Bioactive Peptides from Meretrix lusoria Enzymatic Hydrolysate as a Potential Treatment for Obesity in db/db Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. MLP Enzymatic Hydrolysate Preparation
2.2. MLP Antioxidant Activity and Reducing Power
2.3. Cell Culture and Viability Assay
2.4. Peptide Characterization via LC-MS/MS
2.5. Experimental Design
2.6. Serum Biochemical Analysis
2.7. Hepatic Lipid Profile and Antioxidant Enzyme Activities
2.8. Histopathological Examinations of Liver and Adipose Tissues
2.9. Western Blot Analysis
2.10. qRT-PCR Analysis
2.11. Intraperitoneal Glucose Tolerance Test (IPGTT)
2.12. Statistical Analysis
3. Results
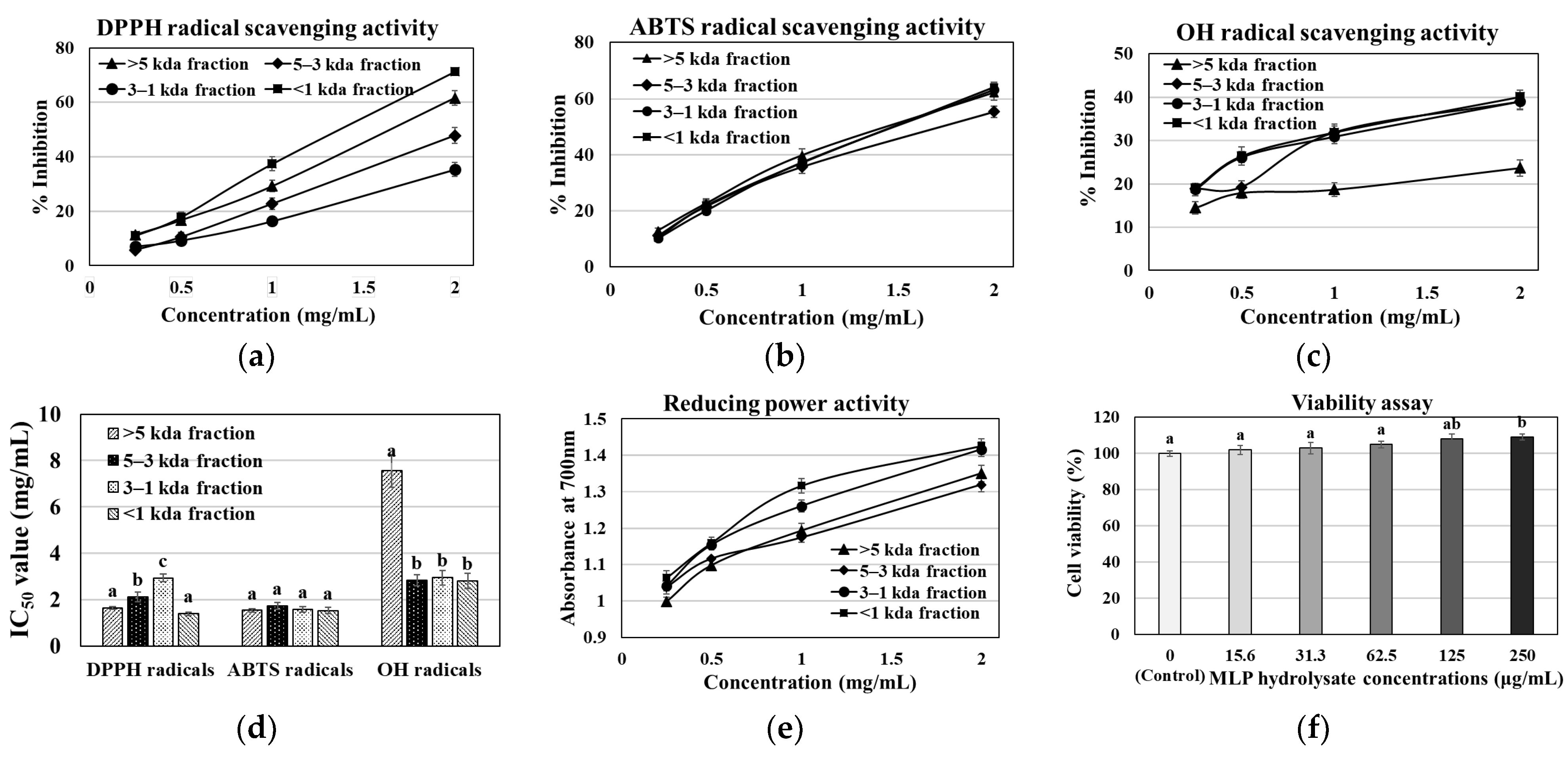
3.1. MLP Enzymatic Hydrolysate Antioxidant, Reducing Power, Viability, and Acute Toxicity Assay
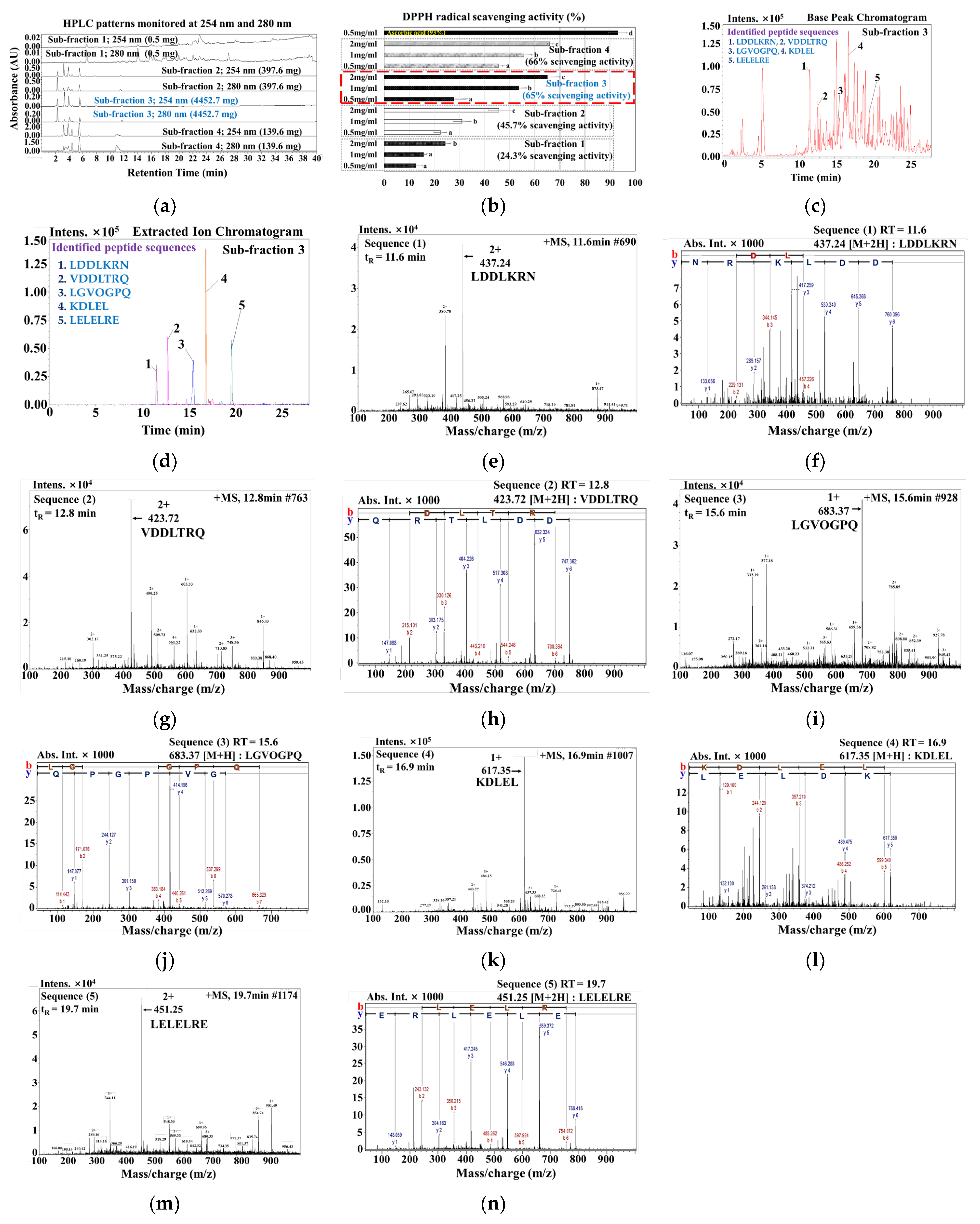
3.2. Identification of Bioactive Peptides in ≤1 kDa MLP Enzymatic Hydrolysate
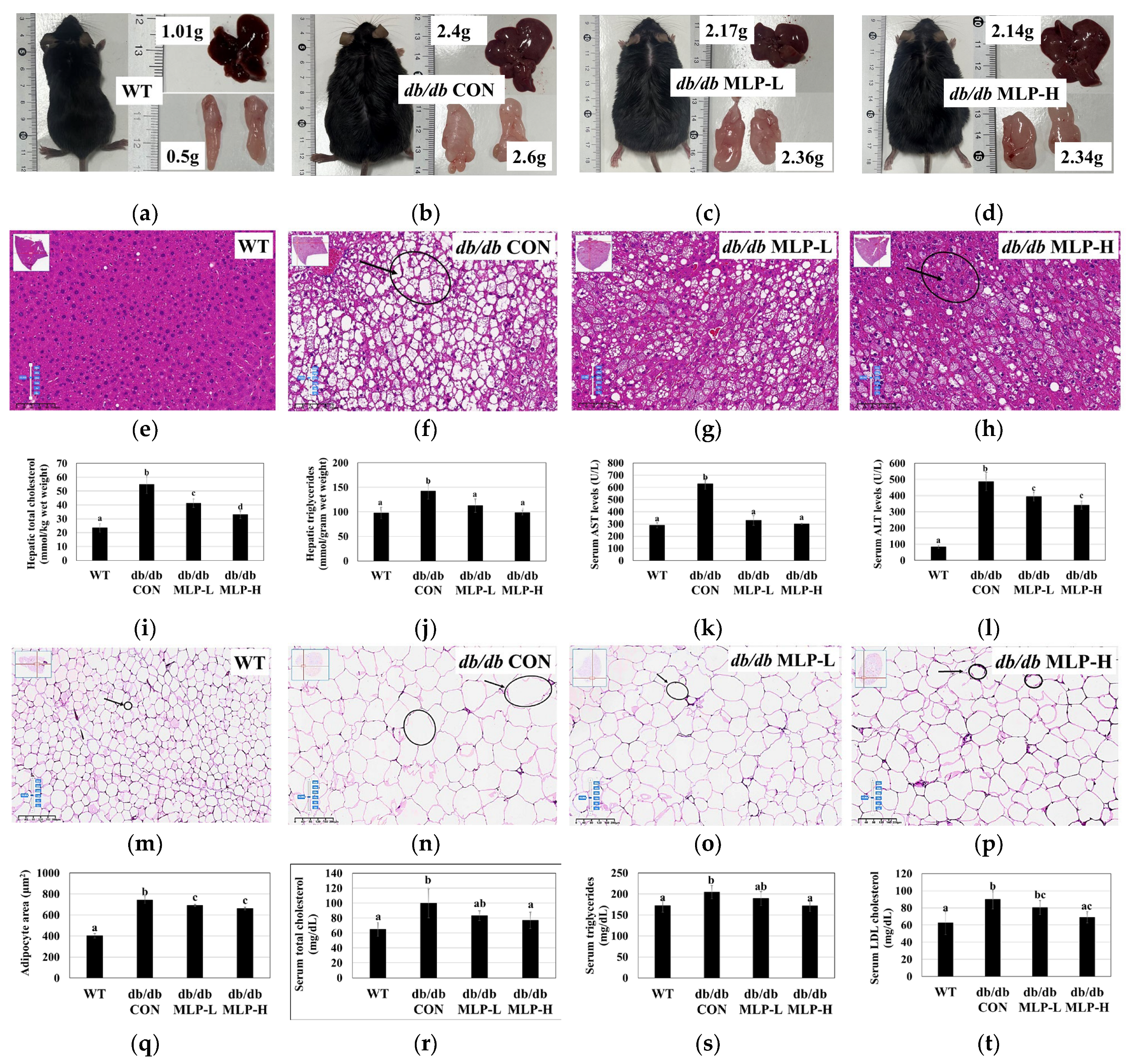
3.3. Effect of MLP on Body Weight, Lipid Changes, and Serum Biochemical Analysis
3.4. MLP Treatment Prevented Obesity and Liver Steatosis in db/db Mice
3.5. Hepatic Antioxidant Enzyme Activities in MLP-Treated Obese Mice
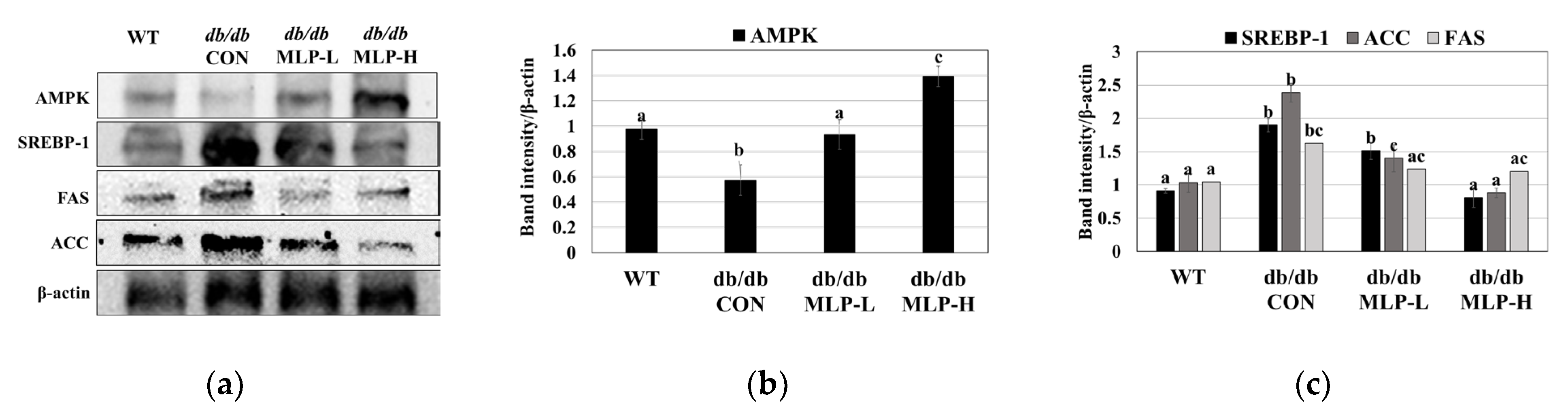
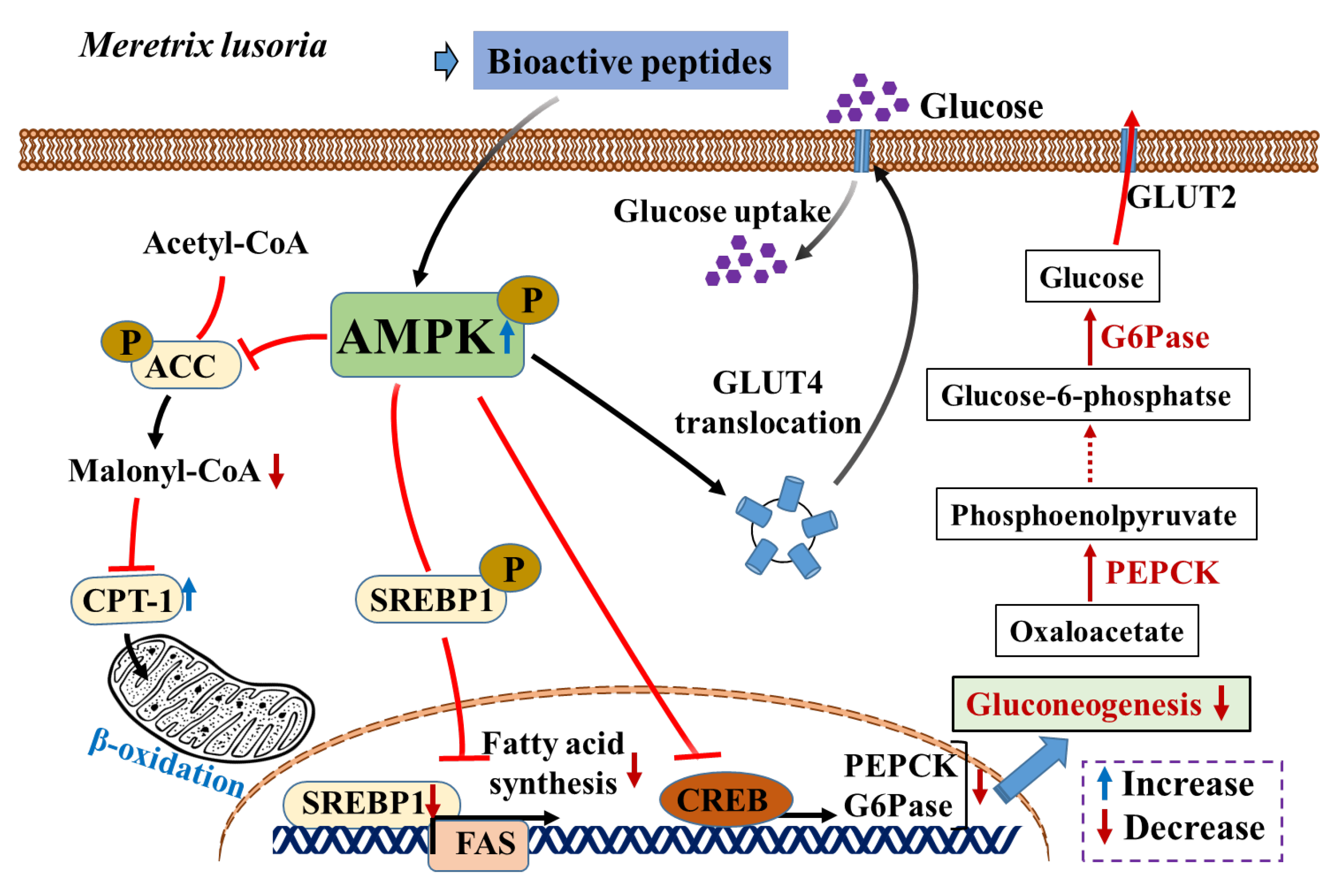
3.6. MLP Regulates Lipid Metabolism by Activating the AMPK Pathway in Obese Mice
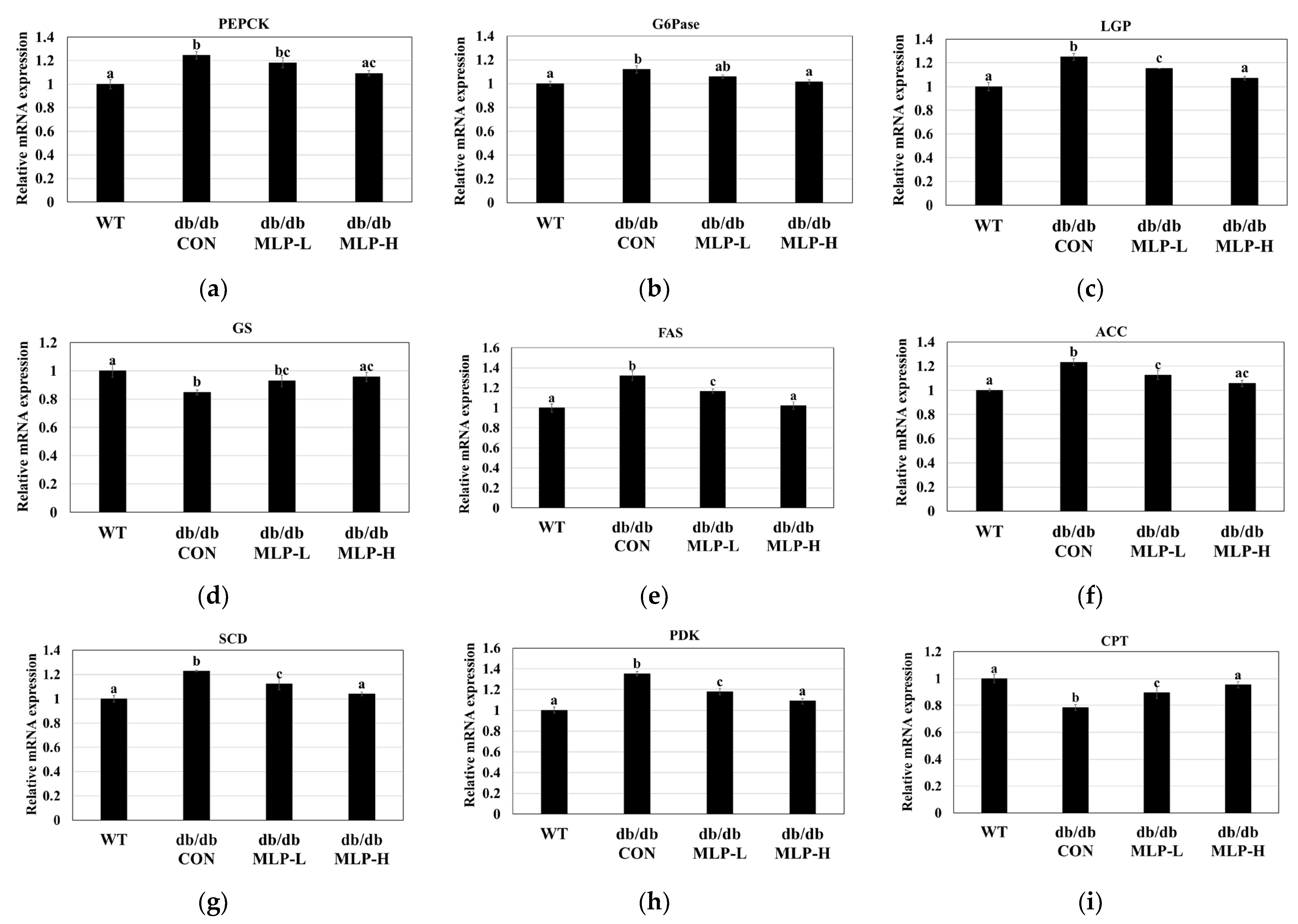
3.7. Lipogenic and Gluconeogenic Enzyme Gene Expressions Using Quantitative RT-PCR
3.8. MLP Improved Glucose Homeostasis in Obese Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, L.; Wang, J.; Xu, T.; Luo, Y.; Cai, Z.; Jiang, Y.; Jin, T.; Bao, H.; Wang, Y. Bicyclol alleviates obesity-induced renal injury by inhibiting JNK and NF-κB-mediated inflammation. Int. Immunopharmacol. 2024, 129, 111609. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Zhang, B.; Wu, B.; Xiao, H.; Li, Z.; Li, R.; Xu, X.; Li, T. Signaling pathways in obesity: Mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2022, 7, 298. [Google Scholar] [CrossRef]
- Caballero, B. Humans against obesity: Who will win? Adv. Nutr. 2019, 10, S4–S9. [Google Scholar] [CrossRef]
- Green, M.; Arora, K.; Prakash, S. Microbial medicine: Prebiotic and probiotic functional foods to target obesity and metabolic syndrome. Int. J. Mol. Sci. 2020, 21, 2890. [Google Scholar] [CrossRef]
- Burke, S.J.; Batdorf, H.M.; Burk, D.H.; Noland, R.C.; Eder, A.E.; Boulos, M.S.; Karlstad, M.D.; Jason Collier, J. db/db mice exhibit features of human type 2 diabetes that are not present in weight-matched C57BL/6J mice fed a western diet. J. Diabetes Res. 2017, 2017, 8503754. [Google Scholar] [CrossRef]
- Sarma, S.; Lipscombe, L.L. In persons with obesity, exercise plus liraglutide improved weight-loss maintenance vs. exercise or placebo. Ann. Intern. Med. 2021, 174, JC102. [Google Scholar] [CrossRef]
- He, H.; Deng, J.; Yang, M.; An, L.; Ye, X.; Li, X. Jatrorrhizine from Rhizoma Coptidis exerts an anti-obesity effect in db/db mice. J. Ethnopharmacol. 2022, 298, 115529. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.S. Peptides and peptidomimetics as potential antiobesity agents: Overview of current status. Front. Nutr. 2019, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Jung, W.-K.; Je, J.-Y. Protective effect of multifunctional peptides PIISVYWK and FSVVPSPK on oxidative stress-mediated HUVEC injury through antioxidant and anti-apoptotic action. Process Biochem. 2023, 125, 121–129. [Google Scholar] [CrossRef]
- Skjånes, K.; Aesoy, R.; Herfindal, L.; Skomedal, H. Bioactive peptides from microalgae: Focus on anti-cancer and immunomodulating activity. Physiol. Plant. 2021, 173, 612–623. [Google Scholar] [CrossRef]
- Suryaningtyas, I.T.; Ahn, C.-B.; Je, J.-Y. Cytoprotective peptides from blue mussel protein hydrolysates: Identification and mechanism investigation in human umbilical vein endothelial cells injury. Mar. Drugs 2021, 19, 609. [Google Scholar] [CrossRef] [PubMed]
- Suryaningtyas, I.T.; Je, J.-Y. Bioactive peptides from food proteins as potential anti-obesity agents: Mechanisms of action and future perspectives. Trends Food Sci. Technol. 2023, 138, 141–152. [Google Scholar] [CrossRef]
- Hyung, J.-H.; Ahn, C.-B.; Je, J.-Y. Ark shell protein hydrolysates inhibit adipogenesis in mouse mesenchymal stem cells through the down-regulation of transcriptional factors. RSC Adv. 2017, 7, 6223–6228. [Google Scholar] [CrossRef]
- Lee, E.J.; Hur, J.; Ham, S.A.; Jo, Y.; Lee, S.; Choi, M.-J.; Seo, H.G. Fish collagen peptide inhibits the adipogenic differentiation of preadipocytes and ameliorates obesity in high fat diet-fed mice. Int. J. Biol. Macromol. 2017, 104, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Bougatef, A.; Ravallec, R.; Nedjar-Arroume, N.; Barkia, A.; Guillochon, D.; Nasri, M. Evidence of in vivo satietogen effect and control of food intake of smooth hound (Mustelus mustelus) muscle protein hydrolysate in rats. J. Funct. Foods 2010, 2, 10–16. [Google Scholar] [CrossRef]
- Mizushige, T.; Komiya, M.; Onda, M.; Uchida, K.; Hayamizu, K.; Kabuyama, Y. Fish protein hydrolysate exhibits anti-obesity activity and reduces hypothalamic neuropeptide Y and agouti-related protein mRNA expressions in rats. Biomed. Res. 2017, 38, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhou, L.; Zhang, Y.; Sheng, N.-J.; Wang, Z.-K.; Wu, T.-Z.; Wang, X.-Z.; Wu, H. Rapid identification of dipeptidyl peptidase-IV (DPP-IV) inhibitory peptides from Ruditapes philippinarum hydrolysate. Molecules 2017, 22, 1714. [Google Scholar] [CrossRef]
- Kim, M.J.; Chilakala, R.; Jo, H.G.; Lee, S.-J.; Lee, D.-S.; Cheong, S.H. Anti-obesity and Anti-hyperglycemic Effects of Meretrix lusoria Protamex Hydrolysate in ob/ob Mice. Int. J. Mol. Sci. 2022, 23, 4015. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, M.; Wu, T.; Fang, L.; Liu, C.; Min, W. Novel anti-obesity peptide (RLLPH) derived from hazelnut (Corylus heterophylla Fisch) protein hydrolysates inhibits adipogenesis in 3T3-L1 adipocytes by regulating adipogenic transcription factors and adenosine monophosphate-activated protein kinase (AMPK) activation. J. Biosci. Bioeng. 2020, 129, 259–268. [Google Scholar]
- Oh, Y.; Ahn, C.-B.; Je, J.-Y. Low molecular weight blue mussel hydrolysates inhibit adipogenesis in mouse mesenchymal stem cells through upregulating HO-1/Nrf2 pathway. Food Res. Int. 2020, 136, 109603. [Google Scholar] [CrossRef]
- Qiao, Q.; Chen, L.; Li, X.; Lu, X.; Xu, Q. Roles of dietary bioactive peptides in redox balance and metabolic disorders. Oxidative Med. Cell. Longev. 2021, 2021, 5582245. [Google Scholar] [CrossRef]
- Fang, K.; Wu, F.; Chen, G.; Dong, H.; Li, J.; Zhao, Y.; Xu, L.; Zou, X.; Lu, F. Diosgenin ameliorates palmitic acid-induced lipid accumulation via AMPK/ACC/CPT-1A and SREBP-1c/FAS signaling pathways in LO2 cells. BMC Complement. Altern. Med. 2019, 19, 255. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.-Y. Oogenesis and sexual maturation in Meretrix lusoria (Röding 1798)(Bivalvia: Veneridae) in western Korea. J. Shellfish Res. 2007, 26, 71–80. [Google Scholar] [CrossRef]
- Xie, W.; Chen, C.; Liu, X.; Wang, B.; Sun, Y.; Yan, M.; Zhang, X. Meretrix meretrix: Active components and their bioactivities. Life Sci. J. 2012, 9, 756–762. [Google Scholar]
- Pan, M.-H.; Huang, Y.-T.; Ho, C.-T.; Chang, C.-I.; Hsu, P.-C.; Pan, B.S. Induction of apoptosis by Meretrix lusoria through reactive oxygen species production, glutathione depletion, and caspase activation in human leukemia cells. Life Sci. 2006, 79, 1140–1152. [Google Scholar] [CrossRef]
- Tsai, J.-S.; Chen, J.-L.; Pan, B.S. ACE-inhibitory peptides identified from the muscle protein hydrolysate of hard clam (Meretrix lusoria). Process Biochem. 2008, 43, 743–747. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kang, H.J.; Kang, J.H.; Hur, S.J. Effect of emulsification on the antioxidant capacity of beef myofibrillar protein-derived bioactive peptides during in vitro human digestion and on the hepatoprotective activity using HepG2 cells. J. Funct. Foods 2021, 81, 104477. [Google Scholar] [CrossRef]
- Park, S.Y.; Je, J.-Y.; Ahn, C.-B. Protein hydrolysates and ultrafiltration fractions obtained from krill (Euphausia superba): Nutritional, functional, antioxidant, and ACE-inhibitory characterization. J. Aquat. Food Prod. Technol. 2016, 25, 1266–1277. [Google Scholar] [CrossRef]
- Ko, W.; Lee, H.; Kim, N.; Jo, H.G.; Woo, E.-R.; Lee, K.; Han, Y.S.; Park, S.R.; Ahn, G.; Cheong, S.H. The anti-oxidative and anti-neuroinflammatory effects of sargassum horneri by heme oxygenase-1 induction in BV2 and HT22 cells. Antioxidants 2021, 10, 859. [Google Scholar] [CrossRef]
- Zhao, H.; Fan, W.; Dong, J.; Lu, J.; Chen, J.; Shan, L.; Lin, Y.; Kong, W. Evaluation of antioxidant activities and total phenolic contents of typical malting barley varieties. Food Chem. 2008, 107, 296–304. [Google Scholar] [CrossRef]
- Zhuang, H.; Tang, N.; Yuan, Y. Purification and identification of antioxidant peptides from corn gluten meal. J. Funct. Foods 2013, 5, 1810–1821. [Google Scholar] [CrossRef]
- Hunsakul, K.; Laokuldilok, T.; Prinyawiwatkul, W.; Utama-ang, N. Effects of thermal processing on antioxidant activities, amino acid composition and protein molecular weight distributions of jasmine rice bran protein hydrolysate. Int. J. Food Sci. Technol. 2021, 56, 3289–3298. [Google Scholar] [CrossRef]
- Joel, C.H.; Sutopo, C.C.; Prajitno, A.; Su, J.-H.; Hsu, J.-L. Screening of angiotensin-I converting enzyme inhibitory peptides derived from Caulerpa lentillifera. Molecules 2018, 23, 3005. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-operation and Development. 425: Acute Oral Toxicity: Up-and-Down Procedure 2008; OECD Publishing: Paris, France, 2022. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Fernando, I.P.S.; Han, E.J.; Kim, M.J.; Jung, K.; Kang, D.-S.; Ahn, C.-B.; Ahn, G. In vivo hepatoprotective effects of a peptide fraction from krill protein hydrolysates against alcohol-induced oxidative damage. Mar. Drugs 2019, 17, 690. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-N.; Kang, M.-C.; Kang, N.; Kim, S.-Y.; Hyun, C.-G.; Roh, S.W.; Ko, E.-Y.; Cho, K.; Jung, W.-K.; Ahn, G. 2, 4, 6-Trihydroxybenzaldehyde, a potential anti-obesity treatment, suppressed adipocyte differentiation in 3T3-L1 cells and fat accumulation induced by high-fat diet in C57BL/6 mice. Environ. Toxicol. Pharmacol. 2015, 39, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Arunkumar, E.; Anuradha, C.V. Genistein promotes insulin action through adenosine monophosphate–activated protein kinase activation and p70 ribosomal protein S6 kinase 1 inhibition in the skeletal muscle of mice fed a high energy diet. Nutr. Res. 2012, 32, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nair, S.; Gagnon, J. Herring Milt and Herring Milt Protein Hydrolysate Are Equally Effective in Improving Insulin Sensitivity and Pancreatic Beta-Cell Function in Diet-Induced Obese-and Insulin-Resistant Mice. Mar. Drugs 2020, 18, 635. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Lin, C.-S.; Zeng, W.-H.; Hwang, C.-C.; Chiu, K.; Ou, T.-Y.; Chang, T.-H.; Tsai, Y.-H. Effect of a novel microwave-assisted induction heating (MAIH) technology on the quality of prepackaged Asian hard clam (Meretrix lusoria). Foods 2021, 10, 2299. [Google Scholar] [CrossRef]
- Pihlanto-Leppälä, A. Bioactive peptides derived from bovine whey proteins: Opioid and ace-inhibitory peptides. Trends Food Sci. Technol. 2000, 11, 347–356. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Bioactive peptides. J. AOAC Int. 2008, 91, 914–931. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-E.; Schmidt, H.; Lai, B.; Ge, K. Transcriptional and epigenomic regulation of adipogenesis. Mol. Cell. Biol. 2019, 39, e00601–e00618. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, N.; Mendis, E.; Jung, W.-K.; Je, J.-Y.; Kim, S.-K. Purification of a radical scavenging peptide from fermented mussel sauce and its antioxidant properties. Food Res. Int. 2005, 38, 175–182. [Google Scholar] [CrossRef]
- Suriano, F.; Vieira-Silva, S.; Falony, G.; Roumain, M.; Paquot, A.; Pelicaen, R.; Régnier, M.; Delzenne, N.M.; Raes, J.; Muccioli, G.G. Novel insights into the genetically obese (ob/ob) and diabetic (db/db) mice: Two sides of the same coin. Microbiome 2021, 9, 147. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Y.; Jeon, B.-T.; Moon, S.-H.; Lee, S.-H. Enzymatic hydrolysate from velvet antler suppresses adipogenesis in 3T3-L1 cells and attenuates obesity in high-fat diet-fed mice. Excli J. 2017, 16, 328. [Google Scholar] [PubMed]
- Kamei, N.; Tobe, K.; Suzuki, R.; Ohsugi, M.; Watanabe, T.; Kubota, N.; Ohtsuka-Kowatari, N.; Kumagai, K.; Sakamoto, K.; Kobayashi, M. Overexpression of monocyte chemoattractant protein-1 in adipose tissues causes macrophage recruitment and insulin resistance. J. Biol. Chem. 2006, 281, 26602–26614. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lu, P.; Ma, Q.; Cao, Y.; Chen, N.; Li, W.; Zhao, S.; Chen, B.; Shi, J.; Sun, Y. CTNNB1/β-catenin dysfunction contributes to adiposity by regulating the cross-talk of mature adipocytes and preadipocytes. Sci. Adv. 2020, 6, eaax9605. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Choi, Y.-J.; Wedamulla, N.E.; Bae, S.M.; Kim, E.-K. Anti-Obesity Effect of Promod 278P Hydrolysate of Allomyrina dichotoma Larvae in Diet-induced Obese Mice. Food Suppl. Biomater. Health 2022, 2, e18. [Google Scholar] [CrossRef]
- Silveira, E.A.; Noll, P.R.E.S.; Mohammadifard, N.; Rodrigues, A.P.S.; Sarrafzadegan, N.; de Oliveira, C. Which diets are effective in reducing cardiovascular and cancer risk in women with obesity? An integrative review. Nutrients 2021, 13, 3504. [Google Scholar] [CrossRef]
- de Melo, D.G.; da Cruz Rodrigues, V.C.; de Sá Pereira, G.J.; de Campos, T.D.P.; dos Santos Canciglieri, R.; Pauli, J.R.; da Silva, A.S.R.; da Costa Fernandes, C.J.; de Moura, L.P. Effects of aerobic exercise on the regulation of mitochondrial carrier homolog-2 and its influence on the catabolic and anabolic activity of lipids in the mesenteric adipose tissue of obese mice. Life Sci. 2024, 345, 122567. [Google Scholar] [CrossRef] [PubMed]
- Marengo, B.; Nitti, M.; Furfaro, A.L.; Colla, R.; Ciucis, C.D.; Marinari, U.M.; Pronzato, M.A.; Traverso, N.; Domenicotti, C. Redox homeostasis and cellular antioxidant systems: Crucial players in cancer growth and therapy. Oxidative Med. Cell. Longev. 2016, 2016, 6235641. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, L.; Sun, Q.; Song, G.; Huang, J. Extraction, identification and structure-activity relationship of antioxidant peptides from sesame (Sesamum indicum L.) protein hydrolysate. Food Res. Int. 2019, 116, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Han, L.; Hong, H.; Pan, J.; Liu, H.; Luo, Y. Purification and identification of novel antioxidant peptides from silver carp muscle hydrolysate after simulated gastrointestinal digestion and transepithelial transport. Food Chem. 2021, 342, 128275. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Ci, A.-T.; Wang, H.; Zhang, Y.-Y.; Zhang, J.-G.; Thakur, K.; Wei, Z.-J. Identification and hydrolysis kinetic of a novel antioxidant peptide from pecan meal using Alcalase. Food Chem. 2018, 261, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Fernando, I.P.S.; Park, S.Y.; Han, E.J.; Kim, H.-S.; Kang, D.-S.; Je, J.-Y.; Ahn, C.-B.; Ahn, G. Isolation of an antioxidant peptide from krill protein hydrolysates as a novel agent with potential hepatoprotective effects. J. Funct. Foods 2020, 67, 103889. [Google Scholar] [CrossRef]
- Lu, M.; Cao, Y.; Xiao, J.; Song, M.; Ho, C.-T. Molecular mechanisms of the anti-obesity effect of bioactive ingredients in common spices: A review. Food Funct. 2018, 9, 4569–4581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, S.-d.; Wang, P.; Guo, N.; Wang, W.; Yao, L.-p.; Yang, Q.; Efferth, T.; Jiao, J.; Fu, Y.-j. Pinolenic acid ameliorates oleic acid-induced lipogenesis and oxidative stress via AMPK/SIRT1 signaling pathway in HepG2 cells. Eur. J. Pharmacol. 2019, 861, 172618. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Pei, R.; Lei, W.; Zhao, M.; Zhang, J.; Tian, L.; Shang, J. Antidiabetic effect of artemether in Db/Db mice involves regulation of AMPK and PI3K/Akt pathways. Front. Endocrinol. 2020, 11, 568864. [Google Scholar] [CrossRef]
- Rabinovitch, R.C.; Samborska, B.; Faubert, B.; Ma, E.H.; Gravel, S.-P.; Andrzejewski, S.; Raissi, T.C.; Pause, A.; Pierre, J.S.; Jones, R.G. AMPK maintains cellular metabolic homeostasis through regulation of mitochondrial reactive oxygen species. Cell Rep. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Bao, Q.; Shen, X.; Qian, L.; Gong, C.; Nie, M.; Dong, Y. Anti-diabetic activities of catalpol in db/db mice. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2016, 20, 153. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Wang, X.; Wu, Z.; Cui, C.; Qi, X.; Yu, Y. Identification of ginsenoside components from adventitious root of ginseng and their hypoglycemic effects on T1DM mice: A network pharmacology and animal experiment study. J. Funct. Foods 2024, 116, 106083. [Google Scholar] [CrossRef]


| Gene 1 | Forward Primer | Reverse Primer |
|---|---|---|
| ACC | GACGTTCGCCATAACCAAGT | CTGCAGGTTCTCAATGCAAA |
| G6Pase | ATGACTTTGGGATCCAGTCG | TGGAACCAGATGGGAAAGAG |
| LGP | CCAGAGTGCTCTACCCCAAT | CCACAAAGTACTCCTGTTTCAGC |
| GS | GACACTGAGCAGGGCTTTTC | GGGCCTGGGATACTTAAAGC |
| PEPCK | CTGGCACCTCAGTGAAGACA | TCGATGCCTTCCCAGTAAAC |
| FAS | CCCTTGATGAAGAGGGATCA | ACTCCACAGGTGGGAACAAG |
| CPT | GAGCCACGAAGCCCTCAAACACAT | GCTGTACAACATGGGCTTCCGACCTG |
| PDK | ATCTAACATCGCCAGAATTAAACC | GGAACGTACACAATGTGGATTG` |
| SCD | AGCTGGTGATGTTCCAGAGG | GTGGGCAGGATGAAGCAC |
| Measurement | WT | db/db CON | db/db MLP-L | db/db MLP-H |
|---|---|---|---|---|
| Initial BW (g) | 23.67 ± 0.94 a | 36.17 ± 1.07 b | 36.67 ± 1.37 b | 36.67 ± 1.37 b |
| Water intake (ml/day) | 5.60 ± 0.07 a | 17.99 ± 2.63 b | 17.25 ± 3.29 b | 17.49 ± 3.00 b |
| Food intake (g/day) | 2.46 ± 0.20 a | 4.67 ± 0.21 b | 4.62 ± 0.48 b | 4.56 ± 0.34 b |
| FER (%) | 1.40 ± 0.62 a | 3.16 ± 1.05 bc | 2.68 ± 0.57 ac | 2.16 ± 1.01 ab |
| BW gain (g/day) | 0.03 ± 0.01 a | 0.14 ± 0.05 b | 0.13 ± 0.02 b | 0.10 ± 0.05 c |
| Heart (g) | 0.110 ± 0.009 a | 0.132 ± 0.004 b | 0.121 ± 0.010 ab | 0.117 ± 0.010 ab |
| Kidney (g) | 0.290 ± 0.024 a | 0.404 ± 0.023 b | 0.363 ± 0.017 b | 0.359 ± 0.030 b |
| Liver (g) | 1.013 ± 0.083 a | 2.359 ± 0.096 b | 2.174 ± 0.201 b | 2.145 ± 0.158 b |
| Gastrocnemius muscle (g) | 0.270 ± 0.013 a | 0.195 ± 0.030 b | 0.220 ± 0.035 ab | 0.215 ± 0.031 ab |
| Mesenteric fat (g) | 0.354 ± 0.013 a | 1.751 ± 0.153 b | 1.690 ± 0.186 b | 1.482 ± 0.101 b |
| Fat retroperitoneal (g) | 0.120 ± 0.019 a | 0.519 ± 0.076 b | 0.454 ± 0.037 b | 0.439 ± 0.065 b |
| Fat epididymal (g) | 0.493 ± 0.068 a | 2.5801 ± 0.051 b | 2.369 ± 0.153 b | 2.346 ± 0.103 b |
| Perirenal fat (g) | 0.060 ± 0.012 a | 0.531 ± 0.037 b | 0.456 ± 0.104 bc | 0.352 ± 0.075 c |
| Fat total (g) | 1.026 ± 0.089 a | 5.382 ± 0.200 b | 4.969 ± 0.358 bc | 4.619 ± 0.192 c |
| Measurement | WT | db/db CON | db/db MLP-L | db/db MLP-H |
|---|---|---|---|---|
| Initial fasting blood glucose (mg/dL) | 120.00 ± 14.04 a | 170.50 ± 17.70 b | 170.33 ± 16.79 b | 170.83 ± 17.72 b |
| Fasting blood glucose at the end of treatment (mg/dL) | 108.16 ± 12.74 a | 476.66 ± 36.99 b | 449.16 ± 43.69 b | 372.5 ± 38.23 c |
| MCP-1 (pg/mL) | 193.47 ± 51.96 a | 963.75 ± 52.43 b | 920.83 ± 45.64 b | 734.58 ± 33.97 c |
| TNF-α (pg/mL) | 372.05 ± 7.45 a | 606.35 ± 7.45 b | 501.96 ± 18.88 c | 433.82 ± 18.49 d |
| Adiponectin (ng/mL) | 1.22 ± 0.07 a | 2.67 ± 0.26 b | 2.59 ± 0.09 b | 2.44 ± 0.13 b |
| TBARS (μmol/L) | 68.52 ± 6.05 a | 90.68 ± 5.95 b | 81.03 ± 5.84 bc | 74.62 ± 7.74 ac |
| HDL-cholesterol (mg/dL) | 166.50 ± 13.50 a | 239.00 ± 18.79 c | 260.00 ± 14.56 b | 262.50 ± 17.04 b |
| Insulin (ng/mL) | 1.30 ± 0.05 a | 3.05 ± 0.04 b | 2.85 ± 0.03 c | 2.63 ± 0.05 d |
| Total protein (g/dL) | 5.58 ± 0.73 a | 6.83 ± 0.40 b | 6.65 ± 0.16 b | 5.70 ± 0.21 a |
| Albumin (g/dL) | 3.75 ± 0.52 a | 4.63 ± 0.28 b | 4.62 ± 0.11 b | 4.02 ± 0.16 a |
| BUN (mg/dL) | 16.50 ± 1.26 a | 28.33 ± 4.71 b | 20.33 ± 1.60 a | 18.33 ± 3.20 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chilakala, R.; Moon, H.J.; Jung, M.S.; Han, J.W.; Ko, K.H.; Lee, D.S.; Cheong, S.H. Bioactive Peptides from Meretrix lusoria Enzymatic Hydrolysate as a Potential Treatment for Obesity in db/db Mice. Nutrients 2024, 16, 1913. https://doi.org/10.3390/nu16121913
Chilakala R, Moon HJ, Jung MS, Han JW, Ko KH, Lee DS, Cheong SH. Bioactive Peptides from Meretrix lusoria Enzymatic Hydrolysate as a Potential Treatment for Obesity in db/db Mice. Nutrients. 2024; 16(12):1913. https://doi.org/10.3390/nu16121913
Chicago/Turabian StyleChilakala, Ramakrishna, Hyeon Jeong Moon, Min Seouk Jung, Jong Won Han, Kang Ho Ko, Dong Sung Lee, and Sun Hee Cheong. 2024. "Bioactive Peptides from Meretrix lusoria Enzymatic Hydrolysate as a Potential Treatment for Obesity in db/db Mice" Nutrients 16, no. 12: 1913. https://doi.org/10.3390/nu16121913
APA StyleChilakala, R., Moon, H. J., Jung, M. S., Han, J. W., Ko, K. H., Lee, D. S., & Cheong, S. H. (2024). Bioactive Peptides from Meretrix lusoria Enzymatic Hydrolysate as a Potential Treatment for Obesity in db/db Mice. Nutrients, 16(12), 1913. https://doi.org/10.3390/nu16121913






