Analysis of Alterations in Intestinal Flora in Chinese Elderly with Cardiovascular Disease and Its Association with Trimethylamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Volunteer Recruitment
2.2. Fecal Material
2.3. Extraction and Detection of TMA
2.4. 16S rRNA Sequencing and Bioinformatic Analysis
2.5. Fecal Sample DNA Extraction and qPCR
2.6. Statistical Analysis
3. Results and Discussion
3.1. Analysis of Fecal TMA Content in Elderly Adults
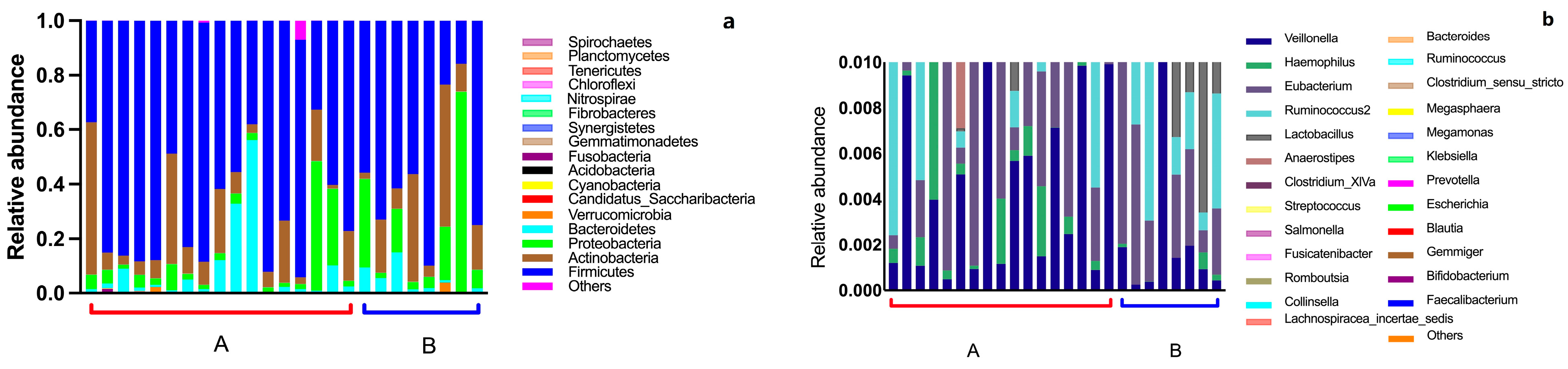
3.2. Intestinal Flora Composition in the Elderly
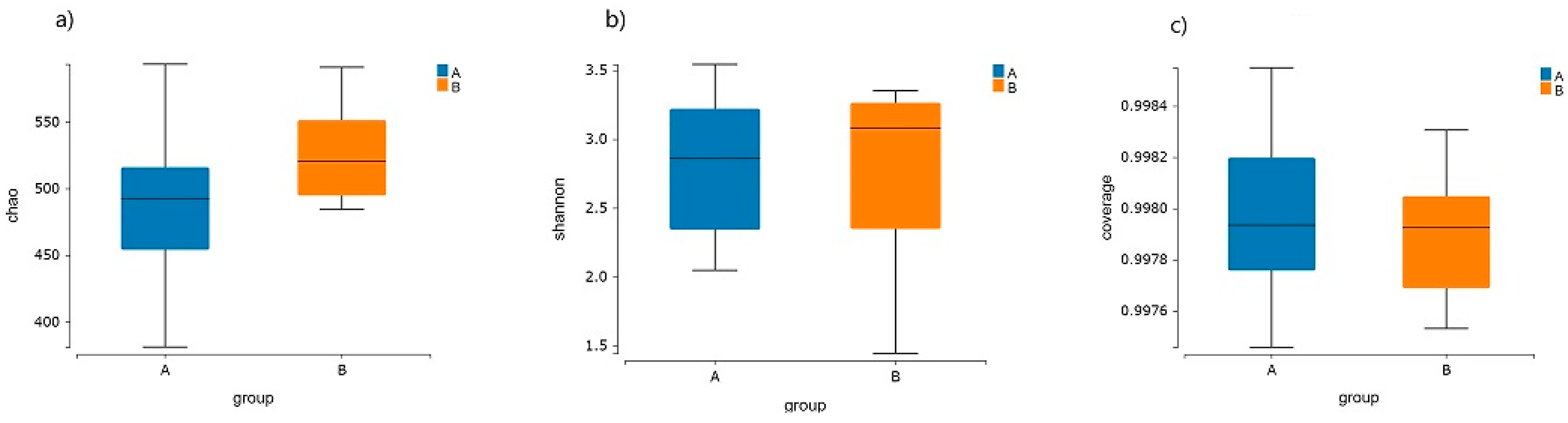
3.3. Intestinal Flora Diversity in the Elderly
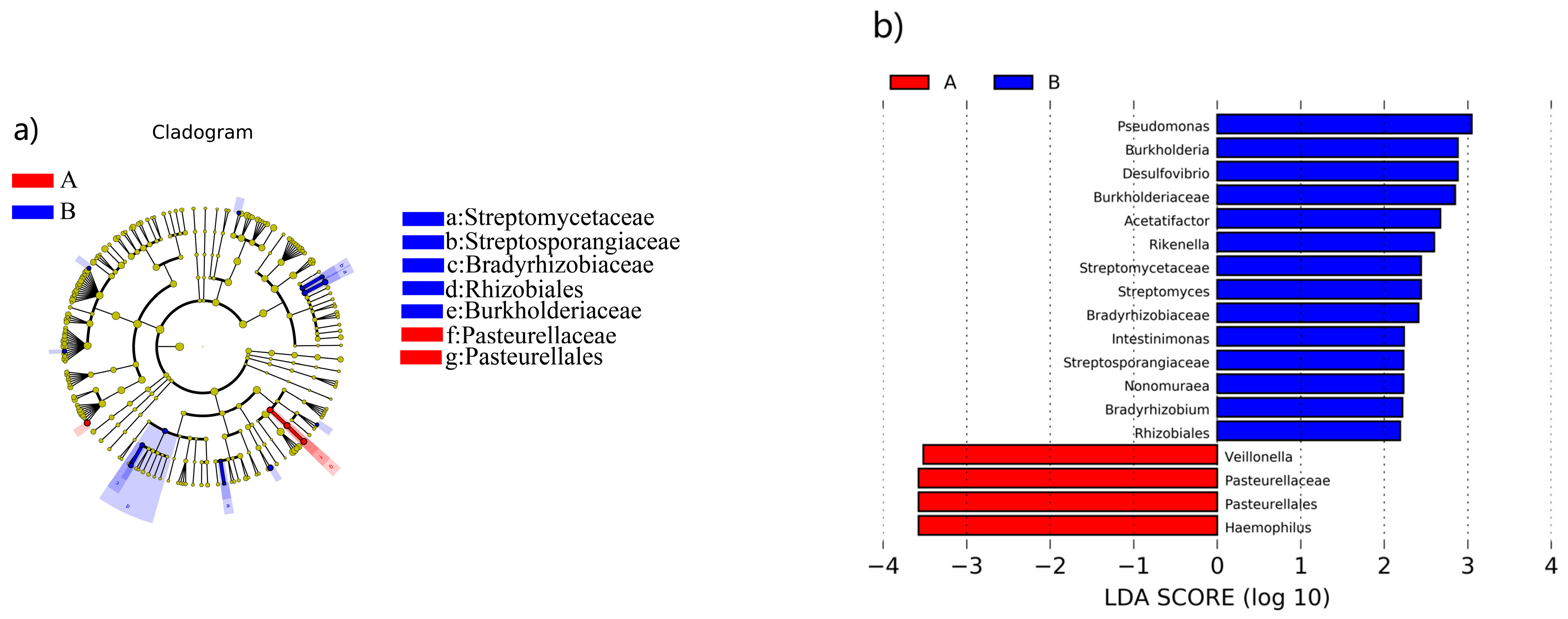
3.4. Analysis of the Characterized Enteric Bacteria That Produce TMA
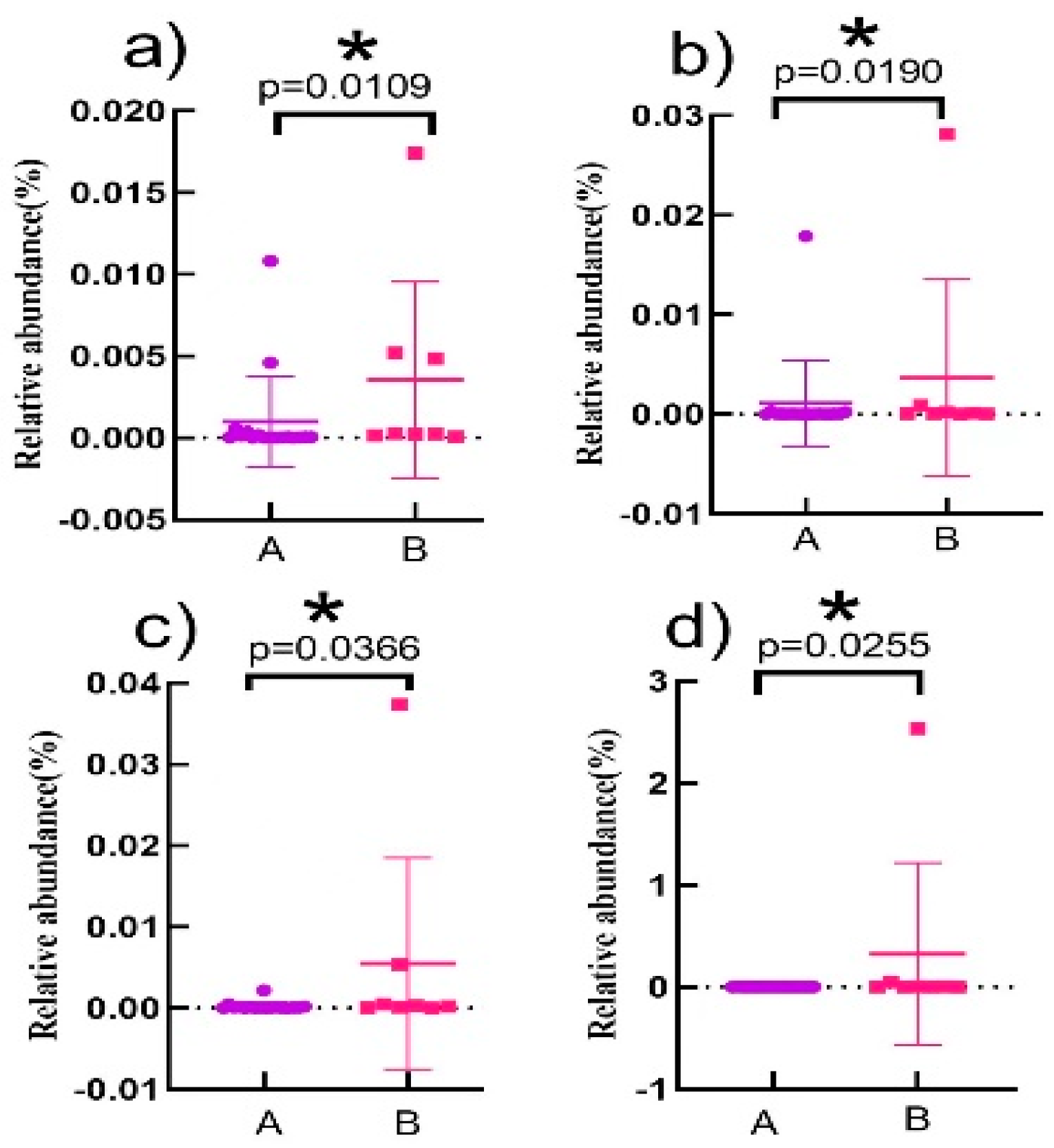
3.5. Correlation Analysis of Characteristic Intestinal Bacteria in the Elderly with TMA
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexander, D.D.; Bylsma, L.C.; Vargas, A.J.; Cohen, S.S.; Doucette, A.; Mohamed, M.; Irvin, S.R.; Miller, P.E.; Watson, H.; Fryzek, J.P. Dairy consumption and CVD: A systematic review and meta-analysis. Br. J. Nutr. 2016, 115, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Ebesunun, M.O.; Agbedana, E.O.; Taylor, G.O.L.; Oladapo, O.O. Plasma lipoprotein (a), homocysteine, and other cardiovascular disease (CVD) risk factors in Nigerians with CVD. Appl. Physiol. Nutr. Metab. 2008, 33, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Tourlouki, E.; Matalas, A.L.; Panagiotakos, D.B. Dietary habits and cardiovascular disease risk in middle-aged and elderly populations: A review of evidence. Clin. Interv. Aging 2009, 4, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Protulipac, J.M.; Sonicki, Z.; Reiner, Z. Cardiovascular disease (CVD) risk factors in older adults—Perception and reality. Arch. Gerontol. Geriatr. 2015, 61, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in Disease: Potential Biomarker or New Therapeutic Target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [PubMed]
- Jaworska, K.; Hering, D.; Mosieniak, G.; Bielak-Zmijewska, A.; Pilz, M.; Konwerski, M.; Gasecka, A.; Kaplon-Cieslicka, A.; Filipiak, K.; Sikora, E.; et al. TMA, A Forgotten Uremic Toxin, but Not TMAO, Is Involved in Cardiovascular Pathology. Toxins 2019, 11, 490. [Google Scholar] [CrossRef] [PubMed]
- Gatarek, P.; Kaluzna-Czaplinska, J. Trimethylamine N-oxide (TMAO) in human health. EXCLI J. 2021, 20, 301–319. [Google Scholar] [CrossRef]
- Li, X.S.M.; Obeid, S.; Klingenberg, R.; Gencer, B.; Mach, F.; Räber, L.; Windecker, S.; Rodondi, N.; Nanchen, D.; Muller, O.; et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: A prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur. Heart J. 2017, 38, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Dalla Via, A.; Gargari, G.; Taverniti, V.; Rondini, G.; Velardi, I.; Gambaro, V.; Visconti, G.L.; De Vitis, V.; Gardana, C.; Ragg, E.; et al. Urinary TMAO Levels Are Associated with the Taxonomic Composition of the Gut Microbiota and with the Choline TMA-Lyase Gene (cutC) Harbored by Enterobacteriaceae. Nutrients 2020, 12, 62. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Charan Reddy, K.V. Heart-gut axis: Targeting proprotein convertase subtilisin/kexin type 9 (PCSK9) to prevent cardiovascular disease through gut microbiota. Med. Microecol. 2021, 7, 100033. [Google Scholar] [CrossRef]
- Katsimichas, T.; Ohtani, T.; Motooka, D.; Tsukamoto, Y.; Kioka, H.; Nakamoto, K.; Konishi, S.; Chimura, M.; Sengoku, K.; Miyawaki, H. Non-Ischemic Heart Failure with Reduced Ejection Fraction Is Associated with Altered Intestinal Microbiota. J. Jpn. Circ. Soc. 2018, 82, 1640–1650. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H.; et al. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2018, 8, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, F.H.; Fåk, F.; Nookaew, I.; Tremaroli, V.; Fagerberg, B.; Petranovic, D.; Bäckhed, F.; Nielsen, J. Symptomatic atherosclerosis is associated with an altered gut metagenome. Nat. Commun. 2012, 3, 1245–1253. [Google Scholar] [CrossRef] [PubMed]
- Ramireddy, L.; Tsen, H.Y.; Chiang, Y.C.; Hung, C.Y.; Chen, F.C.; Sciences, H.T. The gene expression and bioinformatic analysis of choline trimethylamine-lyase (CutC) and its activating enzyme (CutD) for gut microbes and comparison with their TMA production levels. Curr. Res. Microb. Sci. 2021, 2, 10043–10053. [Google Scholar] [CrossRef] [PubMed]
- Bergström, A.; Licht, T.R.; Wilcks, A.; Andersen, J.B.; Schmidt, L.R.; Gronlund, H.A.; Vigsnæs, L.K.; Michaelsen, K.F.; Bahl, M.I. Introducing GUt Low-Density Array (GULDA)—A validated approach for qPCR-based intestinal microbial community analysis. FEMS Microbiol. Lett. 2012, 337, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Simó, C.; Garcia-Canas, V. Dietary bioactive ingredients to modulate the gut microbiota-derived metabolite TMAO. New opportunities for functional food development. Food Funct. 2020, 11, 6745–6776. [Google Scholar] [CrossRef] [PubMed]
- Ufnal, M. Trimethylamine, a Toxic Precursor of Trimethylamine Oxide, Lost in Medical Databases. J. Nutr. 2020, 150, 419. [Google Scholar] [CrossRef] [PubMed]
- Go, A.S.; Chertow, G.M.; Fan, D.J.; McCulloch, C.E.; Hsu, C.Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Drapkina, O.M.; Kaburova, A.N. Gut Microbiota Composition and Metabolites as the new Determinants of Cardiovascular Pathology Development. Stolichnaya Izd. Ko. 2020, 16, 277–285. [Google Scholar] [CrossRef]
- Restini, C.B.A.; Fink, G.D.; Watts, S.W. Vascular reactivity stimulated by TMA and TMAO: Are perivascular adipose tissue and endothelium involved? Pharmacol. Res. 2020, 163, 105273. [Google Scholar] [CrossRef]
- Tang, W.W.; Wang, Z.; Kennedy, D.J.; Wu, Y.; Buffa, J.A.; Agatisa-Boyle, B.; Li, X.S.; Levison, B.S.; Hazen, S.L. Gut Microbiota-Dependent Trimethylamine N-Oxide (TMAO) Pathway Contributes to Both Development of Renal Insufficiency and Mortality Risk in Chronic Kidney Disease. Circ. Res. 2015, 116, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Reiner, M.F.; Müller, D.; Gobbato, S.; Stalder, O.; Limacher, A.; Bonetti, N.R.; Pasterk, L.; Méan, M.; Rodondi, N.; Aujesky, D.; et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) shows a U-shaped association with mortality but not with recurrent venous thromboembolism. Thromb. Res. 2019, 174, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Emoto, T.; Hayashi, T.; Tabata, T.; Yamashita, T.; Watanabe, H.; Takahashi, T.; Gotoh, Y.; Kami, K.; Yoshida, N.; Saito, Y.; et al. Metagenomic analysis of gut microbiota reveals its role in trimethylamine metabolism in heart failure. Int. J. Cardiol. 2021, 338, 138–142. [Google Scholar] [CrossRef]
- Claesson, M.J.; Cusack, S.; O’Sullivan, O.; Greene-Diniz, R.; de Weerd, H.; Flannery, E.; Marchesi, J.R.; Falush, D.; Dinan, T.; Fitzgerald, G.; et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc. Natl. Acad. Sci. USA 2011, 108, 4586–4591. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Nylund, L.; Candela, M.; Ostan, R.; Bucci, L.; Pini, E.; Nikkïla, J.; Monti, D.; Satokari, R.; Franceschi, C.; et al. Through Ageing, and Beyond: Gut Microbiota and Inflammatory Status in Seniors and Centenarians. PLoS ONE 2010, 5, e10667. [Google Scholar] [CrossRef]
- Claesson, M.J.; Jeffery, I.B.; Conde, S.; Power, S.E.; O’Connor, E.M.; Cusack, S.; Harris, H.M.B.; Coakley, M.; Lakshminarayanan, B.; O’Sullivan, D.; et al. Gut microbiota composition correlates with diet and health in the elderly. Nature 2012, 488, 178–184. [Google Scholar] [CrossRef]
- Schirmer, M.; Ijaz, U.Z.; D’Amore, R.; Hall, N.; Sloan, W.T.; Quince, C. Insight into biases and sequencing errors for amplicon sequencing with the Illumina MiSeq platform. Nucleic Acids Res. 2015, 43, e37. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Yu, Z.T. Variations in 16S rRNA-based Microbiome Profiling between Pyro sequencing Runs and between Pyrosequencing Facilities. J. Microbiol. 2014, 52, 355–365. [Google Scholar] [CrossRef]
- Al-Rubaye, H.; Perfetti, G.; Kaski, J.C. The Role of Microbiota in Cardiovascular Risk: Focus on Trimethylamine Oxide. Curr. Probl. Cardiol. 2019, 44, 180–196. [Google Scholar] [CrossRef]
- Kuang, J.J.; Yan, X.; Genders, A.J.; Granata, C.; Bishop, D.J. An overview of technical considerations when using quantitative real-time PCR analysis of gene expression in human exercise research. PLoS ONE 2018, 13, e0196438. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.W.; Chen, M.K.; Yang, B.Y.; Huang, X.J.; Zhang, X.R.; He, L.Q.; Zhang, J.; Hua, Z.C. Use of 16S rRNA Gene-Targeted Group-Specific Primers for Real-Time PCR Analysis of Predominant Bacteria in Mouse Feces. Appl. Environ. Microbiol. 2015, 81, 6749–6756. [Google Scholar] [CrossRef] [PubMed]
- Ohashi, Y.; Fujisawa, T. Analysis of Clostridium cluster XI bacteria in human feces. Biosci. Microbiota Food Health 2019, 38, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Gerges, M.A.; Esmaeel, N.E.; Makram, W.K.; Sharaf, D.M.; Gebriel, M. Altered Profile of Fecal Microbiota in Newly Diagnosed Systemic Lupus Erythematosus Egyptian Patients. Int. J. Microbiol. 2021, 2021, 9934533. [Google Scholar] [CrossRef] [PubMed]
- Jomehzadeh, N.; Javaherizadeh, H.; Amin, M.; Rashno, M.; Teimoori, A.J.C.; Gastroenterology, E. Quantification of Intestinal Lactobacillus Species in Children with Functional Constipation by Quantitative Real-Time PCR. Clin. Exp. Gastroenterol. 2020, 13, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, T.; Kasahara, K.; Emoto, T.; Matsumoto, T.; Mizoguchi, T.; Kitano, N.; Sasaki, N.; Hirata, K.-I. Intestinal Immunity and Gut Microbiota as Therapeutic Targets for Preventing Atherosclerotic Cardiovascular Diseases. Circ. J. 2015, 79, 1882–1890. [Google Scholar] [CrossRef]
- Kailin, H.E.; Haoran, J.I.; Hang, Z.; Tao, H.U.; Ke, X. Heart-to-intestine dialogue: The role of gut bacteria in cardiovascular disease. Chin. J. Arterioscler. 2019, 27, 718–723. [Google Scholar]



| Gender | Age | Height (cm) | Weight (kg) | Physical Condition | Group | |
|---|---|---|---|---|---|---|
| 1 | Female | 65 | 160 | 51 | CVD (diabetes, hypertension, hyperlipidemia and hyperglycemia) | B |
| 2 | Male | 66 | 168 | 71 | CVD (cerebral infarction) | B |
| 3 | Female | 62 | 160 | 61 | healthy | A |
| 4 | Male | 63 | 165 | 70 | CVD (hypertension) | B |
| 5 | Male | 68 | 165 | 65 | healthy | A |
| 6 | Female | 68 | 155 | 60 | CVD (cerebral infarction) | B |
| 7 | Female | 66 | 156 | 60 | CVD (hypertension) | B |
| 8 | Male | 71 | 164 | 65 | CVD (cardiopathy) | B |
| 9 | Female | 60 | 150 | 60 | CVD (hypertension, hyperglycemia) | B |
| 10 | Male | 65 | 171 | 73 | healthy | A |
| 11 | Male | 73 | 165 | 65 | healthy | A |
| 12 | Male | 72 | 171 | 65 | CVD (cardiopathy) | B |
| 13 | Female | 65 | 162 | 65 | healthy | A |
| 14 | Male | 69 | 165 | 70 | healthy | A |
| 15 | Female | 61 | 159 | 60 | healthy | A |
| 16 | Female | 70 | 164 | 65 | healthy | A |
| 17 | Male | 66 | 173 | 60 | healthy | A |
| 18 | Female | 67 | 155 | 48 | healthy | A |
| 19 | Male | 65 | 160 | 65 | healthy | A |
| 20 | Female | 66 | 1.62 | 68 | healthy | A |
| 21 | Female | 70 | 155 | 60 | healthy | A |
| 22 | Male | 63 | 165 | 65 | healthy | A |
| 23 | Female | 60 | 155 | 60 | healthy | A |
| 24 | Male | 70 | 160 | 64 | healthy | A |
| 25 | Male | 60 | 159 | 64.5 | healthy | A |
| Target | Sequence (5′→3′) | Amplicon Length (bp) | References |
|---|---|---|---|
| Klebsiella pneumoniae | F-GATCTGACCTATCTGATTATGG R-TTGTGGAGCATCATCTTGAT | 185 | [9] |
| Clostridium sporogenes | F-TCGTGAAGCAGGAGTATGGG R-GTCAACACGTCCTATAGACATACC | 460 | [15] |
| Escherichia Ferguson | F-AGCGAACTGGGAGCGAAATA- R-TACGACCACGGTTGAGGACA | 421 | [15] |
| Desulfovibrio desulfuricans | F-CGTGTTGACCAGTACATGTA R-GCTGGTAACCTGCGAAGAA | 163 | [9] |
| Total bacteria | F-ACTCCTACGGGAGGCAGCAGT R-GTATTACCGCGGCTGCTGGCAC | 190 | [16] |
| Stage | Cycle | Temperature (°C) | Time (s) |
|---|---|---|---|
| Stage 1 | 95 | 300 | |
| Stage 2 | 40 | 95 | 15 |
| 58 | 20 | ||
| 72 | 40 | ||
| Stage 3 | Melting curve | ||
| Grape | Species Level | Species | p Value |
|---|---|---|---|
| A, B | class | Alphaproteobacteria | 0.038635 |
| order | Pasteurellales | 0.01208 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Chen, S.; Xue, Y.; Lu, H.; Li, Z.; Jia, X.; Ning, Y.; Yuan, Q.; Wang, S. Analysis of Alterations in Intestinal Flora in Chinese Elderly with Cardiovascular Disease and Its Association with Trimethylamine. Nutrients 2024, 16, 1864. https://doi.org/10.3390/nu16121864
He Y, Chen S, Xue Y, Lu H, Li Z, Jia X, Ning Y, Yuan Q, Wang S. Analysis of Alterations in Intestinal Flora in Chinese Elderly with Cardiovascular Disease and Its Association with Trimethylamine. Nutrients. 2024; 16(12):1864. https://doi.org/10.3390/nu16121864
Chicago/Turabian StyleHe, Yannan, Song Chen, Yuling Xue, Han Lu, Ziteng Li, Xianxian Jia, Yibing Ning, Qingbin Yuan, and Shijie Wang. 2024. "Analysis of Alterations in Intestinal Flora in Chinese Elderly with Cardiovascular Disease and Its Association with Trimethylamine" Nutrients 16, no. 12: 1864. https://doi.org/10.3390/nu16121864
APA StyleHe, Y., Chen, S., Xue, Y., Lu, H., Li, Z., Jia, X., Ning, Y., Yuan, Q., & Wang, S. (2024). Analysis of Alterations in Intestinal Flora in Chinese Elderly with Cardiovascular Disease and Its Association with Trimethylamine. Nutrients, 16(12), 1864. https://doi.org/10.3390/nu16121864






