187.7 ± 308.2 | 0.18 |
| Magnesium (mg) | 291.1 ± 96.5 | 277.9 ± 85.9 | 272.7 ± 82.9 | 0.23 |
| Iron (mg) | 42.3 ± 43.7 | 21.7 ± 18.9 | 19.5 ± 18.2 | <0.01 * |
| Zinc (mg) | 22.3 ± 13.9 | 16.1 ± 11.4 | 15.9 ± 14.1 | <0.01 * |
| Copper (mg) | 1.1 ± 0.4 | 1.2 ± 0.6 | 1.2 ± 1 | 0.47 |
| Selenium (mcg) | 107.6 ± 37.4 | 112 ± 39.6 | 108.3 ± 40.8 | 0.58 |
| Sodium (mg) | 2562.9 ± 930.6 | 2689.5 ± 1031.5 | 2545.4 ± 844.3 | 0.41 |
| Potassium (mg) | 2403.1 ± 850.8 | 2296 ± 734.8 | 2152 ± 639.9 | 0.08 * |
| Manganese (mg) | 2.9 ± 1.3 | 2.7 ± 1.2 | 2.6 ± 1.4 | 0.22 |
| Beta-Coefficient | 95% CI | p-Value | |
|---|---|---|---|
| 2′-FL | |||
| Carbohydrate (g) | 0.743 | [−9.635, 11.121] | 0.88 |
| Total Sugar (g) | −6.597 | [−18.626, 5.432] | 0.28 |
| Soluble fiber (g) | 94.789 | [25.710, 163.868] | <0.01 * |
| SSB plus juice (servings/day) | 1.400 | [−118.417, 121.217] | 0.98 |
| Vitamin A (mcg) | −0.068 | [−1.049, 0.913] | 0.89 |
| Vitamin C (mg) | 1.586 | [−2.249, 5.421] | 0.41 |
| Niacin (mg) | 31.355 | [1.672, 61.038] | 0.03 * |
| Folate (mcg) | 0.228 | [−1.766, 2.223] | 0.82 |
| Calcium (mg) | 0.720 | [−0.155, 1.595] | 0.10 |
| Phosphorus (mg) | 0.387 | [−0.897, 1.672] | 0.55 |
| Iron (mg) | 2.449 | [−5.797, 10.696] | 0.56 |
| Zinc (mg) | −40.196 | [−79.614, −0.778] | 0.04 * |
| Potassium (mg) | −0.070 | [−0.696, 0.555] | 0.82 |
| 3′-FL | |||
| Carbohydrate (g) | −2.107 | [−9.249, 5.036] | 0.56 |
| Total Sugar (g) | −5.501 | [−13.205, 2.203] | 0.21 |
| Soluble fiber (g) | 16.031 | [−9.126, 41.188] | 0.68 |
| SSB plus juice (servings/day) | 29.473 | [−53.852, 112.799] | 0.48 |
| Vitamin A (mcg) | 0.487 | [−0.219, 1.193] | 0.17 |
| Vitamin C (mg) | 2.885 | [−0.585, 6.356] | 0.10 |
| Niacin (mg) | −10.136 | [−32.644, 12.373] | 0.37 |
| Folate (mcg) | −0.036 | [−1.449, 1.375] | 0.95 |
| Calcium (mg) | −0.097 | [−0.997, 0.803] | 0.91 |
| Phosphorus (mg) | 0.751 | [−0.169, 1.671] | 0.10 |
| Iron (mg) | −0.879 | [−7.462, 5.704] | 0.78 |
| Zinc (mg) | 1.818 | [−27.255, 30.891] | 0.90 |
| Potassium (mg) | −0.258 | [−0.695, 0.179] | 0.24 |
| 3′-SL | |||
| Carbohydrate (g) | 0.220 | [−1.919, 2.358] | 0.84 |
| Total Sugar (g) | −2.039 | [−4.769, 0.690] | 0.14 |
| Soluble fiber (g) | 2.300 | [−21.747, 26.347] | 0.85 |
| SSB plus juice (servings/day) | 17.281 | [−9.197, 43.758] | 0.20 |
| Vitamin A (mcg) | −0.005 | [−0.220, 0.209] | 0.96 |
| Vitamin C (mg) | −0.399 | [−1.437, 0.639] | 0.45 |
| Niacin (mg) | −2.520 | [−9.794, 4.755] | 0.49 |
| Folate (mcg) | −0.326 | [−0.774, 0.122] | 0.15 |
| Calcium (mg) | 0.022 | [−0.169, 0.213] | 0.82 |
| Phosphorus (mg) | −0.133 | [−0.430, 0.163] | 0.37 |
| Iron (mg) | −0.327 | [−2.272, 1.618] | 0.74 |
| Zinc (mg) | 2.010 | [−6.894, 10.914] | 0.65 |
| Potassium (mg) | 0.101 | [−0.039, 0.241] | 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokhtari, P.; Schmidt, K.A.; Zamanian, H.; Babaei, M.; Machle, C.J.; Trifonova, D.; Alderete, T.L.; Holzhausen, E.A.; Ottino-González, J.; Chalifour, B.N.; et al. Maternal Diet Associated with Oligosaccharide Abundances in Human Milk from Latina Mothers. Nutrients 2024, 16, 1795. https://doi.org/10.3390/nu16121795
Mokhtari P, Schmidt KA, Zamanian H, Babaei M, Machle CJ, Trifonova D, Alderete TL, Holzhausen EA, Ottino-González J, Chalifour BN, et al. Maternal Diet Associated with Oligosaccharide Abundances in Human Milk from Latina Mothers. Nutrients. 2024; 16(12):1795. https://doi.org/10.3390/nu16121795
Chicago/Turabian StyleMokhtari, Pari, Kelsey A. Schmidt, Hashem Zamanian, Mahsa Babaei, Christopher J. Machle, Diana Trifonova, Tanya L. Alderete, Elizabeth A. Holzhausen, Jonatan Ottino-González, Bridget N. Chalifour, and et al. 2024. "Maternal Diet Associated with Oligosaccharide Abundances in Human Milk from Latina Mothers" Nutrients 16, no. 12: 1795. https://doi.org/10.3390/nu16121795
APA StyleMokhtari, P., Schmidt, K. A., Zamanian, H., Babaei, M., Machle, C. J., Trifonova, D., Alderete, T. L., Holzhausen, E. A., Ottino-González, J., Chalifour, B. N., Jones, R. B., Furst, A., Yonemitsu, C., Bode, L., & Goran, M. I. (2024). Maternal Diet Associated with Oligosaccharide Abundances in Human Milk from Latina Mothers. Nutrients, 16(12), 1795. https://doi.org/10.3390/nu16121795






