The Effect of Sargassum fusiforme and Fucus vesiculosus on Continuous Glucose Levels in Overweight Patients with Type 2 Diabetes Mellitus: A Feasibility Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
:1. Introduction
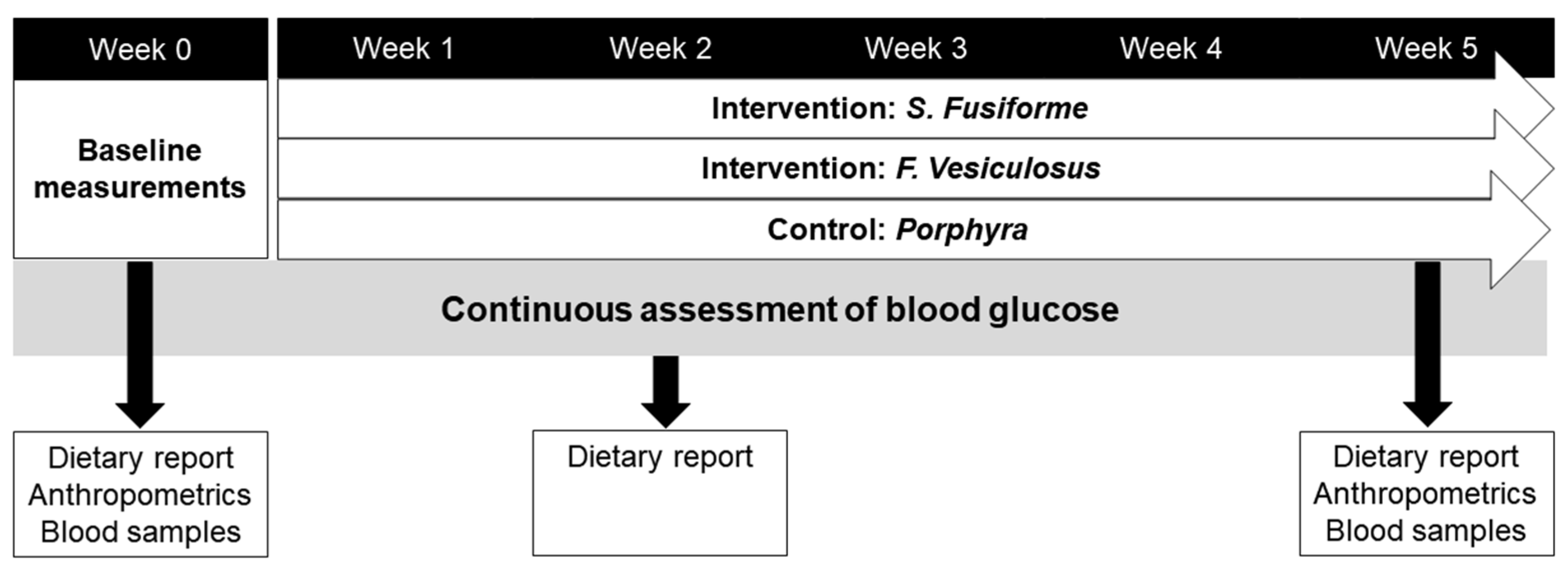
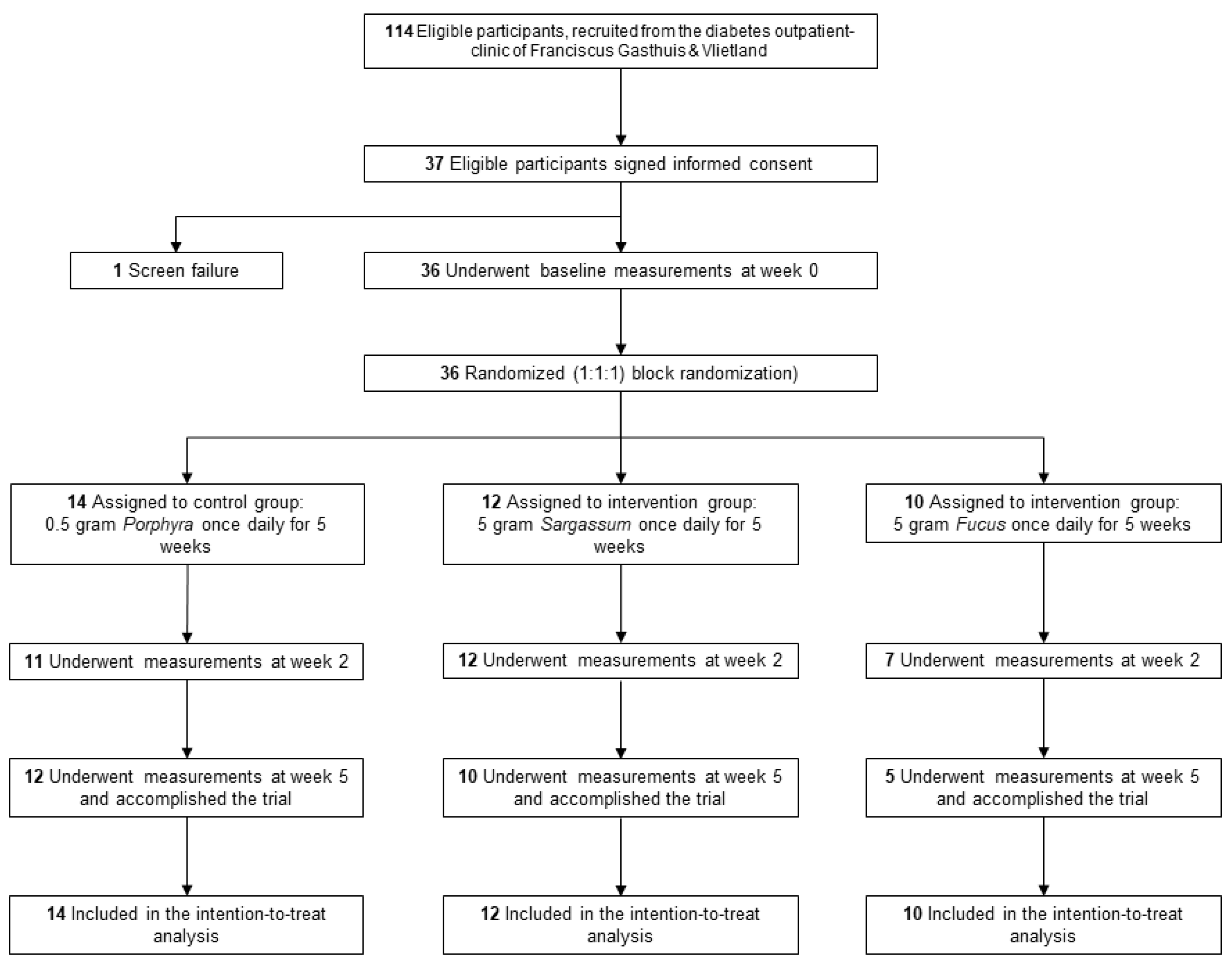
2. Materials and Methods
3. Results
3.1. The Effect of S. fusiforme and F. vesiculosus Intake on Blood Glucose
3.2. Effect of Seaweed on Use of Medication, Anthropometrics, and Energy Intake
3.3. Effect of S. fusiforme and F. vesiculosus on Plasma Lipids
3.4. Effect of Brown Seaweed on Plasma Sterol Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E. Risk factors for type 2 diabetes mellitus: An exposure-wide umbrella review of meta-analyses. PLoS ONE 2018, 13, e0194127. [Google Scholar] [CrossRef] [PubMed]
- Carey, V.J.; Walters, E.E.; Colditz, G.A.; Solomon, C.G.; Willett, W.C.; Rosner, B.A.; Speizer, F.E.; Manson, J.E. Body fat distribution and risk of non-insulin-dependent diabetes mellitus in women. The Nurses’ Health Study. Am. J. Epidemiol. 1997, 145, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martin, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Colditz, G.; Liu, S.; Solomon, C.G.; Willett, W.C. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N. Engl. J. Med. 2001, 345, 790–797. [Google Scholar] [CrossRef] [PubMed]
- Schellenberg, E.S.; Dryden, D.M.; Vandermeer, B.; Ha, C.; Korownyk, C. Lifestyle interventions for patients with and at risk for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2013, 159, 543–551. [Google Scholar] [CrossRef]
- Gomez-Zavaglia, A.; Prieto Lage, M.A.; Jimenez-Lopez, C.; Mejuto, J.C.; Simal-Gandara, J. The Potential of Seaweeds as a Source of Functional Ingredients of Prebiotic and Antioxidant Value. Antioxidants 2019, 8, 406. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.S.; Allsopp, P.J.; Magee, P.J.; Gill, C.I.; Nitecki, S.; Strain, C.R.; McSorley, E.M. Seaweed and human health. Nutr. Rev. 2014, 72, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.M.; Patel, I.C. A review on phytoconstituents of marine brown algae. Future J. Pharm. Sci. 2020, 6, 129. [Google Scholar] [CrossRef]
- Schepers, M.; Martens, N.; Tiane, A.; Vanbrabant, K.; Liu, H.-B.; Lütjohann, D.; Mulder, M.; Vanmierlo, T. Edible seaweed-derived constituents: An undisclosed source of neuroprotective compounds. Neural Regen. Res. 2020, 15, 790–795. [Google Scholar]
- Begum, R.; Howlader, S.; Mamun-Or-Rashid, A.N.M.; Rafiquzzaman, S.M.; Ashraf, G.M.; Albadrani, G.M.; Sayed, A.A.; Peluso, I.; Abdel-Daim, M.M.; Uddin, M.S. Antioxidant and Signal-Modulating Effects of Brown Seaweed-Derived Compounds against Oxidative Stress-Associated Pathology. Oxid. Med. Cell Longev. 2021, 2021, 9974890. [Google Scholar] [CrossRef]
- Chater, P.I.; Wilcox, M.D.; Houghton, D.; Pearson, J.P. The role of seaweed bioactives in the control of digestion: Implications for obesity treatments. Food Funct. 2015, 6, 3420–3427. [Google Scholar] [CrossRef] [PubMed]
- Gabbia, D.; Dall’Acqua, S.; Di Gangi, I.M.; Bogialli, S.; Caputi, V.; Albertoni, L.; Marsilio, I.; Paccagnella, N.; Carrara, M.; Giron, M.C.; et al. The Phytocomplex from Fucus vesiculosus and Ascophyllum nodosum Controls Postprandial Plasma Glucose Levels: An In Vitro and In Vivo Study in a Mouse Model of NASH. Mar. Drugs 2017, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kim, J.Y.; Choi, W.H.; Lee, S.S. Effects of seaweed supplementation on blood glucose concentration, lipid profile, and antioxidant enzyme activities in patients with type 2 diabetes mellitus. Nutr. Res. Pract. 2008, 2, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Lange, K.W.; Hauser, J.; Nakamura, Y.; Kanaya, S. Dietary seaweeds and obesity. Food Sci. Hum. Wellness 2015, 4, 87–96. [Google Scholar] [CrossRef]
- Mikami, N.; Hosokawa, M.; Miyashita, K.; Sohma, H.; Ito, Y.M.; Kokai, Y. Reduction of HbA1c levels by fucoxanthin-enriched akamoku oil possibly involves the thrifty allele of uncoupling protein 1 (UCP1): A randomised controlled trial in normal-weight and obese Japanese adults. J. Nutr. Sci. 2017, 6, e5. [Google Scholar] [CrossRef] [PubMed]
- Murai, U.; Yamagishi, K.; Kishida, R.; Iso, H. Correction: Impact of seaweed intake on health. Eur. J. Clin. Nutr. 2021, 75, 862. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, K.; Mitamura, R. Effects of Undaria pinnatifida (Wakame) on Postprandial Glycemia and Insulin Levels in Humans: A Randomized Crossover Trial. Plant Foods Hum. Nutr. 2019, 74, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zuo, J.; Cheng, Y.; Zhang, Y.; Zhang, Z.; Wu, M.; Yang, Y.; Tong, H. Ethanol extract of Sargarsum fusiforme alleviates HFD/STZ-induced hyperglycemia in association with modulation of gut microbiota and intestinal metabolites in type 2 diabetic mice. Food Res. Int. 2021, 147, 110550. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, S.; Cheng, Y.; Liu, Q.; Su, L.; Yang, Y.; Zhang, X.; Wu, M.; Choi, J.I.; Tong, H. Sargassum fusiforme Alginate Relieves Hyperglycemia and Modulates Intestinal Microbiota and Metabolites in Type 2 Diabetic Mice. Nutrients 2021, 13, 2887. [Google Scholar] [CrossRef]
- Kim, K.T.; Rioux, L.E.; Turgeon, S.L. Alpha-amylase and alpha-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef]
- Lordan, S.; Smyth, T.J.; Soler-Vila, A.; Stanton, C.; Ross, R.P. The alpha-amylase and alpha-glucosidase inhibitory effects of Irish seaweed extracts. Food Chem. 2013, 141, 2170–2176. [Google Scholar] [CrossRef] [PubMed]
- Apostolidis, E.; Lee, C.M. In vitro potential of Ascophyllum nodosum phenolic antioxidant-mediated alpha-glucosidase and alpha-amylase inhibition. J. Food Sci. 2010, 75, H97–H102. [Google Scholar] [CrossRef] [PubMed]
- Ramel, A.; Sveinsdóttir, K.; Ingadóttir, B.; As, E.S.; Og, G.; Pv, J. In Vitro and In VivoEffects of Seaweed Extract on Carbohydrate Digestion and Availability. Int. J. Food Sci. Nutr. Res. 2019, 1, 1003. [Google Scholar] [CrossRef]
- Zang, L.; Baharlooeian, M.; Terasawa, M.; Shimada, Y.; Nishimura, N. Beneficial effects of seaweed-derived components on metabolic syndrome via gut microbiota modulation. Front. Nutr. 2023, 10, 1173225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, N.; Longhe, Y.; Hong, Z.; Cai, B.; Le, Q.; Yang, T.; Shi, L.; He, J.; Cui, C.-B. Insoluble dietary fiber derived from brown seaweed Laminaria japonica ameliorate obesity-related features via modulating gut microbiota dysbiosis in high-fat diet–fed mice. Food Funct. 2021, 12, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Shannon, E.; Conlon, M.; Hayes, M. Seaweed Components as Potential Modulators of the Gut Microbiota. Mar. Drugs 2021, 19, 358. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.R.; Park, M.J.; Park, S.Y.; Kim, J.Y. Brown Seaweed Consumption as a Promising Strategy for Blood Glucose Management: A Comprehensive Meta-Analysis. Nutrients 2023, 15, 4987. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, Y. Method of removing inorganic arsenic from dried hijiki seaweed products. Nippon Suisan Gakkaishi 2014, 80, 973–978. [Google Scholar] [CrossRef]
- Bogie, J.; Hoeks, C.; Schepers, M.; Tiane, A.; Cuypers, A.; Leijten, F.; Chintapakorn, Y.; Suttiyut, T.; Pornpakakul, S.; Struik, D.; et al. Dietary Sargassum fusiforme improves memory and reduces amyloid plaque load in an Alzheimer’s disease mouse model. Sci. Rep. 2019, 9, 4908. [Google Scholar] [CrossRef]
- Martens, N.; Zhan, N.; Voortman, G.; Leijten, F.P.J.; van Rheenen, C.; van Leerdam, S.; Geng, X.; Huybrechts, M.; Liu, H.; Jonker, J.W.; et al. Activation of Liver X Receptors and Peroxisome Proliferator-Activated Receptors by Lipid Extracts of Brown Seaweeds: A Potential Application in Alzheimer’s Disease? Nutrients 2023, 15, 3004. [Google Scholar] [CrossRef]
- Lutjohann, D.; Brzezinka, A.; Barth, E.; Abramowski, D.; Staufenbiel, M.; von Bergmann, K.; Beyreuther, K.; Multhaup, G.; Bayer, T.A. Profile of cholesterol-related sterols in aged amyloid precursor protein transgenic mouse brain. J. Lipid Res. 2002, 43, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Zaharudin, N.; Tullin, M.; Pekmez, C.T.; Sloth, J.J.; Rasmussen, R.R.; Dragsted, L.O. Effects of brown seaweeds on postprandial glucose, insulin and appetite in humans—A randomized, 3-way, blinded, cross-over meal study. Clin. Nutr. 2021, 40, 830–838. [Google Scholar] [CrossRef] [PubMed]
- van den Driessche, J.J.; Plat, J.; Konings, M.; Mensink, R.P. Effects of spirulina and wakame consumption on intestinal cholesterol absorption and serum lipid concentrations in non-hypercholesterolemic adult men and women. Eur. J. Nutr. 2020, 59, 2229–2236. [Google Scholar] [CrossRef] [PubMed]
- van den Driessche, J.J.; Mensink, R.P.; Plat, J. Spirulina, wakame or goji berries do not lower markers of low-grade systemic inflammation in healthy subjects. Funct. Foods Health Dis. 2021, 11, 627–640. [Google Scholar] [CrossRef]
- Cho, T.J.; Rhee, M.S. Health Functionality and Quality Control of Laver (Porphyra, Pyropia): Current Issues and Future Perspectives as an Edible Seaweed. Mar. Drugs 2019, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Kim, H.C.; Vitek, L.; Nam, C.M. Algae consumption and risk of type 2 diabetes: Korean National Health and Nutrition Examination Survey in 2005. J. Nutr. Sci. Vitaminol. 2010, 56, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Zeng, A.; Yang, R.; Yu, S.; Zhao, W. A novel hypoglycemic agent: Polysaccharides from laver (Porphyra spp.). Food Funct. 2020, 11, 9048–9056. [Google Scholar] [CrossRef]
- Kitano, Y.; Murazumi, K.; Duan, J.; Kurose, K.; Kobayashi, S.; Sugawara, T.; Hirata, T. Effect of Dietary Porphyran from the Red Alga, Porphyra yezoensis, on Glucose Metabolism in Diabetic KK-Ay Mice. J. Nutr. Sci. Vitaminol. 2012, 58, 14–19. [Google Scholar] [CrossRef]
- Cao, C.; Chen, M.; Liang, B.; Xu, J.; Ye, T.; Xia, Z. Hypoglycemic effect of abandoned Porphyra haitanensis polysaccharides in alloxan-induced diabetic mice. Bioact. Carbohydr. Diet. Fibre 2016, 8, 1–6. [Google Scholar] [CrossRef]
- Chao, P.-C.; Hsu, C.-C.; Liu, W.-H. Renal protective effects of Porphyra dentate aqueous extract in diabetic mice. Biomedicine 2014, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Andre, R.; Pacheco, R.; Bourbon, M.; Serralheiro, M.L. Brown Algae Potential as a Functional Food against Hypercholesterolemia: Review. Foods 2021, 10, 234. [Google Scholar] [CrossRef] [PubMed]
- Neto, R.T.; Marcal, C.; Queiros, A.S.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Screening of Ulva rigida, Gracilaria sp., Fucus vesiculosus and Saccharina latissima as Functional Ingredients. Int. J. Mol. Sci. 2018, 19, 2987. [Google Scholar] [CrossRef] [PubMed]
- Ara, J.; Sultana, V.; Qasim, R.; Ahmad, V.U. Hypolipidaemic activity of seaweed from Karachi coast. Phytother. Res. 2002, 16, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.V.; Tsou, Y.C.; Chen, Y.T.; Lu, W.J.; Hwang, P.A. Effects of Low-Molecular-Weight Fucoidan and High Stability Fucoxanthin on Glucose Homeostasis, Lipid Metabolism, and Liver Function in a Mouse Model of Type II Diabetes. Mar. Drugs 2017, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K.; Beppu, F.; Hosokawa, M.; Liu, X.; Wang, S. Nutraceutical characteristics of the brown seaweed carotenoid fucoxanthin. Arch. Biochem. Biophys. 2020, 686, 108364. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Banuelos, T.; Gutierrez-Rodriguez, A.G.; Mendez-Bellido, R.; Tovar-Miranda, R.; Arroyo-Helguera, O.; Juarez-Portilla, C.; Meza-Menchaca, T.; Aguilar-Rosas, L.E.; Hernandez-Kelly, L.C.R.; Ortega, A.; et al. Brown Seaweed Egregia menziesii’s Cytotoxic Activity against Brain Cancer Cell Lines. Molecules 2019, 24, 260. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, M.Y.; Shim, B.J.; Youn, H.J.; Hwang, H.J.; Shin, H.C.; Jeon, H.K. Effects of Ecklonia cava polyphenol in individuals with hypercholesterolemia: A pilot study. J. Med. Food 2012, 15, 1038–1044. [Google Scholar] [CrossRef] [PubMed]
- Trigo, J.P.; Palmnas-Bedard, M.; Juanola, M.V.; Undeland, I. Effects of whole seaweed consumption on humans: Current evidence from randomized-controlled intervention trials, knowledge gaps, and limitations. Front. Nutr. 2023, 10, 1226168. [Google Scholar] [CrossRef]
- Gylling, H.; Simonen, P. Phytosterols, Phytostanols, and Lipoprotein Metabolism. Nutrients 2015, 7, 7965–7977. [Google Scholar] [CrossRef]
- Din, N.A.S.; Mohd Alayudin, S.; Sofian-Seng, N.S.; Rahman, H.A.; Mohd Razali, N.S.; Lim, S.J.; Wan Mustapha, W.A. Brown Algae as Functional Food Source of Fucoxanthin: A Review. Foods 2022, 11, 2235. [Google Scholar] [CrossRef] [PubMed]
- Ha, A.W.; Kim, W.K. The effect of fucoxanthin rich power on the lipid metabolism in rats with a high fat diet. Nutr. Res. Pract. 2013, 7, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.H.; Shiue, S.J.; Chen, C.N.; Cheng, S.W.; Lin, H.Y.; Wu, L.W.; Wu, M.S. Fucoidan and Fucoxanthin Attenuate Hepatic Steatosis and Inflammation of NAFLD through Modulation of Leptin/Adiponectin Axis. Mar. Drugs 2021, 19, 148. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Si, X.; Yuan, Z.; Xu, X.; Li, G. Isolation of fucoxanthin from edible brown algae by microwave-assisted extraction coupled with high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 2313–2317. [Google Scholar] [CrossRef]
- Sohn, S.I.; Rathinapriya, P.; Balaji, S.; Jaya Balan, D.; Swetha, T.K.; Durgadevi, R.; Alagulakshmi, S.; Singaraj, P.; Pandian, S. Phytosterols in Seaweeds: An Overview on Biosynthesis to Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 2691. [Google Scholar] [CrossRef]

| Chemical Composition | S. fusiforme | F. vesiculosus | Porphyra |
|---|---|---|---|
| Water (%) | 15.0 | 9.29 | 6.5 |
| Lipid (%) | 4.23 | 6.43 | 1.5 |
| Carbohydrate (%) | 2.53 | 5.57 | 10.5 |
| Arsenic (As) (%) | 16.1 | 23.42 | 13.6 |
| Mercury (Hg) (mg/kg) | 0.055 | 0.016 | - |
| Cadmium (Cd) (mg/kg) | 0.361 | 0.282 | - |
| Lead (Pb) (mg/kg) | 0.347 | 0.512 | - |
| Beryllium (Be) (mg/kg) | 0.013 | 0.014 | - |
| As (V, mg/kg) | 16.86 | 36.8 | - |
| iAs (V and III, mg/kg) | 0.45 | 0.21 | - |
| Phytosterols (%) | 0.416 | 0.35 | - |
| Parameter | Control (n = 14) | S. fusiforme (n = 12) | F. vesiculosus (n = 10) | p-Value |
|---|---|---|---|---|
| Sex, n (%) men | 8 (57) | 11 (92) | 6 (60) | 0.1 |
| Age (Years) | 65 (54–71) | 60 (46–67) | 66 (54–74) | 0.7 |
| Diabetes duration (years) | 13 (11–21) | 14 (11–19) | 9 (6–14) | 0.2 |
| Insulin users, n (%) | 10 (71) | 8 (67) | 4 (40) | 0.3 |
| Insulin use (units/day) | 76 (57–108) | 59 (46–98) | 56 (15–74) | 0.1 |
| Antidiabetic drug use, n (%) | 7 (50) | 8 (67) | 5 (46) | 0.2 |
| Body mass (kg) | 103 ± 16.9 | 95 ± 7.5 | 102 ± 18.1 | 0.8 |
| Energy intake | 1543 ± 374 | 1748 ± 318 | 1428 ± 595 | 0.2 |
| BMI (kg/m2) | 34 ± 5.1 | 30 ± 2.8 | 34 ± 7.1 | 0.2 |
| Waist circumference (cm) | 119 ± 13.3 | 104 ± 5.5 | 115 ± 12.4 | 0.008 |
| SBP (mm/Hg) | 131 (127–148) | 134 (125–137) | 135 (125–146) | 1.0 |
| DBP (mm/Hg) | 78 ± 7.8 | 82 ± 6.8 | 76 ± 3.7 | 0.1 |
| Outcome | Group | Baseline | Week 2 | Week 5 | p-Value Within-Group Effect | p-Value Between-Group Effect over Time Compared to Control |
|---|---|---|---|---|---|---|
| Avg glucose (mmol/L) | Control (n = 12) | 8.9 ± 1.7 | 8.4 ± 0.5 | 8.6 ± 0.9 | 0.78 | |
| S. fusiforme (n = 10) | 8.2 ± 2.1 | 8.5 ± 0.5 | 9.0 ± 0.7 | 0.18 | 0.25 | |
| F. vesiculosus (n = 5) | 10.1 ± 3.3 | 9.7 ± 0.9 | 9.2 ± 0.7 | 0.86 | 0.90 | |
| Postprandial glucose (mmol/L) | Control (n = 8) | 9.1 ± 1.6 | 9.5 ± 1.1 | 8.5 ± 0.6 | 0.64 | |
| S. fusiforme (n = 9) | 9.7 ± 2.0 | 9.7 ± 1.0 | 9.6 ± 0.9 | 0.92 | 0.97 | |
| F. vesiculosus (n = 2) | 9.6 ± 2.9 | 9.5 ± 1.4 | 11.6 ± 2.1 | 0.37 | 0.92 | |
| TIR (%) | Control (n = 12) | 69 ± 24 | 74 ± 7.1 | 76 ± 7.6 | 0.38 | |
| S. fusiforme (n = 10) | 67 ± 23 | 68 ± 6.4 | 69 ± 8.2 | 0.58 | 0.87 | |
| F. vesiculosus (n = 5) | 55 ± 31 | 53 ± 10.4 | 58 ± 10.8 | 0.78 | 0.75 | |
| TAR (%) | Control (n = 12) | 29 ± 24 | 24 ± 6.7 | 23 ± 7.8 | 0.44 | |
| S. fusiforme (n = 10) | 26 ± 23 | 28 ± 6.5 | 29 ± 8.3 | 0.47 | 0.45 | |
| F. vesiculosus (n = 5) | 42 ± 33 | 43 ± 11.5 | 37 ± 11.3 | 0.63 | 0.86 | |
| TUR (%) | Control (n = 12) | 0.0 (0–1.3) | 0.5 (0–1.5) | 0.7 (0–3.0) | 0.26 | |
| S. fusiforme (n = 10) | 0.0 (0–6.3) | 0.6 (0–3.9) | 0 (0–2.0) | 0.50 | 0.39 | |
| F. vesiculosus (n = 5) | 0.0 (0–13.3) | 0 (0–3.5) | 0 (0–14) | 0.18 | 0.40 | |
| eHbA1c % (mmol/mol) | Control (n = 13) | 7.2 ± 1.1 | - | 6.8 ± 0.3 | 0.25 | |
| S. fusiforme (n = 11) | 6.7 ± 1.4 | - | 7.2 ± 0.4 | 0.13 | 0.05 | |
| F. vesiculosus (n = 9) | 8.1 ± 2.2 | - | 7.4 ± 0.5 | 0.78 | 0.82 |
| Outcome | Group | Baseline | Week 5 | Within-Group Effect (p-Value) | Between-Group Effect over Time Compared to Control (p-Value) |
|---|---|---|---|---|---|
| TC (mmol/L) | Control (n = 10) | 4.1 ± 0.8 | 3.9 ± 0.3 | <0.05 | |
| S. fusiforme (n = 9) | 4.0 ± 1.2 | 3.8 ± 0.2 | 0.17 | 0.48 | |
| F. vesiculosus (n = 5) | 3.7 ± 0.6 | 3.8 ± 0.4 | 0.47 | 0.08 | |
| TG (mmol/L) | Control (n = 10) | 2.0 ± 1.0 | 1.7 ± 0.3 | <0.05 | |
| S. fusiforme (n = 9) | 2.7 ± 1.9 | 3.0 ± 0.8 | <0.001 | 0.25 | |
| F. vesiculosus (n = 5) | 1.0 ± 0.4 | 1.1 ± 0.2 | <0.05 | 0.06 | |
| HDLc (mmol/L) | Control (n = 10) | 1.4 (0.9–1.5) | 1.4 (0.8–1.6) | 0.68 | |
| S. fusiforme (n = 9) | 1.0 (0.8–1.4) | 1.2 (0.7–1.5) | 0.91 | 0.51 | |
| F. vesiculosus (n = 5) | 1.2 (1.2–1.6) | 1.2 (1.2–1.5) | <0.05 | 0.93 | |
| LDLc (mmol/L) | Control (n = 10) | 2.2 (1.9–2.6) | 2.1 (1.7– 2.7) | 0.68 | |
| S. fusiforme (n = 9) | 1.9 (1.6–2.3) | 1.9 (1.5–2.4) | 0.95 | 0.93 | |
| F. vesiculosus (n = 5) | 2.2 (1.8–2.8) | 2.1 (1.8–2.9) | 0.89 | 0.89 | |
| Lp(a) (g/L) | Control (n = 10) | 0.1 (0.07–0.22) | 0.1 (0.09–0.30) | 0.41 | |
| S. fusiforme (n = 10) | 0.1 (0.08–0.13) | 0.1 (0.07–0.21) | 0.55 | 0.72 | |
| F. vesiculosus (n = 5) | 0.4 (0.21–0.49) | 0.3 (0.1–0.5) | 0.34 | 0.40 | |
| ApoA-I (g/L) | Control (n = 10) | 1.6 (1.2–1.8) | 1.5 (1.2–1.8) | <0.001 | |
| S. fusiforme (n = 10) | 1.3 (1.2–1.4) | 1.3 (1.2–1.4) | <0.05 | 0.78 | |
| F. vesiculosus (n = 5) | 1.2 (1.2–1.3) | 1.3 (1.2–1.6) | <0.05 | 0.44 | |
| ApoB (g/L) | Control (n = 10) | 0.8 ± 0.2 | 0.8 ± 0.08 | <0.001 | |
| S. fusiforme (n = 10) | 0.7 ± 0.2 | 0.7 ± 0.09 | 0.28 | 0.57 | |
| F. vesiculosus (n = 5) | 0.7 ± 0.2 | 0.6 ± 0.09 | <0.05 | 0.89 |
| Outcome | Control | S. fusiforme | F. vesiculosus | Between-Group Effects (p-Value) | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline (n = 10) | Week 5 (n = 10) | Baseline (n = 10) | Week 5 (n = 10) | Baseline (n = 5) | Week 5 (n = 5) | Control vs. S. fusiforme | Control vs. F. vesiculosus | |
| Lathosterol (mg/dL) | 0.12 (0.01–0.23) | 0.10 (0.07–0.12) | 0.13 (0.06–0.19) | 0.11 (0.05–0.17) | 0.10 (0.05–0.11) | 0.11 (0.07–0.15) | 0.325 | 0.270 |
| Campesterol (mg/dL) | 0.46 ± 0.07 | 0.36 ± 0.06 | 0.48 ± 0.06 | 0.52 ± 0.07 | 0.60 ± 0.09 | 0.56 ± 0.08 | 0.017 | 0.228 |
| Stigmasterol (µg/dL) | 8.2 ± 1.7 | 7.1 ± 1.4 | 5.8 ± 0.8 | 6.5 ± 1.0 | 10 ± 2.1 | 9.9 ± 2.1 | 0.056 | 0.298 |
| Sitosterol (mg/dL) | 0.27 ± 0.04 | 0.22 ± 0.03 | 0.30 ± 0.03 | 0.30 ± 0.04 | 0.36 ± 0.06 | 0.35 ± 0.06 | 0.097 | 0.174 |
| Avenasterol (µg/dL) | 44 ± 5.1 | 38 ± 3.7 | 50 ± 5.5 | 50 ± 6.5 | 59 ± 10 | 61 ± 12 | 0.145 | 0.144 |
| Brassicasterol (µg/dL) | 10 (7.5–13) | 6.4 (3.9–8.9) | 8.6 (5.2–12) | 12 (9.7–14) | 11 (6.3–15) | 12 (8.1–15) | 0.003 | 0.192 |
| Desmosterol (mg/dL) | 0.16 (0.06–0.27) | 0.12 (0.06–0.17) ** | 0.18 (0.05–0.30) | 0.16 (0.07–0.25) ** | 0.11 (0.07–0.16) | 0.13 (0.11–0.16) | 0.097 | 0.050 |
| 24OH Cholesterol (ng/mL) | 44 ± 4.2 | 39 ± 4.1 | 38 ± 4.5 | 37 ± 4.5 | 41 ± 1.0 | 39 ± 2.9 | 0.304 | 0.595 |
| 7αOH-Cholesterol (ng/mL) | 105 ± 19 | 81.8 ± 11 | 125 ± 22 | 116 ± 20 | 93.3 ± 13 | 86.4 ± 12 | 0.391 | 0.351 |
| 27OH-Cholesterol (ng/mL) | 128 ± 13 | 117 ± 13 ** | 114 ± 10 | 110 ± 13 ** | 133 ± 5.7 | 125 ± 11 | 0.461 | 0.786 |
| Cholesterol (GC) (mg/dL) | 185 ± 13 | 165 ± 14 ** | 171 ± 12 | 159 ± 9.3 ** | 174 ± 5.9 | 173 ± 20 | 0.460 | 0.394 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geurts, K.A.M.; Meijer, S.; Roeters van Lennep, J.E.; Wang, X.; Özcan, B.; Voortman, G.; Liu, H.; Castro Cabezas, M.; Berk, K.A.; Mulder, M.T. The Effect of Sargassum fusiforme and Fucus vesiculosus on Continuous Glucose Levels in Overweight Patients with Type 2 Diabetes Mellitus: A Feasibility Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients 2024, 16, 1837. https://doi.org/10.3390/nu16121837
Geurts KAM, Meijer S, Roeters van Lennep JE, Wang X, Özcan B, Voortman G, Liu H, Castro Cabezas M, Berk KA, Mulder MT. The Effect of Sargassum fusiforme and Fucus vesiculosus on Continuous Glucose Levels in Overweight Patients with Type 2 Diabetes Mellitus: A Feasibility Randomized, Double-Blind, Placebo-Controlled Trial. Nutrients. 2024; 16(12):1837. https://doi.org/10.3390/nu16121837
Chicago/Turabian StyleGeurts, Karlijn A. M., Sjoerd Meijer, Jeanine E. Roeters van Lennep, Xi Wang, Behiye Özcan, Gardi Voortman, Hongbing Liu, Manuel Castro Cabezas, Kirsten A. Berk, and Monique T. Mulder. 2024. "The Effect of Sargassum fusiforme and Fucus vesiculosus on Continuous Glucose Levels in Overweight Patients with Type 2 Diabetes Mellitus: A Feasibility Randomized, Double-Blind, Placebo-Controlled Trial" Nutrients 16, no. 12: 1837. https://doi.org/10.3390/nu16121837





