Association between Childhood Overweight and Altered Concentrations of Circulating Amino Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Anthropometric and Body Composition Evaluation
2.3. Blood Pressure Measures
2.4. Collection of Blood Samples and Biochemical Analyses
2.5. Metabolomics Analysis and Data Processing
2.6. Data Quality and Data Selection
2.7. Statistical Analyses
3. Results
3.1. Chromatographic Analyses
3.2. Study Design and Participants’ Clinical Features
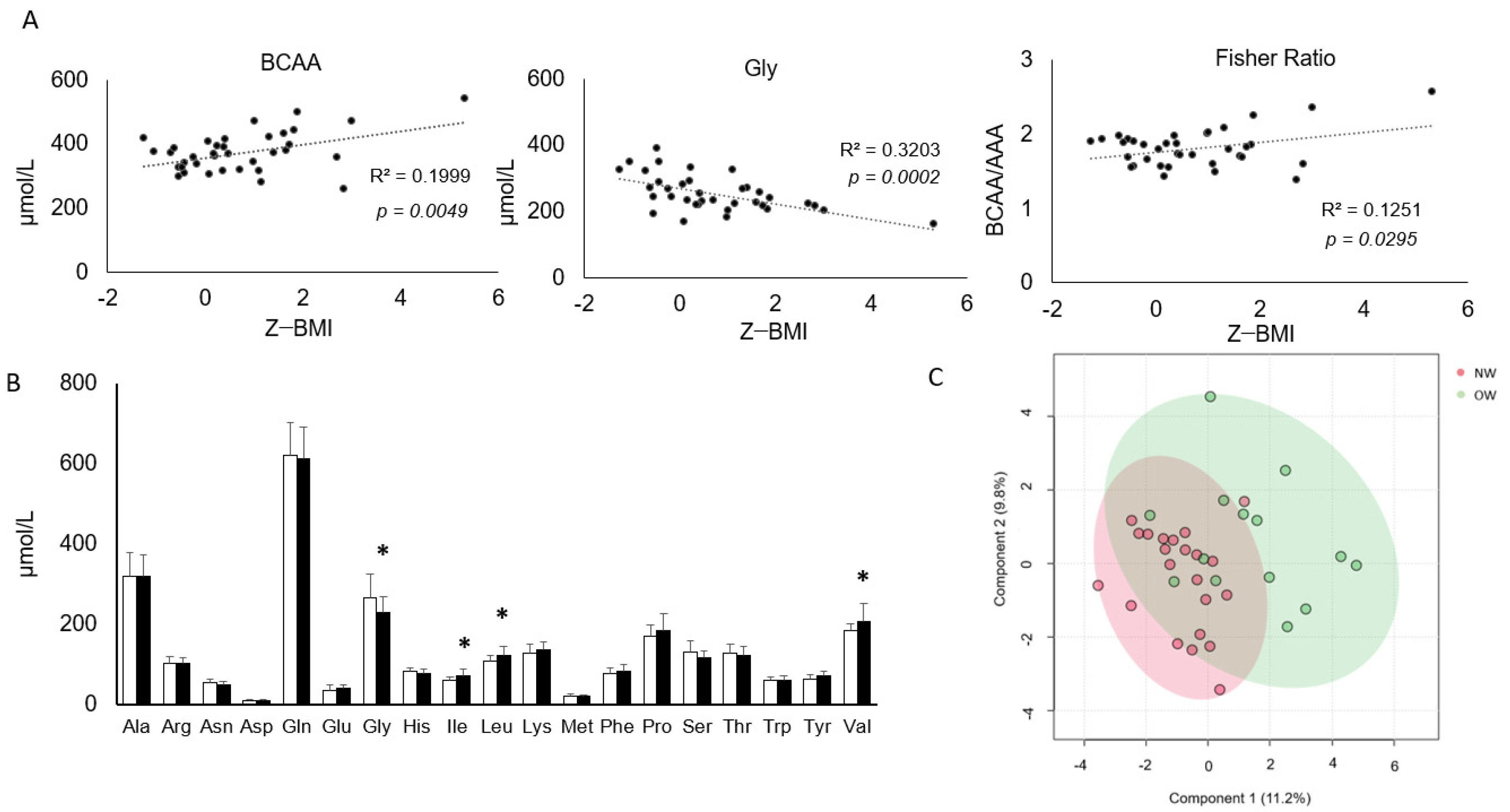
3.3. A Subset of Circulating AA Shows a Correlation with BMI and Is Significantly Altered in Overweight Children
3.4. Correlations among Clinical, Anthropometric Parameters and Amino Acids Profiles
3.5. Amino Acid Profiles Change with Age
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szczyrska, J. Pediatric obesity—Time to act as early as possible. Pediatr. Endocrinol. Diabetes Metab. 2023, 29, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Nishtar, S.; Gluckman, P.; Armstrong, T. Ending childhood obesity: A time for action. Lancet 2016, 387, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Felig, P.; Marliss, E.; Cahill, G.F. Plasma amino acid levels and insulin secretion in obesity. N. Engl. J. Med. 1969, 281, 811–816. [Google Scholar] [CrossRef]
- Newgard, C.B.; An, J.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Lien, L.F.; Haqq, A.M.; Shah, S.H.; Arlotto, M.; Slentz, C.A.; et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009, 9, 311–326. [Google Scholar] [CrossRef]
- Zheng, Y.; Ceglarek, U.; Huang, T.; Li, L.; Rood, J.; Ryan, D.H.; Bray, G.A.; Sacks, F.M.; Schwarzfuchs, D.; Thiery, J.; et al. Weight-loss diets and 2-y changes in circulating amino acids in 2 randomized intervention trials. Am. J. Clin. Nutr. 2016, 103, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Glynn, E.L.; Piner, L.W.; Huffman, K.M.; Slentz, C.A.; Elliot-Penry, L.; AbouAssi, H.; White, P.J.; Bain, J.R.; Muehlbauer, M.J.; Ilkayeva, O.R.; et al. Impact of combined resistance and aerobic exercise training on branched-chain amino acid turnover, glycine metabolism and insulin sensitivity in overweight humans. Diabetologia 2015, 58, 2324–2335. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, M.; Carli, F.; Rosso, C.; Buzzigoli, E.; Marietti, M.; Della Latta, V.; Ciociaro, D.; Abate, M.L.; Gambino, R.; Cassader, M.; et al. Altered amino acid concentrations in NAFLD: Impact of obesity and insulin resistance. Hepatology 2018, 67, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Kivelä, J.; Meinilä, J.; Uusitupa, M.; Tuomilehto, J.; Lindström, J. Longitudinal Branched-Chain Amino Acids, Lifestyle Intervention, and Type 2 Diabetes in the Finnish Diabetes Prevention Study. J. Clin. Endocrinol. Metab. 2022, 107, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Fu, L.; Li, R.; Wang, L.-N.; Yang, Y.; Liu, N.-N.; Zhang, C.-M.; Wang, Y.; Liu, P.; Tu, B.-B.; et al. Metabolic profiles characterizing different phenotypes of polycystic ovary syndrome: Plasma metabolomics analysis. BMC Med. 2012, 10, 153. [Google Scholar] [CrossRef]
- Bacha, F.; El-Ayash, H.; Mohamad, M.; Sharma, S.; Puyau, M.; Kanchi, R.; Coarfa, C. Distinct Amino Acid Profile Characterizes Youth with or at risk for Type 2 Diabetes. Diabetes 2024, 73, 628–636. [Google Scholar] [CrossRef]
- Wang, S.M.; Yang, R.Y.; Wang, M.; Ji, F.; Li, H.; Tang, Y.; Chen, W.; Dong, J. Identification of serum metabolites associated with obesity and traditional risk factors for metabolic disease in Chinese adults. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.; Kim, O.Y.; Jo, G.; Shin, M.-J. Alterations in Circulating Amino Acid Metabolite Ratio Associated with Arginase Activity Are Potential Indicators of Metabolic Syndrome: The Korean Genome and Epidemiology Study. Nutrients 2017, 9, 740. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.H.W.; Wang, Z.; Cho, L.; Brennan, D.M.; Hazen, S.L. Diminished global arginine bioavailability and increased arginine catabolism as metabolic profile of increased cardiovascular risk. J. Am. Coll. Cardiol. 2009, 53, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Lohman, T.G. Applicability of body composition techniques and constants for children and youths. Exerc. Sport. Sci. Rev. 1986, 14, 325–357. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, M.H.; Lohman, T.G.; Boileau, R.A.; Horswill, C.A.; Stillman, R.J.; Van Loan, M.D.; Bemben, D.A. Skinfold equations for estimation of body fatness in children and youth. Hum. Biol. 1988, 60, 709–723. [Google Scholar]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004, 114 (Suppl. S2), 555–576. [CrossRef]
- Malachias, M.V.B.; Koch, V.; Colombo, F.C.; Silva, A.C.S.; Guimarães, I.C.B.; Nogueira, P.K. 7a Diretriz Brasileira de Hipertensão Arterial: Capítulo 10-Hipertensão na Criança e no Adolescente. Arq. Bras. Cardiol. 2016, 107, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.; Benson, M.D.; Long, J.Z.; Chen, Z.-Z.; Wang, R.; Nath, A.K.; Keyes, M.J.; Shen, D.; Sinha, S.; Kuhn, E.; et al. Proteomic profiling reveals biomarkers and pathways in type 2 diabetes risk. JCI Insight 2021, 6, e144392. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.H.; Bain, J.R.; Muehlbauer, M.J.; Stevens, R.D.; Crosslin, D.R.; Haynes, C.; Dungan, J.; Newby, L.K.; Hauser, E.R.; Ginsburg, G.S.; et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ. Cardiovasc. Genet. 2010, 3, 207–214. [Google Scholar] [CrossRef]
- Corbin, L.J.; Hughes, D.A.; Bull, C.J.; Vincent, E.E.; Smith, M.L.; McConnachie, A.; Messow, C.-M.; Welsh, P.; Taylor, R.; Lean, M.E.J.; et al. The metabolomic signature of weight loss and remission in the Diabetes Remission Clinical Trial (DiRECT). Diabetologia 2024, 67, 74–87. [Google Scholar] [CrossRef]
- Sawicki, K.T.; Ning, H.; Allen, N.B.; Carnethon, M.R.; Wallia, A.; Otvos, J.D.; Ben-Sahra, I.; McNally, E.M.; Snell-Bergeon, J.K.; Wilkins, J.T. Longitudinal trajectories of branched chain amino acids through young adulthood and diabetes in later life. JCI Insight 2023, 8, e166956. [Google Scholar] [CrossRef]
- McCormack, S.E.; Shaham, O.; McCarthy, M.A.; Deik, A.A.; Wang, T.J.; Gerszten, R.E.; Clish, C.B.; Mootha, V.K.; Grinspoon, S.K.; Fleischman, A. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatr. Obes. 2013, 8, 52–61. [Google Scholar] [CrossRef]
- Mahbub, M.H.; Yamaguchi, N.; Hase, R.; Takahashi, H.; Ishimaru, Y.; Watanabe, R.; Saito, H.; Shimokawa, J.; Yamamoto, H.; Kikuchi, S.; et al. Plasma Branched-Chain and Aromatic Amino Acids in Relation to Hypertension. Nutrients 2020, 12, 3791. [Google Scholar] [CrossRef]
- Hamaya, R.; Mora, S.; Lawler, P.R.; Cook, N.R.; Ridker, P.M.; Buring, J.E.; Lee, I.-M.; Manson, J.E.; Tobias, D.K. Association of Plasma Branched-Chain Amino Acid with Biomarkers of Inflammation and Lipid Metabolism in Women. Circ. Genom. Precis. Med. 2021, 14, e003330. [Google Scholar] [CrossRef]
- Adeva-Andany, M.; Souto-Adeva, G.; Ameneiros-Rodríguez, E.; Fernández-Fernández, C.; Donapetry-García, C.; Domínguez-Montero, A. Insulin resistance and glycine metabolism in humans. Amino Acids 2018, 50, 11–27. [Google Scholar] [CrossRef]
- do Prado, W.L.; Josephson, S.; Cosentino, R.G.; Churilla, J.R.; Hossain, J.; Balagopal, P.B. Preliminary evidence of glycine as a biomarker of cardiovascular disease risk in children with obesity. Int. J. Obes. 2023, 47, 1023–1026. [Google Scholar] [CrossRef]
- Alves, A.; Bassot, A.; Bulteau, A.-L.; Pirola, L.; Morio, B. Glycine Metabolism and Its Alterations in Obesity and Metabolic Diseases. Nutrients 2019, 11, 1356. [Google Scholar] [CrossRef]
- White, P.J.; Lapworth, A.L.; McGarrah, R.W.; Kwee, L.C.; Crown, S.B.; Ilkayeva, O.; An, J.; Carson, M.W.; Christopher, B.A.; Davies, M.N.; et al. Muscle-Liver Trafficking of BCAA-Derived Nitrogen Underlies Obesity-Related Glycine Depletion. Cell Rep. 2020, 33, 108375. [Google Scholar] [CrossRef]
- Ntzouvani, A.; Nomikos, T.; Panagiotakos, D.; Fragopoulou, E.; Pitsavos, C.; McCann, A.; Ueland, P.; Antonopoulou, S. Amino acid profile and metabolic syndrome in a male Mediterranean population: A cross-sectional study. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 1021–1030. [Google Scholar] [CrossRef]
- Huffman, K.M.; Shah, S.H.; Stevens, R.D.; Bain, J.R.; Muehlbauer, M.; Slentz, C.A.; Tanner, C.J.; Kuchibhatla, M.; Houmard, J.A.; Newgard, C.B.; et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care 2009, 32, 1678–1683. [Google Scholar] [CrossRef]
- Sourij, H.; Meinitzer, A.; Pilz, S.; Grammer, T.B.; Winkelmann, B.R.; Boehm, B.O.; März, W. Arginine bioavailability ratios are associated with cardiovascular mortality in patients referred to coronary angiography. Atherosclerosis 2011, 218, 220–225. [Google Scholar] [CrossRef]
- Yu, E.; Ruiz-Canela, M.; Hu, F.B.; Clish, C.B.; Corella, D.; Salas-Salvadó, J.; Hruby, A.; Fitó, M.; Liang, L.; Toledo, E.; et al. Plasma Arginine/Asymmetric Dimethylarginine Ratio and Incidence of Cardiovascular Events: A Case-Cohort Study. J. Clin. Endocrinol. Metab. 2017, 102, 1879–1888. [Google Scholar] [CrossRef]
- Goli, P.; Yazdi, M.; Heidari-Beni, M.; Kelishadi, R. Growth Hormone Response to L-Arginine Alone and Combined with Different Doses of Growth Hormone-Releasing Hormone: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2022, 2022, 8739289. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, Y.; Guasch-Ferré, M.; Ruiz-Canela, M.; Toledo, E.; Clish, C.; Liang, L.; Razquin, C.; Corella, D.; Estruch, R.; et al. High plasma glutamate and low glutamine-to-glutamate ratio are associated with type 2 diabetes: Case-cohort study within the PREDIMED trial. Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1040–1049. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, F.B.; Ruiz-Canela, M.; Clish, C.B.; Dennis, C.; Salas-Salvado, J.; Hruby, A.; Liang, L.; Toledo, E.; Corella, D.; et al. Metabolites of Glutamate Metabolism Are Associated with Incident Cardiovascular Events in the PREDIMED PREvención con DIeta MEDiterránea (PREDIMED) Trial. J. Am. Heart Assoc. 2016, 5, e003755. [Google Scholar] [CrossRef]
- Kouchiwa, T.; Wada, K.; Uchiyama, M.; Kasezawa, N.; Niisato, M.; Murakami, H.; Fukuyama, K.; Yokogoshi, H. Age-related changes in serum amino acids concentrations in healthy individuals. Clin. Chem. Lab. Med. 2012, 50, 861–870. [Google Scholar] [CrossRef]
- De Bandt, J.-P.; Coumoul, X.; Barouki, R. Branched-Chain Amino Acids and Insulin Resistance, from Protein Supply to Diet-Induced Obesity. Nutrients 2022, 15, 68. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Normal Weight n = 22 | Overweight n = 14 | p Value |
|---|---|---|---|
| Age 1 | 9.4 ± 1.1 | 9.9 ± 0.9 | 0.181 |
| Height 1 | 1.38 ± 0.07 | 1.4 ± 0.09 | 0.049 |
| BMI 2 | 16.2 ± 1.3 | 22.2 ± 3.8 | <0.001 |
| BMI Z-Score 1 | −0.09 ± 0.56 | 2.05 ± 1.14 | <0.001 |
| WC 2 | 71.0 ± 5.18 | 85.0 ± 10.1 | <0.001 |
| Waist/Height 2 | 0.43 ± 0.024 | 0.53 ± 0.05 | <0.001 |
| BF 2 | 19.0 ± 5.7 | 32.4 ± 10.5 | <0.001 |
| TC 1 | 158.1 ± 32.4 | 152.0 ± 28.4 | 0.575 |
| HDL 1 | 46.6 ± 13.1 | 38.7 ± 13.8 | 0.104 |
| TG 2 | 64.5 ± 21.2 | 84.2 ± 32.4 | 0.050 |
| LDL 1 | 98.4 ± 22.4 | 96.5 ± 21.2 | 0.858 |
| FG 1 | 87.0 ± 6.99 | 89.5 ± 7.05 | 0.324 |
| SBP 1 | 95.1 ± 6.47 | 98.8 ± 10.9 | 0.214 |
| DBP 1 | 59.2 ± 6.76 | 61.5 ± 10.4 | 0.417 |
| Variables | TC | HDL | LDL | TG | FG | PAS | PAD | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| Glutamine | −0.012 | 0.948 | 0.399 | 0.026 | −0.153 | 0.411 | −0.306 | 0.094 | −0.144 | 0.439 | −0.007 | 0.97 | −0.106 | 0.569 |
| Glutamic acid | −0.225 | 0.225 | −0.274 | 0.136 | −0.126 | 0.499 | 0.126 | 0.5 | 0.254 | 0.168 | 0.222 | 0.23 | 0.393 | 0.029 |
| Glycine | −0.112 | 0.549 | 0.304 | 0.096 | −0.23 | 0.213 | −0.293 | 0.11 | −0.298 | 0.103 | −0.208 | 0.26 | −0.218 | 0.239 |
| Isoleucine | 0.072 | 0.701 | −0.082 | 0.661 | 0.037 | 0.845 | 0.467 | 0.008 | −0.088 | 0.639 | 0.289 | 0.115 | 0.198 | 0.285 |
| Leucine | 0.027 | 0.884 | −0.041 | 0.828 | −0.009 | 0.962 | 0.352 | 0.052 | −0.094 | 0.614 | 0.147 | 0.431 | 0.073 | 0.697 |
| Phenylalanine | −0.019 | 0.918 | −0.216 | 0.243 | 0.068 | 0.718 | 0.117 | 0.531 | −0.021 | 0.91 | −0.194 | 0.295 | 0.046 | 0.807 |
| Tryptophan | 0.048 | 0.798 | 0.239 | 0.196 | −0.053 | 0.777 | −0.067 | 0.721 | 0.081 | 0.664 | −0.204 | 0.27 | −0.373 | 0.039 |
| Tyrosine | 0.036 | 0.847 | 0.061 | 0.746 | −0.029 | 0.877 | 0.251 | 0.173 | 0.248 | 0.178 | 0.144 | 0.438 | 0.132 | 0.479 |
| Valine | 0.019 | 0.921 | −0.232 | 0.208 | 0.063 | 0.738 | 0.442 | 0.013 | −0.144 | 0.438 | 0.339 | 0.062 | 0.212 | 0.252 |
| BCAA | 0.034 | 0.857 | −0.153 | 0.411 | 0.038 | 0.84 | 0.446 | 0.012 | −0.125 | 0.502 | 0.287 | 0.117 | 0.177 | 0.34 |
| Fisher_Ratio | 0.027 | 0.887 | −0.174 | 0.348 | 0.053 | 0.775 | 0.354 | 0.051 | −0.254 | 0.168 | 0.409 | 0.022 | 0.229 | 0.216 |
| GABR | −0.127 | 0.496 | −0.047 | 0.802 | −0.122 | 0.513 | 0.058 | 0.756 | 0.443 | 0.013 | −0.298 | 0.104 | −0.094 | 0.614 |
| Gln/Glu | 0.263 | 0.153 | 0.378 | 0.036 | 0.135 | 0.468 | −0.225 | 0.223 | −0.163 | 0.382 | −0.168 | 0.367 | −0.363 | 0.044 |
| Variables | Z-BMI | WC | RCE | %BF | ||||
|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | |
| Glutamine | −0.217 | 0.217 | −0.165 | 0.352 | −0.256 | 0.144 | −0.238 | 0.176 |
| Glutamic acid | 0.235 | 0.182 | 0.236 | 0.179 | 0.232 | 0.186 | 0.196 | 0.268 |
| Glycine | −0.570 | 0.000 | −0.449 | 0.008 | −0.497 | 0.003 | −0.519 | 0.002 |
| Isoleucine | 0.345 | 0.045 | 0.357 | 0.038 | 0.271 | 0.121 | 0.253 | 0.149 |
| Leucine | 0.253 | 0.149 | 0.237 | 0.178 | 0.168 | 0.341 | 0.189 | 0.285 |
| Phenylalanine | 0.055 | 0.757 | 0.036 | 0.842 | 0.069 | 0.7 | 0.074 | 0.677 |
| Tryptophan | −0.133 | 0.455 | −0.181 | 0.306 | −0.201 | 0.255 | −0.239 | 0.174 |
| Tyrosine | 0.244 | 0.164 | 0.248 | 0.158 | 0.224 | 0.203 | 0.284 | 0.104 |
| Valine | 0.463 | 0.006 | 0.457 | 0.007 | 0.342 | 0.048 | 0.393 | 0.021 |
| BCAA | 0.397 | 0.020 | 0.391 | 0.022 | 0.291 | 0.096 | 0.32 | 0.065 |
| Fisher_Ratio | 0.362 | 0.035 | 0.372 | 0.030 | 0.257 | 0.143 | 0.284 | 0.103 |
| GABR | 0.097 | 0.587 | 0.114 | 0.521 | 0.124 | 0.484 | 0.284 | 0.103 |
| Gln/Glu | −0.551 | 0.001 | −0.493 | 0.005 | −0.478 | 0.007 | −0.413 | 0.021 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, J.d.O.; Oliveira, T.L.P.S.d.A.; Vitalis, O.; Pereira, J.G.; Nogueira, I.d.C.R.; Santos, G.C.J.; Chikh, K.; Leandro, C.G.; Costa-Silva, J.H.d.; Pirola, L. Association between Childhood Overweight and Altered Concentrations of Circulating Amino Acids. Nutrients 2024, 16, 1843. https://doi.org/10.3390/nu16121843
Campos JdO, Oliveira TLPSdA, Vitalis O, Pereira JG, Nogueira IdCR, Santos GCJ, Chikh K, Leandro CG, Costa-Silva JHd, Pirola L. Association between Childhood Overweight and Altered Concentrations of Circulating Amino Acids. Nutrients. 2024; 16(12):1843. https://doi.org/10.3390/nu16121843
Chicago/Turabian StyleCampos, Jéssica de Oliveira, Tafnes Laís Pereira Santos de Almeida Oliveira, Oriane Vitalis, Jéssica Gonzaga Pereira, Isabella da Costa Ribeiro Nogueira, Gabriela Carvalho Jurema Santos, Karim Chikh, Carol Gois Leandro, João Henrique da Costa-Silva, and Luciano Pirola. 2024. "Association between Childhood Overweight and Altered Concentrations of Circulating Amino Acids" Nutrients 16, no. 12: 1843. https://doi.org/10.3390/nu16121843





