Sub-Optimal Paternal Diet at the Time of Mating Disrupts Maternal Adaptations to Pregnancy in the Late Gestation Mouse
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Treatments and Tissue Collection
2.2. Assessment of Maternal Metabolic Status
2.3. Maternal Gut Microbiota Sequencing
2.4. Maternal Liver RNA-Seq
2.5. Maternal Femur µ-CT Analysis
2.6. Maternal Cardiac Gene Expression
2.7. Statistical Analyses
3. Results
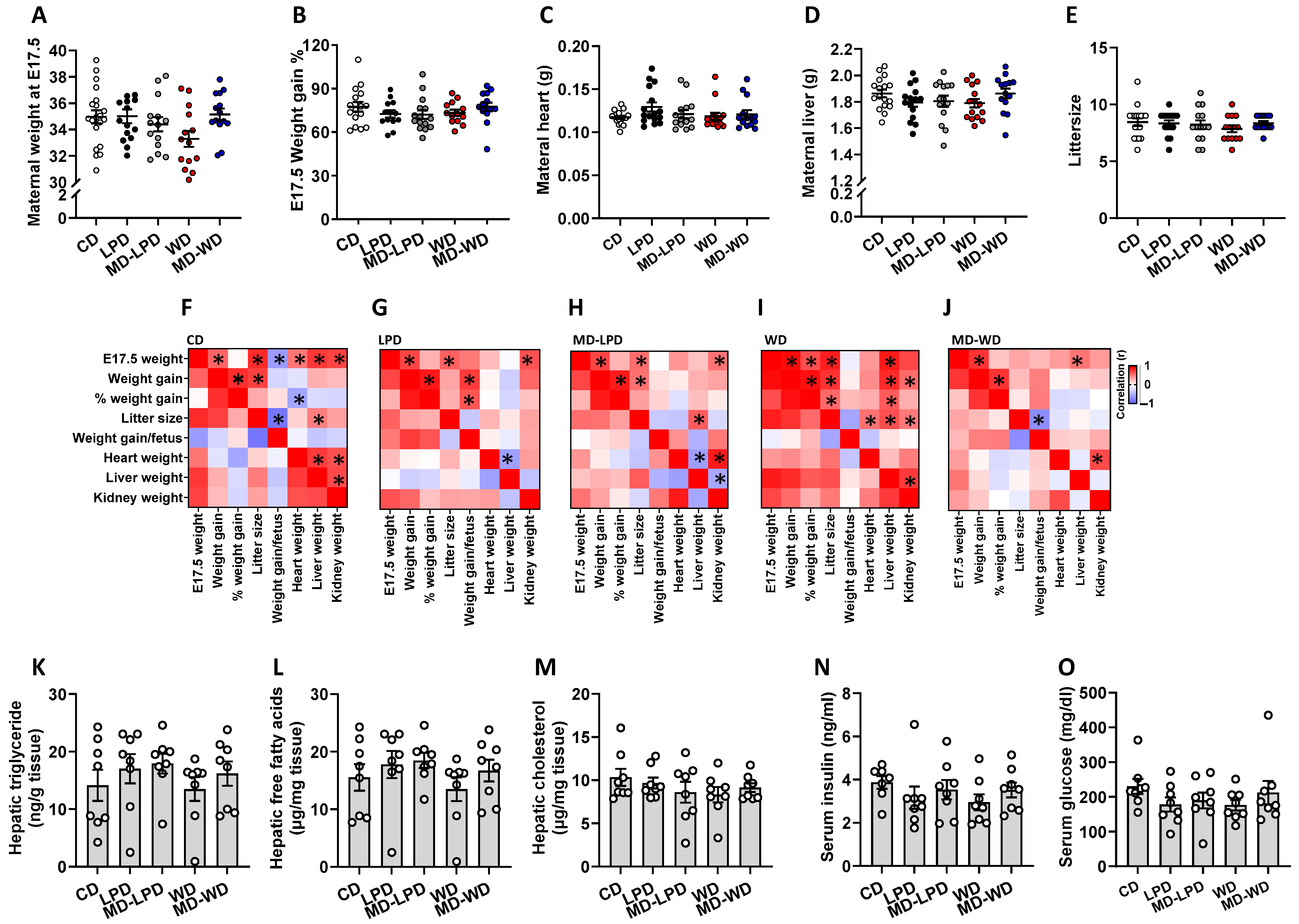
3.1. Maternal Physiology and Metabolic Status
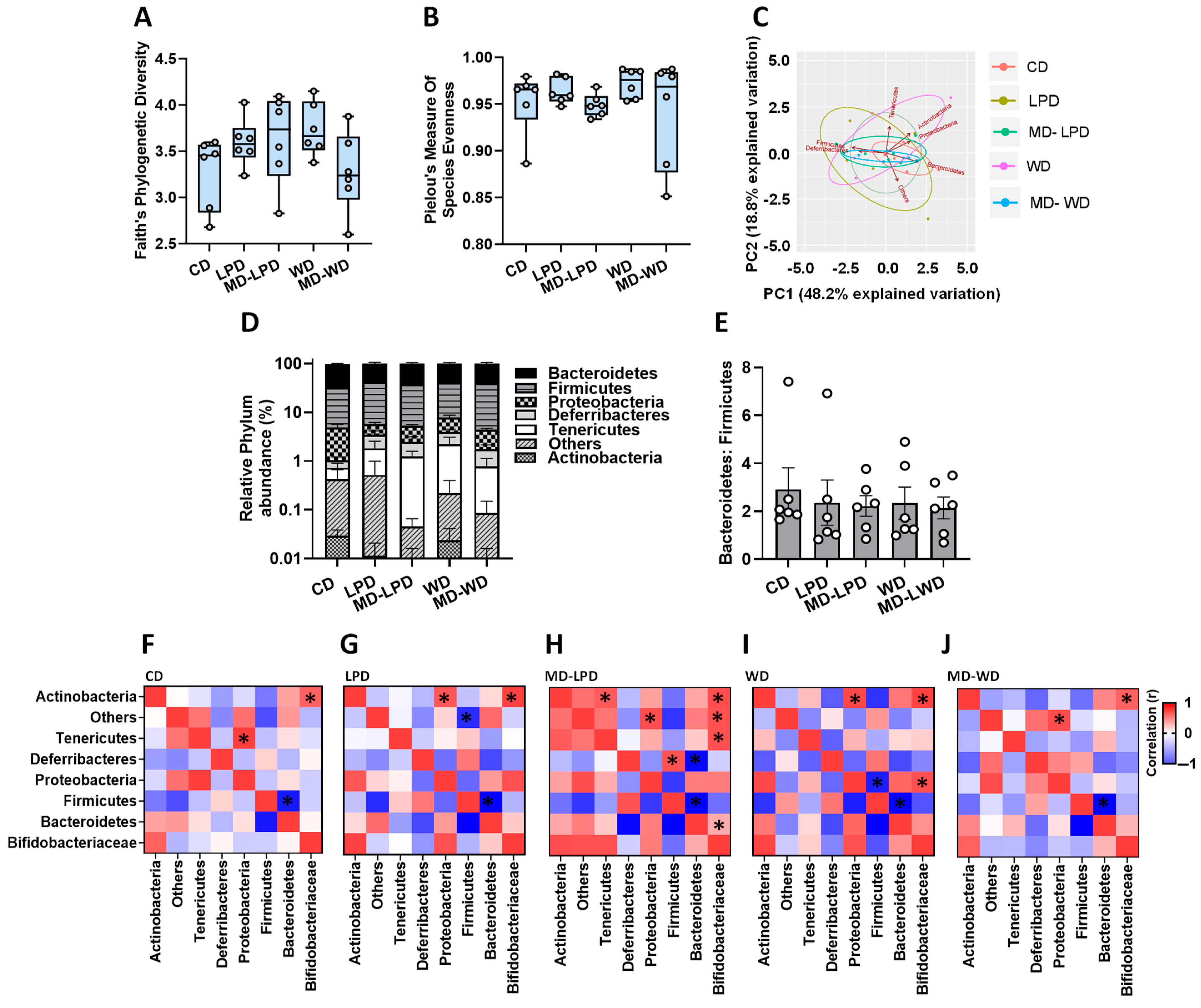
3.2. Impact of Paternal Diet on Late Gestation Maternal Gut Microbiota
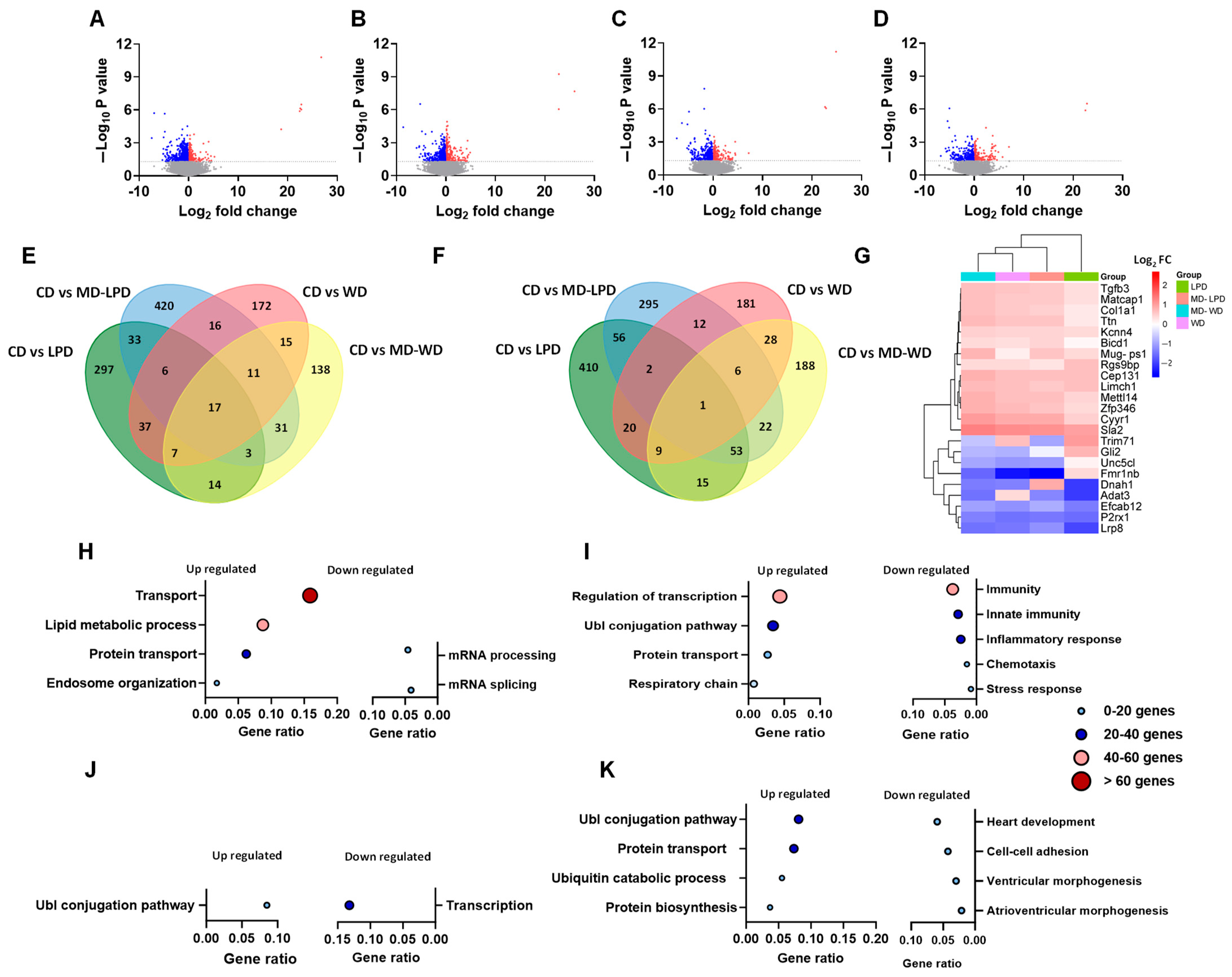
3.3. Comparison of Maternal Late Gestation Liver Transcriptome
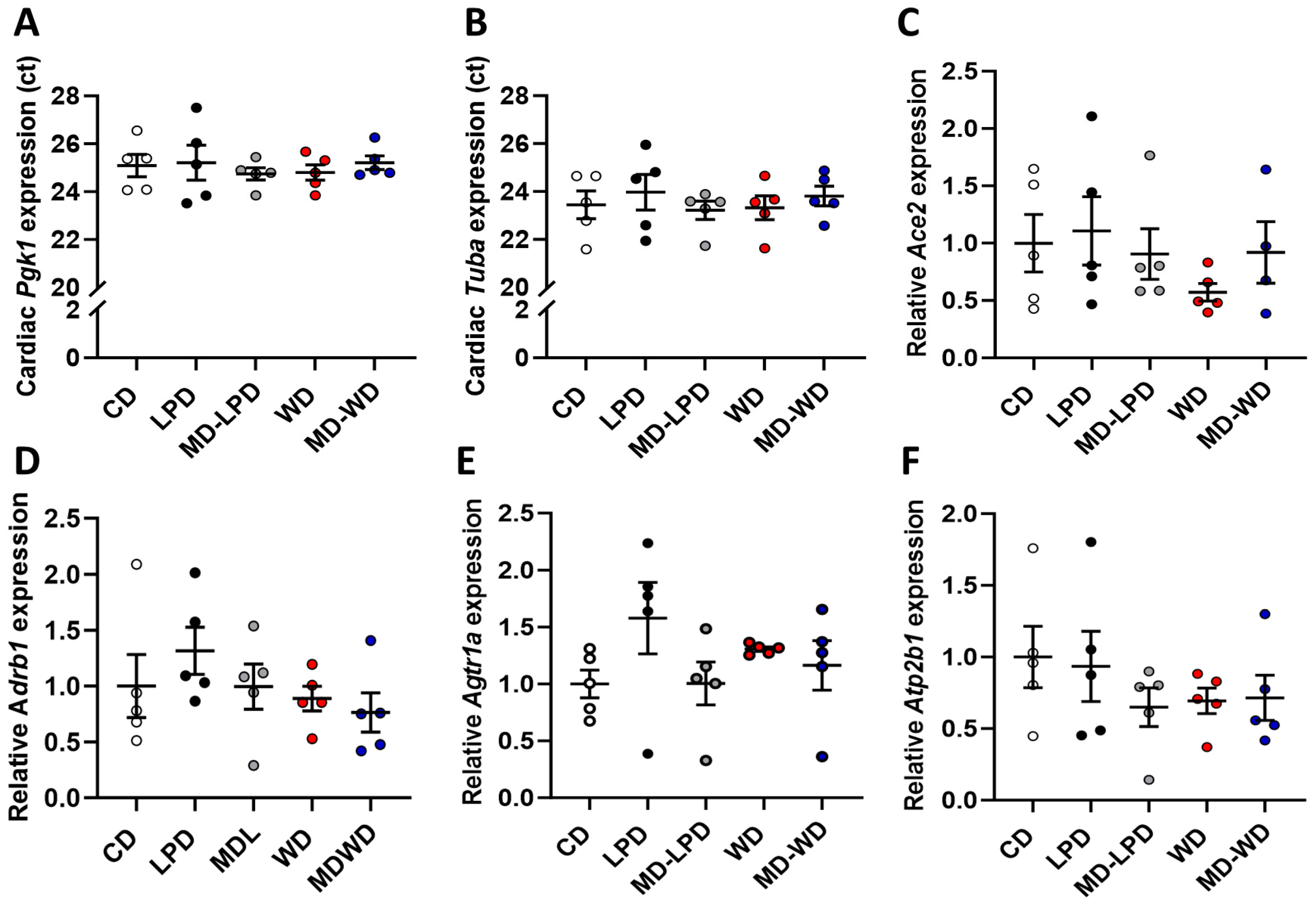
3.4. Maternal Late Gestation Cardiac Gene Expression
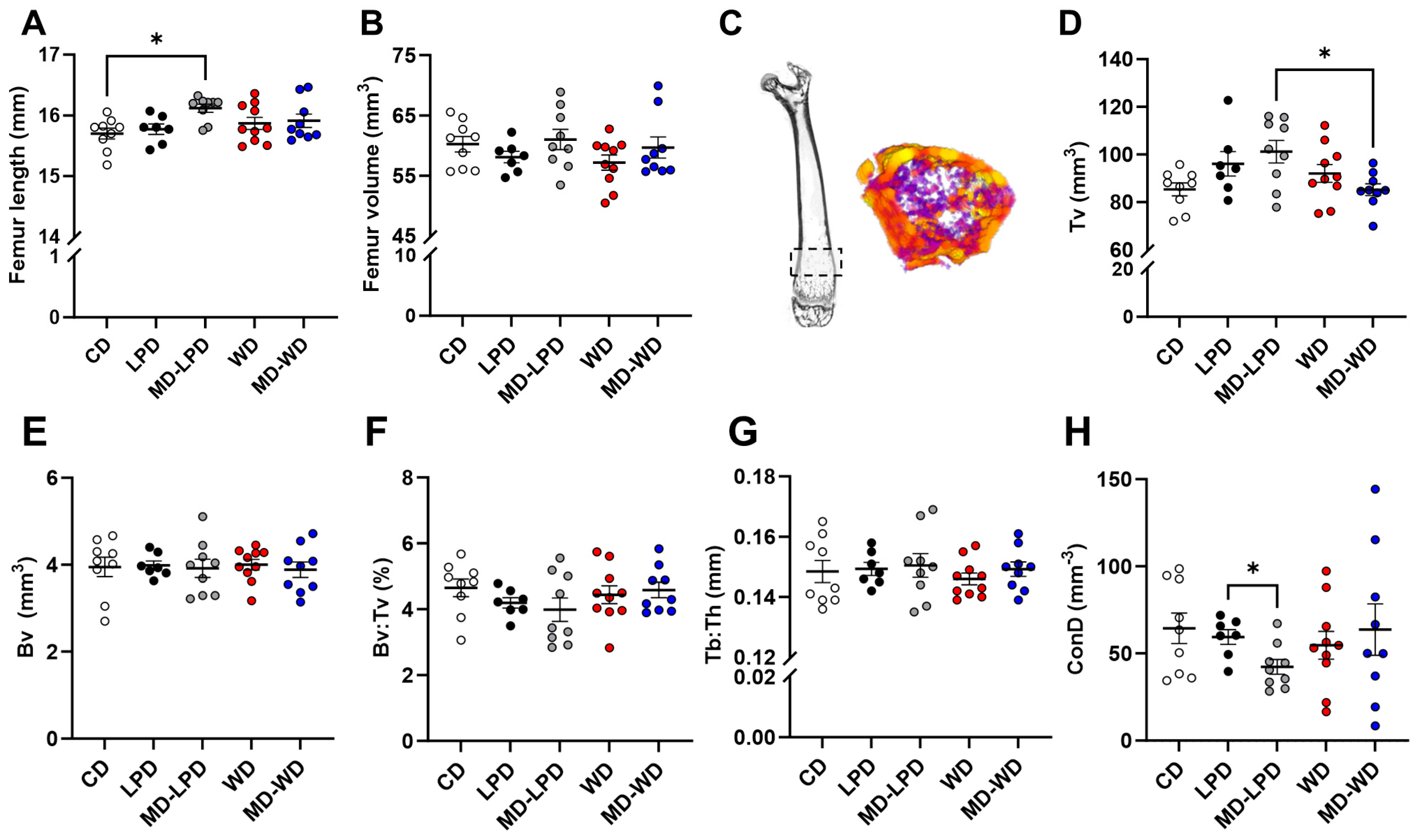
3.5. Maternal Late Gestation Femur Trabecular Bone Morphology
3.6. Maternal Late Gestation Femur Cortical Bone Morphology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinnars, M.T.; Bixo, M.; Damdimopoulou, P. Pregnancy-related maternal physiological adaptations and fetal chemical exposure. Mol. Cell. Endocrinol. 2023, 578, 112064. [Google Scholar] [CrossRef]
- Casagrande, S.S.; Linder, B.; Cowie, C.C. Prevalence of gestational diabetes and subsequent Type 2 diabetes among U.S. women. Diabetes Res. Clin. Pract. 2018, 141, 200–208. [Google Scholar] [CrossRef]
- Say, L.; Chou, D.; Gemmill, A.; Tunçalp, Ö.; Moller, A.-B.; Daniels, J.; Gülmezoglu, A.M.; Temmerman, M.; Alkema, L. Global causes of maternal death: A WHO systematic analysis. Lancet Glob. Health 2014, 2, e323–e333. [Google Scholar] [CrossRef]
- Brett, K.E.; Ferraro, Z.M.; Yockell-Lelievre, J.; Gruslin, A.; Adamo, K.B. Maternal-fetal nutrient transport in pregnancy pathologies: The role of the placenta. Int. J. Mol. Sci. 2014, 15, 16153–16185. [Google Scholar] [CrossRef]
- Dimasuay, K.G.; Boeuf, P.; Powell, T.L.; Jansson, T. Placental Responses to Changes in the Maternal Environment Determine Fetal Growth. Front. Physiol. 2016, 7, 12. [Google Scholar] [CrossRef]
- Kingdom, J.C.; Audette, M.C.; Hobson, S.R.; Windrim, R.C.; Morgen, E. A placenta clinic approach to the diagnosis and management of fetal growth restriction. Am. J. Obstet. Gynecol. 2018, 218, S803–S817. [Google Scholar] [CrossRef]
- Mulder, J.; Kusters, D.M.; Roeters van Lennep, J.E.; Hutten, B.A. Lipid metabolism during pregnancy: Consequences for mother and child. Curr. Opin. Lipidol. 2024, 35, 133–140. [Google Scholar] [CrossRef]
- Napso, T.; Yong, H.E.J.; Lopez-Tello, J.; Sferruzzi-Perri, A.N. The Role of Placental Hormones in Mediating Maternal Adaptations to Support Pregnancy and Lactation. Front. Physiol. 2018, 9, 1091. [Google Scholar] [CrossRef]
- Wiznitzer, A.; Mayer, A.; Novack, V.; Sheiner, E.; Gilutz, H.; Malhotra, A.; Novack, L. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: A population-based study. Am. J. Obstet. Gynecol. 2009, 201, 482.e1–482.e8. [Google Scholar] [CrossRef] [PubMed]
- Ernst, S.; Demirci, C.; Valle, S.; Velazquez-Garcia, S.; Garcia-Ocana, A. Mechanisms in the adaptation of maternal beta-cells during pregnancy. Diabetes Manag. 2011, 1, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Dathan-Stumpf, A.; Vogel, M.; Jank, A.; Thiery, J.; Kiess, W.; Stepan, H. Reference intervals of serum lipids in the second and third trimesters of pregnancy in a Caucasian cohort: The LIFE Child study. Arch. Gynecol. Obstet. 2019, 300, 1531–1539. [Google Scholar] [CrossRef]
- Emet, T.; Üstüner, I.; Güven, S.G.; Balık, G.; Ural, M.; Tekin, Y.B.; Şentürk, Ş.; Şahin, F.K.; Avşar, A.F. Plasma lipids and lipoproteins during pregnancy and related pregnancy outcomes. Arch. Gynecol. Obstet. 2013, 288, 49–55. [Google Scholar] [CrossRef]
- Friis Petersen, J.; Friis-Hansen, L.J.; Jensen, A.K.; Nyboe Andersen, A.; Lokkegaard, E.C.L. Early pregnancy reference intervals; 29 serum analytes from 4 to 12 weeks’ gestation in naturally conceived and uncomplicated pregnancies resulting in live births. Clin. Chem. Lab. Med. 2019, 57, 1956–1967. [Google Scholar] [CrossRef]
- Zheng, W.; Huang, W.; Zhang, L.; Tian, Z.; Wang, T.; Zhang, T.; Zhang, Z.; Zhang, W.; Li, G. Changes in Serum Lipid Levels During Pregnancy and Association with Neonatal Outcomes: A Large Cohort Study. Reprod. Sci. 2018, 25, 1406–1412. [Google Scholar] [CrossRef]
- Jo, Y.S.; Cheon, J.Y.; Ahn, J.W.; Kim, Y.; Lee, G.S.R. Pentraxin 3 and lipid profile status in pregnancy. J. Obstet. Gynaecol. 2017, 37, 727–730. [Google Scholar] [CrossRef]
- Alvarez, J.J.; Montelongo, A.; Iglesias, A.; Lasuncion, M.A.; Herrera, E. Longitudinal study on lipoprotein profile, high density lipoprotein subclass, and postheparin lipases during gestation in women. J. Lipid Res. 1996, 37, 299–308. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Lin, L. Maternal lipid profiles in women with and without gestational diabetes mellitus. Medicine 2019, 98, e15320. [Google Scholar] [CrossRef]
- Roeters van Lennep, J.E.; Tokgözoğlu, L.S.; Badimon, L.; Dumanski, S.M.; Gulati, M.; Hess, C.N.; Holven, K.B.; Kavousi, M.; Kayıkçıoğlu, M.; Lutgens, E.; et al. Women, lipids, and atherosclerotic cardiovascular disease: A call to action from the European Atherosclerosis Society. Eur. Heart J. 2023, 44, 4157–4173. [Google Scholar] [CrossRef]
- Pijnenborg, R.; Vercruysse, L.; Hanssens, M. The uterine spiral arteries in human pregnancy: Facts and controversies. Placenta 2006, 27, 939–958. [Google Scholar]
- Lash, G.E.; Robson, S.C.; Bulmer, J.N. Review: Functional role of uterine natural killer (uNK) cells in human early pregnancy decidua. Placenta 2010, 31, S87–S92. [Google Scholar] [CrossRef]
- Sferruzzi-Perri, A.N.; Macpherson, A.M.; Roberts, C.T.; Robertson, S.A. Csf2 null mutation alters placental gene expression and trophoblast glycogen cell and giant cell abundance in mice. Biol. Reprod. 2009, 81, 207–221. [Google Scholar] [CrossRef]
- Hunter, S.; Robson, S.C. Adaptation of the maternal heart in pregnancy. Br. Heart J. 1992, 68, 540–543. [Google Scholar] [CrossRef]
- Duvekot, J.J.; Peeters, L.L. Maternal cardiovascular hemodynamic adaptation to pregnancy. Obstet. Gynecol. Surv. 1994, 49 (Suppl. S12), S1–S14. [Google Scholar] [CrossRef]
- Watkins, A.J.; Sirovica, S.; Stokes, B.; Isaacs, M.; Addison, O.; Martin, R.A. Paternal low protein diet programs preimplantation embryo gene expression, fetal growth and skeletal development in mice. Biochim. Biophys. Acta 2017, 1863, 1371–1381. [Google Scholar] [CrossRef]
- Watkins, A.J.; Dias, I.; Tsuro, H.; Allen, D.; Emes, R.D.; Moreton, J.; Wilson, R.; Ingram, R.J.; Sinclair, K.D. Paternal diet programs offspring health through sperm- and seminal plasma-specific pathways in mice. Proc. Natl. Acad. Sci. USA 2018, 115, 10064–10069. [Google Scholar] [CrossRef]
- Morgan, H.L.; Paganopoulou, P.; Akhtar, S.; Urquhart, N.; Philomin, R.; Dickinson, Y.; Watkins, A.J. Paternal diet impairs F1 and F2 offspring vascular function through sperm and seminal plasma specific mechanisms in mice. J. Physiol. 2020, 598, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Morgan, H.L.; Aljumah, A.; Rouillon, C.; Watkins, A.J. Paternal low protein diet and the supplementation of methyl-donors impact fetal growth and placental development in mice. Placenta 2021, 103, 124–133. [Google Scholar] [CrossRef]
- Morgan, H.L.; Furse, S.; Dias, I.H.K.; Shabir, K.; Castellanos, M.; Khan, I.; May, S.T.; Holmes, N.; Carlile, M.; Sang, F.; et al. Paternal low protein diet perturbs inter-generational metabolic homeostasis in a tissue-specific manner in mice. Commun. Biol. 2022, 5, 929. [Google Scholar] [CrossRef]
- Morgan, H.L.; Watkins, A.J. The influence of seminal plasma on offspring development and health. Semin. Cell Dev. Biol. 2020, 97, 131–137. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Gonzalez Peña, A.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordelières, F.P.; Dougherty, R.P.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef]
- Lucas, E.S.; Watkins, A.J.; Cox, A.L.; Marfy-Smith, S.J.; Smyth, N.; Fleming, T.P. Tissue-specific selection of reference genes is required for expression studies in the mouse model of maternal protein undernutrition. Theriogenology 2011, 76, 558–569. [Google Scholar] [CrossRef]
- Sferruzzi-Perri, A.N.; Lopez-Tello, J.; Napso, T.; Yong, H.E.J. Exploring the causes and consequences of maternal metabolic maladaptations during pregnancy: Lessons from animal models. Placenta 2020, 98, 43–51. [Google Scholar] [CrossRef]
- Pham, V.T.; Dold, S.; Rehman, A.; Bird, J.K.; Steinert, R.E. Vitamins, the gut microbiome and gastrointestinal health in humans. Nutr. Res. 2021, 95, 35–53. [Google Scholar] [CrossRef]
- Brown, E.M.; Clardy, J.; Xavier, R.J. Gut microbiome lipid metabolism and its impact on host physiology. Cell Host Microbe 2023, 31, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.C.; Manasson, J.; Scher, J.U. The role of the gut microbiome in systemic inflammatory disease. BMJ 2018, 360, j5145. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Masenga, S.K.; Hamooya, B.; Hangoma, J.; Hayumbu, V.; Ertuglu, L.A.; Ishimwe, J.; Rahman, S.; Saleem, M.; Laffer, C.L.; Elijovich, F.; et al. Recent advances in modulation of cardiovascular diseases by the gut microbiota. J. Hum. Hypertens. 2022, 36, 952–959. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A relevant minority for the maintenance of gut homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef]
- Teixeira, T.F.S.; Grześkowiak, Ł.M.; Salminen, S.; Laitinen, K.; Bressan, J.; Peluzio, M.D.C.G. Faecal levels of Bifidobacterium and Clostridium coccoides but not plasma lipopolysaccharide are inversely related to insulin and HOMA index in women. Clin. Nutr. 2013, 32, 1017–1022. [Google Scholar] [CrossRef] [PubMed]
- Duca, F.A.; Sakar, Y.; Covasa, M. The modulatory role of high fat feeding on gastrointestinal signals in obesity. J. Nutr. Biochem. 2013, 24, 1663–1677. [Google Scholar] [CrossRef] [PubMed]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef] [PubMed]
- Houten, S.M.; Violante, S.; Ventura, F.V.; Wanders, R.J. The Biochemistry and Physiology of Mitochondrial Fatty Acid Beta-Oxidation and Its Genetic Disorders. Annu. Rev. Physiol. 2016, 78, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.-Y.; Watt, M.J.; Rensen, S.S.; Greve, J.W.; Huynh, K.; Jayawardana, K.S.; Meikle, P.J.; Meex, R.C.R. Mitochondrial dysfunction-related lipid changes occur in nonalcoholic fatty liver disease progression. J. Lipid Res. 2018, 59, 1977–1986. [Google Scholar] [CrossRef]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Elenkov, I.J.; Wilder, R.L.; Bakalov, V.K.; Link, A.A.; Dimitrov, M.A.; Fisher, S.; Crane, M.; Kanik, K.S.; Chrousos, G.P. IL-12, TNF-alpha, and hormonal changes during late pregnancy and early postpartum: Implications for autoimmune disease activity during these times. J. Clin. Endocrinol. Metab. 2001, 86, 4933–4938. [Google Scholar] [PubMed]
- Confavreux, C.; Hutchinson, M.; Hours, M.M.; Cortinovis-Tourniaire, P.; Moreau, T.; Pregnancy in Multiple Sclerosis Group. Rate of pregnancy-related relapse in multiple sclerosis. N. Engl. J. Med. 1998, 339, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Rodriguez-Garcia, M.; Patel, M.V.; Wira, C.R. Direct and Indirect endocrine-mediated suppression of human endometrial CD8+T cell cytotoxicity. Sci. Rep. 2021, 11, 1773. [Google Scholar] [CrossRef]
- Hellberg, S.; Raffetseder, J.; Rundquist, O.; Magnusson, R.; Papapavlou, G.; Jenmalm, M.C.; Ernerudh, J.; Gustafsson, M. Progesterone Dampens Immune Responses in In Vitro Activated CD4+ T Cells and Affects Genes Associated with Autoimmune Diseases That Improve During Pregnancy. Front. Immunol. 2021, 12, 672168. [Google Scholar] [CrossRef]
- Flint, E.J.; Cerdeira, A.S.; Redman, C.W.; Vatish, M. The role of angiogenic factors in the management of preeclampsia. Acta Obstet. Gynecol. Scand. 2019, 98, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Dimas, A.; Politi, A.; Bargiota, A.; Panoskaltsis, T.; Vlahos, N.F.; Valsamakis, G. The Gestational Effects of Maternal Bone Marker Molecules on Fetal Growth, Metabolism and Long-Term Metabolic Health: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 8328. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S. Maternal Mineral and Bone Metabolism During Pregnancy, Lactation, and Post-Weaning Recovery. Physiol. Rev. 2016, 96, 449–547. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, C.S. Osteoporosis presenting in pregnancy, puerperium, and lactation. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Masiukiewicz, U.S.; Mitnick, M.; Gulanski, B.I.; Insogna, K.L. Evidence that the IL-6/IL-6 soluble receptor cytokine system plays a role in the increased skeletal sensitivity to PTH in estrogen-deficient women. J. Clin. Endocrinol. Metab. 2002, 87, 2892–2898. [Google Scholar] [CrossRef] [PubMed]
- Moller, U.K.; Vieth Streym, S.; Mosekilde, L.; Rejnmark, L. Changes in bone mineral density and body composition during pregnancy and postpartum. A controlled cohort study. Osteoporos. Int. 2012, 23, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.M.; Nelson, E.A.; Leung, S.S.; Cheng, J.C. Bone mineral density and calcium metabolism of Hong Kong Chinese postpartum women—A 1-y longitudinal study. Eur. J. Clin. Nutr. 2005, 59, 868–876. [Google Scholar] [CrossRef]
- Sowers, M. Pregnancy and lactation as risk factors for subsequent bone loss and osteoporosis. J. Bone Miner. Res. 1996, 11, 1052–1060. [Google Scholar] [CrossRef]
- Lambrot, R.; Xu, C.; Saint-Phar, S.; Chountalos, G.; Cohen, T.; Paquet, M.; Suderman, M.; Hallett, M.; Kimmins, S. Low paternal dietary folate alters the mouse sperm epigenome and is associated with negative pregnancy outcomes. Nat. Commun. 2013, 4, 2889. [Google Scholar] [CrossRef] [PubMed]
- Alio, A.P.; Salihu, H.M.; McIntosh, C.; August, E.M.; Weldeselasse, H.; Sanchez, E.; Mbah, A.K. The effect of paternal age on fetal birth outcomes. Am. J. Men’s Health 2012, 6, 427–435. [Google Scholar] [CrossRef]
- Bellinger, D.C. Teratogen update: Lead and pregnancy. Birth Defects Res. Part A Clin. Mol. Teratol. 2005, 73, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Burton, G.J.; Fowden, A.L. The placenta: A multifaceted, transient organ. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140066. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khoshkerdar, A.; Eid, N.; Batra, V.; Baker, N.; Holmes, N.; Henson, S.; Sang, F.; Wright, V.; McLaren, J.; Shakesheff, K.; et al. Sub-Optimal Paternal Diet at the Time of Mating Disrupts Maternal Adaptations to Pregnancy in the Late Gestation Mouse. Nutrients 2024, 16, 1879. https://doi.org/10.3390/nu16121879
Khoshkerdar A, Eid N, Batra V, Baker N, Holmes N, Henson S, Sang F, Wright V, McLaren J, Shakesheff K, et al. Sub-Optimal Paternal Diet at the Time of Mating Disrupts Maternal Adaptations to Pregnancy in the Late Gestation Mouse. Nutrients. 2024; 16(12):1879. https://doi.org/10.3390/nu16121879
Chicago/Turabian StyleKhoshkerdar, Afsaneh, Nader Eid, Vipul Batra, Nichola Baker, Nadine Holmes, Sonal Henson, Fei Sang, Victoria Wright, Jane McLaren, Kevin Shakesheff, and et al. 2024. "Sub-Optimal Paternal Diet at the Time of Mating Disrupts Maternal Adaptations to Pregnancy in the Late Gestation Mouse" Nutrients 16, no. 12: 1879. https://doi.org/10.3390/nu16121879
APA StyleKhoshkerdar, A., Eid, N., Batra, V., Baker, N., Holmes, N., Henson, S., Sang, F., Wright, V., McLaren, J., Shakesheff, K., Woad, K. J., Morgan, H. L., & Watkins, A. J. (2024). Sub-Optimal Paternal Diet at the Time of Mating Disrupts Maternal Adaptations to Pregnancy in the Late Gestation Mouse. Nutrients, 16(12), 1879. https://doi.org/10.3390/nu16121879





