AI-Assisted Body Composition Assessment Using CT Imaging in Colorectal Cancer Patients: Predictive Capacity for Sarcopenia and Malnutrition Diagnosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Screening, Assessment, and Nutritional Intervention
- Height, weight, and body mass index (BMI), as well as weight loss in the previous six months.
- Morphofunctional assessment:
- -
- Impedance measurement using a portable device (Akern BIA-101/Nutrilab analyzer, Akern SRL, Pontassieve, Florence, Italy). The variables collected were fat mass, fat-free mass, fat-free mass index (FFMI), appendicular skeletal muscle mass (ASMM), phase angle, and body cell mass (BCM).
- -
- Handgrip strength measurement using a Jamar dynamometer (Asimow Engineering Co., Los Angeles, CA, USA). Low handgrip strength was considered when values were below the cut-off points indicated in the EWGSOP2 criteria for sarcopenia [16].
- Diagnosis of malnutrition using GLIM criteria. It was estimated that all patients presented at least one etiological criterion, as they had colorectal neoplasia (considered a chronic inflammatory process). For the phenotypic criteria, patients with low BMI, weight loss greater than 5% in six months, and low FFMI by BIA were identified according to the cutoff points recommended by the consensus itself [3].
- Diagnosis of sarcopenia according to EWGSOP2 criteria. Cutoff points for low handgrip strength stipulated by the consensus were used, and ASMM assessed by BIA was used as a parameter for low muscle mass [16].
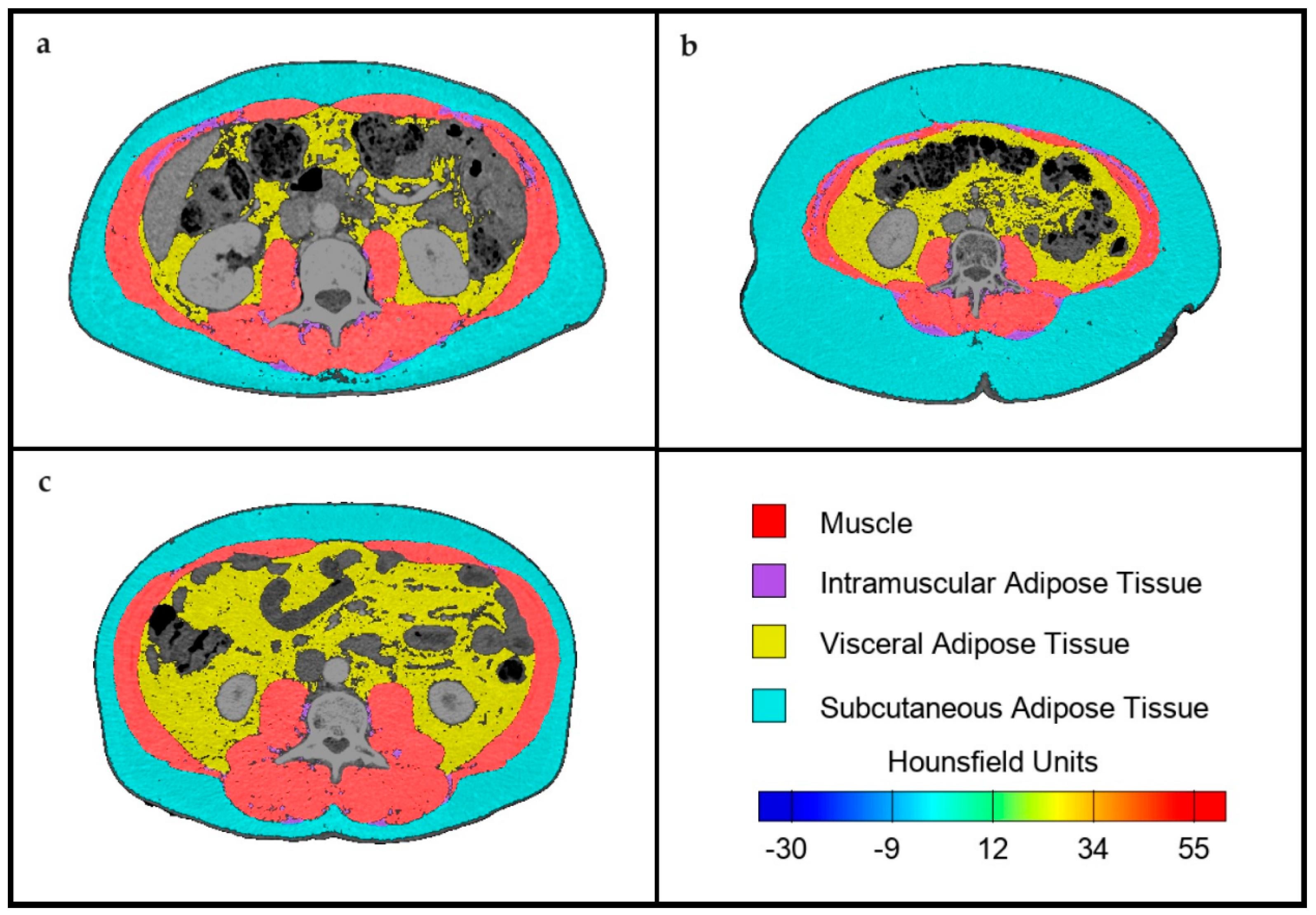
2.2. Body Composition Assessment by CT
2.3. Statistical Analysis
2.4. Ethics
3. Results
Assessment of Body Composition and Anthropometric Measures
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Gastroenterol. Rev. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Gillis, C.; Richer, L.; Fenton, T.R.; Gramlich, L.; Keller, H.; Culos-Reed, S.N.; Sajobi, T.T.; Awasthi, R.; Carli, F. Colorectal cancer patients with malnutrition suffer poor physical and mental health before surgery. Surgery 2021, 170, 841–847. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.J.S.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Ruan, X.; Wang, X.; Zhang, Q.; Nakyeyune, R.; Shao, Y.; Shen, Y.; Niu, C.; Zhu, L.; Zang, Z.; Wei, T.; et al. The performance of three nutritional tools varied in colorectal cancer patients: A retrospective analysis. J. Clin. Epidemiol. 2022, 149, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Song, H.N.; Wang WBin Luo, X.; Huang, D.D.; Ruan, X.J.; Xing, C.G.; Chen, W.Z.; Dong, Q.T.; Chen, X.L. Effect of GLIM-defined malnutrition on postoperative clinical outcomes in patients with colorectal cancer. Jpn. J. Clin. Oncol. 2022, 52, 466–474. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Carli, F.; Higashiguchi, T.; Hübner, M.; Klek, S.; Laviano, A.; Ljungqvist, O.; Lobo, D.N.; Martindale, R.G.; et al. ESPEN practical guideline: Clinical nutrition in surgery. Clin. Nutr. 2021, 40, 4745–4761. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torralvo, F.J.; González-Poveda, I.; García-Olivares, M.; Porras, N.; Gonzalo-Marín, M.; Tapia, M.J.; Mera-Velasco, S.; Toval-Mata, J.A.; Ruiz-López, M.; Carrasco-Campos, J.; et al. Poor Physical Performance Is Associated with Postoperative Complications and Mortality in Preoperative Patients with Colorectal Cancer. Nutrients 2022, 14, 1484. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.D.; Wang, S.L.; Zhuang, C.L.; Zheng, B.S.; Lu, J.X.; Chen, F.F.; Zhou, C.J.; Shen, X.; Yu, Z. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Color. Dis. 2015, 17, O256–O264. [Google Scholar] [CrossRef]
- Nakanishi, R.; Oki, E.; Sasaki, S.; Hirose, K.; Jogo, T.; Edahiro, K.; Korehisa, S.; Taniguchi, D.; Kudo, K.; Kurashige, J.; et al. Sarcopenia is an independent predictor of complications after colorectal cancer surgery. Surg. Today 2018, 48, 151–157. [Google Scholar] [CrossRef]
- Almeida, J.M.G.; García, C.G.; Aguilar, I.M.V.; Castañeda, V.B.; Guerrero, D.B. Morphofunctional assessment of patient’s nutritional status: A global approach. Nutr. Hosp. 2021, 38, 592–600. [Google Scholar] [CrossRef]
- Tolonen, A.; Pakarinen, T.; Sassi, A.; Kyttä, J.; Cancino, W.; Rinta-Kiikka, I.; Pertuz, S.; Arponen, O. Methodology, clinical applications, and future directions of body composition analysis using computed tomography (CT) images: A review. Eur. J. Radiol. 2021, 145, 109943. [Google Scholar] [CrossRef]
- Sánchez-Torralvo, F.J.; Ruiz-García, I.; Contreras-Bolívar, V.; González-Almendros, I.; Ruiz-Vico, M.; Abuín-Fernández, J.; Barrios, M.; Alba, E.; Olveira, G. CT-Determined Sarcopenia in GLIM-Defined Malnutrition and Prediction of 6-Month Mortality in Cancer Inpatients. Nutrients 2021, 13, 2647. [Google Scholar] [CrossRef]
- Nunes, G.D.; Cardenas, L.Z.; Miola, T.M.; Souza, J.O.; Carniatto, L.N.; Bitencourt, A.G.V. Preoperative evaluation of sarcopenia in patients with colorectal cancer: A prospective study. Rev. Assoc. Med. Bras. (1992) 2023, 69, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Baba, Y.; Sakamoto, Y.; Ohuchi, M.; Tokunaga, R.; Kurashige, J.; Hiyoshi, Y.; Iwagami, S.; Yoshida, N.; Yoshida, M.; et al. Sarcopenia is a Negative Prognostic Factor After Curative Resection of Colorectal Cancer. Ann. Surg. Oncol. 2015, 22, 2663–2668. [Google Scholar] [CrossRef] [PubMed]
- Golder, A.M.; Sin, L.K.E.; Alani, F.; Alasadi, A.; Dolan, R.; Mansouri, D.; Horgan, P.G.; McMillan, D.C.; Roxburgh, C.S. The relationship between the mode of presentation, CT-derived body composition, systemic inflammatory grade and survival in colon cancer. J. Cachexia Sarcopenia Muscle 2022, 13, 2863–2874. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Jochum, S.B.; Kistner, M.; Wood, E.H.; Hoscheit, M.; Nowak, L.; Poirier, J.; Eberhardt, J.M.; Saclarides, T.J.; Hayden, D.M. Is sarcopenia a better predictor of complications than body mass index? Sarcopenia and surgical outcomes in patients with rectal cancer. Color. Dis. 2019, 21, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Torralvo, F.J.; Pérez-del-Río, V.; García-Olivares, M.; Porras, N.; Abuín-Fernández, J.; Bravo-Bardají, M.F.; García-de-Quevedo, D.; Olveira, G. Global Subjective Assessment and Mini Nutritional Assessment Short Form Better Predict Mortality Than GLIM Malnutrition Criteria in Elderly Patients with Hip Fracture. Nutrients 2023, 15, 1828. [Google Scholar] [CrossRef]
- García-Almeida, J.M.; García-García, C.; Vegas-Aguilar, I.M.; Pomar, M.D.B.; Cornejo-Pareja, I.M.; Medina, B.F.; de Luis Román, D.A.; Guerrero, D.B.; Lesmes, I.B.; Madueño, F.J.T. Nutritional ultrasound®: Conceptualisation, technical considerations and standardisation. Endocrinol. Diabetes Nutr. 2022, 70, 74–84. [Google Scholar] [CrossRef]
- Tewari, N.; Awad, S.; Macdonald, I.A.; Lobo, D.N. A comparison of three methods to assess body composition. Nutrition 2018, 47, 1–5. [Google Scholar] [CrossRef]
- Barazzoni, R.; Jensen, G.L.; Correia, M.I.T.; Gonzalez, M.C.; Higashiguchi, T.; Shi, H.P.; Bischoff, S.C.; Boirie, Y.; Carrasco, F.; Cruz-Jentoft, A.; et al. Guidance for assessment of the muscle mass phenotypic criterion for the Global Leadership Initiative on Malnutrition (GLIM) diagnosis of malnutrition. Clin. Nutr. 2022, 41, 1425–1433. [Google Scholar] [CrossRef] [PubMed]
- da Silva Couto, A.; Gonzalez, M.C.; Martucci, R.B.; Feijó, P.M.; Rodrigues, V.D.; de Pinho, N.B.; Souza, N.C. Predictive validity of GLIM malnutrition diagnosis in patients with colorectal cancer. JPEN J. Parenter. Enter. Nutr 2023, 47, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.L.S.; Santos, B.C.; Frazão, L.N.; Miranda, A.L.; Fayh, A.P.T.; Silva, F.M.; Gonzalez, M.C.; Correia, M.I.T.D.; Souza, N.C.; Anastácio, L.R.; et al. Validity of the GLIM criteria for the diagnosis of malnutrition in patients with colorectal cancer: A multicenter study on the diagnostic performance of different indicators of reduced muscle mass and disease severity. Nutrition 2024, 119, 112324. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.; Dolan, R.D.; Horgan, P.G.; Laird, B.J.; McMillan, D.C. Computed tomography-defined low skeletal muscle index and density in cancer patients: Observations from a systematic review. J. Cachexia Sarcopenia Muscle 2021, 12, 1408–1417. [Google Scholar] [CrossRef]
- Souza, B.U.D.; Souza, N.C.S.; Martucci, R.B.; Rodrigues, V.D.; Pinho, N.B.D.; Gonzalez, M.C.; Avesani, C.M. Factors Associated with Sarcopenia in Patients with Colorectal Cancer. Nutr. Cancer 2018, 70, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Saino, Y.; Kawase, F.; Nagano, A.; Ueshima, J.; Kobayashi, H.; Murotani, K.; Inoue, T.; Nagami, S.; Suzuki, M.; Maeda, K. Diagnosis and prevalence of sarcopenic obesity in patients with colorectal cancer: A scoping review. Clin. Nutr. 2023, 42, 1595–1601. [Google Scholar] [CrossRef]
- Prado, C.M.; Lieffers, J.R.; McCargar, L.J.; Reiman, T.; Sawyer, M.B.; Martin, L.; Baracos, V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008, 9, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Chai, V.W.; Chia, M.; Cocco, A.; Bhamidipaty, M.; D’Souza, B. Sarcopenia is a strong predictive factor of clinical and oncological outcomes following curative colorectal cancer resection. ANZ J. Surg. 2021, 91, E292–E297. [Google Scholar] [CrossRef] [PubMed]
- Olmez, T.; Karakose, E.; Bozkurt, H.; Pence, H.H.; Gulmez, S.; Aray, E.; Bulut, C.I.; Sert, O.Z.; Polat, E.; Duman, M. Sarcopenia is associated with increased severe postoperative complications after colon cancer surgery. Arch. Med. Sci. 2021, 17, 361. [Google Scholar] [CrossRef]
- Dolan, R.D.; Almasaudi, A.S.; Dieu, L.B.; Horgan, P.G.; McSorley, S.T.; McMillan, D.C. The relationship between computed tomography-derived body composition, systemic inflammatory response, and survival in patients undergoing surgery for colorectal cancer. J. Cachexia Sarcopenia Muscle 2019, 10, 111–122. [Google Scholar] [CrossRef]
- Aro, R.; Mäkäräinen-Uhlbäck, E.; Ämmälä, N.; Rautio, T.; Ohtonen, P.; Saarnio, J.; Meriläinen, S. The impact of sarcopenia and myosteatosis on postoperative outcomes and 5-year survival in curatively operated colorectal cancer patients—A retrospective register study. Eur. J. Surg. Oncol. 2020, 46, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.M.; Kang, J. Prognostic impact of myosteatosis in patients with colorectal cancer: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 1270–1282. [Google Scholar] [CrossRef] [PubMed]
- Benedek, Z.; Coroș, M.F. The impact of sarcopenia on the postoperative outcome in colorectal cancer surgery. Med. Pharm. Rep. 2023, 96, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Palmas, F.; Ciudin, A.; Guerra, R.; Eiroa, D.; Espinet, C.; Roson, N.; Burgos, R.; Simó, R. Comparison of computed tomography and dual-energy X-ray absorptiometry in the evaluation of body composition in patients with obesity. Front. Endocrinol. 2023, 14, 1161116. [Google Scholar] [CrossRef] [PubMed]


| Total (n = 586) | Men (n = 365) | Women (n = 221) | p Value | ||
|---|---|---|---|---|---|
| Age (years) | mean ± SD | 68.4 ± 10.2 | 68.3 ± 10.7 | 69.1 ± 10.1 | 0.36 |
| BMI (kg/m2) | mean ± SD | 27 ± 5.1 | 27.1 ± 4.7 | 26.9 ± 5.6 | 0.63 |
| Type of cancer | n (%) | 0.93 | |||
| Colon | 423 (72.2) | 263 (72.1) | 160 (72.4) | ||
| Rectum | 163 (27.8) | 102(27.9) | 61 (27.6) | ||
| Stage | n (%) | 0.49 | |||
| Unknown | 37 (6.3) | 26 (7.1) | 11 (5) | ||
| I | 102 (17.4) | 69 (18.9) | 33 (14.9) | ||
| II | 184 (31.4) | 113 (31) | 71 (32.1) | ||
| III | 214 (36.5) | 126 (34.5) | 88 (39.8) | ||
| IV | 49 (8.4) | 31 (8.5) | 18 (8.1) | ||
| Type of surgery | n (%) | 0.75 | |||
| Open | 84 (14.3) | 51 (14) | 33 (14.9) | ||
| Laparoscopic | 502 (85.7) | 314 (86) | 188 (85.1) |
| Total (n = 586) | Men (n = 365) | Women (n = 221) | p-Value | ||
|---|---|---|---|---|---|
| BMI (kg/m2) | mean ± SD | 27.1 ± 5.1 | 27.1 ± 4.7 | 26.9 ± 5.9 | 0.63 |
| Low BMI | n (%) | 53 (9) | 26 (7.1) | 27 (12.2) | 0.037 |
| Weight loss > 5% | n (%) | 189 (32.2) | 119 (32.5) | 70 (31.8) | 0.9 |
| FFMI (kg/m2) | mean ± SD | 19.4 ± 2.9 | 20.5 ± 2.6 | 17.6 ± 2.4 | <0.001 |
| Low FFMI | n (%) | 55 (9.4) | 24 (6.7) | 31 (13.9) | 0.006 |
| Malnourished (GLIM criteria) | n (%) | 245 (41.8) | 140 (38.4) | 105 (47.5) | 0.029 |
| Handgrip strength (kg) | mean ± SD | 28.4 ± 10.3 | 33.6 ± 8.9 | 19.9 ± 5.9 | <0.001 |
| Low handgrip strength | n (%) | 142 (24.3) | 82 (22.8) | 60 (27) | 0.25 |
| ASMM (kg) | mean ± SD | 21.1 ± 4.8 | 22.9 ± 3.9 | 16.7 ± 2.6 | <0.001 |
| Low ASMM | n (%) | 127 (21.6) | 78 (21.2) | 49 (22.2) | 0.81 |
| Sarcopenia (EWGSOP2 criteria) | n (%) | 56 (9.6) | 35 (9.5) | 21 (9.7) | 0.94 |
| Men (n = 365) | Women (n = 221) | p Value | ||
|---|---|---|---|---|
| Muscle area (SMA) (cm2) | mean ± SD | 130.96 ± 25.48 | 90.59 ± 16.39 | p < 0.001 |
| Muscle percentage | mean ± SD | 17.63 ± 4.42 | 14.36 ± 3.95 | p < 0.001 |
| Muscle Hounsfield Units | mean ± SD | 39.44 ± 9.19 | 36.37 ± 9.99 | p < 0.001 |
| SMI (cm2/m2) | mean ± SD | 45.52 ± 8.79 | 36.79 ± 6.29 | p < 0.001 |
| IMAT area (cm2) | mean ± SD | 16.41 ± 11.09 | 15.81 ± 9.58 | p = 0.51 |
| IMAT percentage | mean ± SD | 2.13 ± 1.47 | 2.34 ± 1.21 | p = 0.08 |
| IMAT Hounsfield Units | mean ± SD | −62.89 ± 6.54 | −63.06 ± 6.37 | p = 0.76 |
| VAT area (cm2) | mean ± SD | 192.57 ± 107.09 | 180.88 ± 104.19 | p = 0.19 |
| VAT percentage | mean ± SD | 24.19 ± 13.6 | 26.09 ± 11.71 | p = 0.09 |
| VAT Hounsfield Units | mean ± SD | −93.79 ± 10.65 | −94.48 ± 10.62 | p = 0.31 |
| SAT area (cm2) | mean ± SD | 193.58 ± 103.75 | 177.53 ± 118.22 | p = 0.09 |
| SAT percentage | mean ± SD | 24.59 ± 14.38 | 24.54 ± 11.89 | p = 0.96 |
| SAT Hounsfield Units | mean ± SD | −93.79 ± 10.65 | −93.68 ± 11.78 | p = 0.9 |
| Normo-Nourished (n = 341) | Malnourished (n = 245) | p-Value | Nonsarcopenic (n = 514) | Sarcopenic (n = 72) | p-Value | ||
|---|---|---|---|---|---|---|---|
| Muscle area (SMA) (cm2) | m ± SD | 120.9 ± 29.5 | 108.6 ± 28.8 | p < 0.001 | 115.9 ± 29.8 | 103.7 ± 23.3 | p = 0.005 |
| Muscle percentage | m ± SD | 15.9 ± 4.2 | 17.1 ± 4.8 | p = 0.003 | 16.2 ± 4.4 | 18.8 ± 5.8 | p < 0.001 |
| Muscle HU | m ± SD | 38.1 ± 9.1 | 38.5 ± 10.3 | p = 0.672 | 38.5 ± 9.7 | 39.2 ± 8.4 | p = 0.592 |
| SMI (cm2/m2) | m ± SD | 43.5 ± 9.2 | 40.4 ± 8.3 | p < 0.001 | 42.3 ± 8.9 | 36.5 ± 6.4 | p < 0.001 |
| IMAT area (cm2) | m ± SD | 17.2 ± 10.8 | 14.8 ± 10 | p = 0.006 | 16.7 ± 11.2 | 13.5 ± 7.3 | p = 0.05 |
| IMAT percentage | m ± SD | 2.23 ± 1.45 | 2.16 ± 1.27 | p = 0.723 | 2.25 ± 1.48 | 2.32 ± 1.1 | p = 0.727 |
| IMAT HU | m ± SD | −63.8 ± 5.9 | −61.8 ± 6.9 | p < 0.001 | −63.7 ± 6.6 | −61.5 ± 6.2 | p = 0.028 |
| VAT area (cm2) | m ± SD | 209.4 ± 104.8 | 158.6 ± 100.9 | p < 0.001 | 193.1 ± 104.2 | 107.9 ± 71.4 | p < 0.001 |
| VAT percentage | m ± SD | 26.6 ± 13.9 | 22.6 ± 10.9 | p < 0.001 | 25.6 ± 14.1 | 17.4 ± 9.1 | p < 0.001 |
| VAT HU | m ± SD | −96.2 ± 7.3 | −90.8 ± 11.7 | p < 0.001 | −94.3 ± 9.5 | −87.1 ± 11.3 | p < 0.001 |
| SAT area (cm2) | m ± SD | 210.9 ± 107 | 154.9 ± 104.9 | p < 0.001 | 189.9 ± 108.9 | 125.9 ± 82.9 | p < 0.001 |
| SAT percentage | m ± SD | 26.8 ± 14.9 | 21.4 ± 10.4 | p < 0.001 | 24.9 ± 14.8 | 20.4 ± 10.9 | p = 0.036 |
| SAT HU | m ± SD | −96.3 ± 9.2 | −90.1 ± 12.4 | p = 0.014 | −95.1 ± 10.9 | −88.4 ± 14.2 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soria-Utrilla, V.; Sánchez-Torralvo, F.J.; Palmas-Candia, F.X.; Fernández-Jiménez, R.; Mucarzel-Suarez-Arana, F.; Guirado-Peláez, P.; Olveira, G.; García-Almeida, J.M.; Burgos-Peláez, R. AI-Assisted Body Composition Assessment Using CT Imaging in Colorectal Cancer Patients: Predictive Capacity for Sarcopenia and Malnutrition Diagnosis. Nutrients 2024, 16, 1869. https://doi.org/10.3390/nu16121869
Soria-Utrilla V, Sánchez-Torralvo FJ, Palmas-Candia FX, Fernández-Jiménez R, Mucarzel-Suarez-Arana F, Guirado-Peláez P, Olveira G, García-Almeida JM, Burgos-Peláez R. AI-Assisted Body Composition Assessment Using CT Imaging in Colorectal Cancer Patients: Predictive Capacity for Sarcopenia and Malnutrition Diagnosis. Nutrients. 2024; 16(12):1869. https://doi.org/10.3390/nu16121869
Chicago/Turabian StyleSoria-Utrilla, Virginia, Francisco José Sánchez-Torralvo, Fiorella Ximena Palmas-Candia, Rocío Fernández-Jiménez, Fernanda Mucarzel-Suarez-Arana, Patricia Guirado-Peláez, Gabriel Olveira, José Manuel García-Almeida, and Rosa Burgos-Peláez. 2024. "AI-Assisted Body Composition Assessment Using CT Imaging in Colorectal Cancer Patients: Predictive Capacity for Sarcopenia and Malnutrition Diagnosis" Nutrients 16, no. 12: 1869. https://doi.org/10.3390/nu16121869
APA StyleSoria-Utrilla, V., Sánchez-Torralvo, F. J., Palmas-Candia, F. X., Fernández-Jiménez, R., Mucarzel-Suarez-Arana, F., Guirado-Peláez, P., Olveira, G., García-Almeida, J. M., & Burgos-Peláez, R. (2024). AI-Assisted Body Composition Assessment Using CT Imaging in Colorectal Cancer Patients: Predictive Capacity for Sarcopenia and Malnutrition Diagnosis. Nutrients, 16(12), 1869. https://doi.org/10.3390/nu16121869






