Abstract
Probiotics are posited to enhance exercise performance by influencing muscle protein synthesis, augmenting glycogen storage, and reducing inflammation. This double-blind study randomized 88 participants to receive a six-week intervention with either a placebo, Lactococcus lactis subsp. lactis LY-66, Lactobacillus plantarum PL-02, or a combination of both strains, combined with a structured exercise training program. We assessed changes in maximal oxygen consumption (VO2max), exercise performance, and gut microbiota composition before and after the intervention. Further analyses were conducted to evaluate the impact of probiotics on exercise-induced muscle damage (EIMD), muscle integrity, and inflammatory markers in the blood, 24 and 48 h post-intervention. The results demonstrated that all probiotic groups exhibited significant enhancements in exercise performance and attenuation of muscle strength decline post-exercise exhaustion (p < 0.05). Notably, PL-02 intake significantly increased muscle mass, whereas LY-66 and the combination therapy significantly reduced body fat percentage (p < 0.05). Analysis of intestinal microbiota revealed an increase in beneficial bacteria, especially a significant rise in Akkermansia muciniphila following supplementation with PL-02 and LY-66 (p < 0.05). Overall, the combination of exercise training and supplementation with PL-02, LY-66, and their combination improved muscle strength, explosiveness, and endurance performance, and had beneficial effects on body composition and gastrointestinal health, as evidenced by data obtained from non-athlete participants.
1. Introduction
Regular exercise has been extensively studied and scientifically validated for its numerous health benefits across a range of physical conditions, including cardiovascular disease, lung disease, metabolic syndrome, cancer, and sexual dysfunction [1]. Among the array of benefits conferred by exercise, it is particularly noteworthy that it can enhance maximal oxygen uptake, thereby fortifying cardiovascular robustness. Maximal oxygen uptake (VO2max), the maximum rate of oxygen consumption during intense and maximal exercise, serves as a crucial measure of cardiovascular health and aerobic endurance [2,3]. The ability to transform nutrients into adenosine triphosphate (ATP) via aerobic pathways is directly linked to how effectively oxygen is utilized by the muscles, thereby enhancing athletic performance and supporting cardiovascular health, potentially reducing the risk of developing atherosclerosis [4]. Exercise plays a pivotal role in positively influencing the musculoskeletal system by enhancing muscular strength, endurance, and flexibility. These benefits not only help mitigate muscle loss but also serve as preventive measures against falls in the elderly [5]. Furthermore, regular physical activity significantly contributes to enhanced social well-being, improved quality of life [6], and increased longevity [7]. It is noteworthy that exercise can also be associated with elevated self-esteem and a reduced risk of anxiety in younger individuals [8,9]. Despite these advantages, many individuals experience post-exercise fatigue, which can hinder the maintenance of a regular exercise routine. This form of fatigue typically arises from exercise-induced muscle damage (EIMD). During the initial phases of muscle damage, mechanical trauma triggers an acute inflammatory cascade, resulting in the release of pro-inflammatory cytokines such as interleukin 6 (IL-6), tumor necrosis factor-alpha (TNF-α), and IL-1β, alongside heightened levels of creatine kinase (CK) and myoglobin [10,11]. Beyond the acute inflammatory response, prolonged endurance exercise precipitates oxidative and metabolic stress, further exacerbating inflammation [12,13]. These physiological alterations result in a temporary decrease in muscle strength and performance. To modulate the dysregulated inflammatory response while continually optimizing athletic performance and preserving health, athletes have increasingly turned to dietary supplements in recent years. Among these, the utilization of probiotics for health enhancement and performance augmentation has attracted growing interest.
Scientists have previously proposed the concept of the “gut–muscle axis”, suggesting a connection between the gut microbiota and muscle cells. It is believed that the gut microbiota, composed of four main phyla including Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, may play a role in producing beneficial nutrients such as short-chain fatty acids (SCFAs) that can impact muscle cells [14,15]. The human gut microbiota consists of an incredibly vast number of microorganisms, with over 1014 cells, which is approximately ten times the number of bacterial cells compared to the total count of human cells. Furthermore, the gut microbiota has a genome size that is 150 times larger than the human genome [16]. This highlights the significant presence and potential impact of the gut microbiota on the human body’s normal homeostasis. However, the specific mechanisms by which the gut microbiota regulates the metabolism of muscle cells are still not fully understood. Further research is needed to uncover the intricate workings of the gut–muscle axis and the specific interactions between the gut microbiota and muscle cells.
A human study reported that the probiotic strain Bacillus coagulans demonstrated potential in reducing exercise-induced muscle damage [17]. Another study focused on a probiotic strain isolated from humans, Bifidobacterium longum subsp. longum OLP-01, which was found to enhance endurance running distance in middle- and long-distance runners [18]. Furthermore, this probiotic strain was associated with increased muscle grip strength and a reduction in fatigue induced by exhaustive exercise [19]. Additionally, our previous animal study investigated two probiotic strains, Lactococcus lactis subsp. lactis LY-66 and Lactobacillus plantarum PL-02, which were isolated from an Olympic elite athlete. These strains were found to significantly elevate grip strength and endurance in tested mice. The mechanisms underlying these effects were proposed to involve improved fatty acid metabolism and increased glycogen storage levels in the liver and muscles, leading to enhanced exercise performance in the mouse model [20]. However, whether these two probiotic strains can similarly promote exercise performance in humans remains uncertain. While these studies show promising findings regarding the potential benefits of specific probiotic strains on exercise-related outcomes, it is important to note that further research is needed to validate and understand the precise mechanisms involved. Additionally, individual variations, the specific strains and dosages of probiotics, and other factors should be considered.
The aim of this study was to investigate the effects of PL-02, LY-66, and their combined supplementation on exercise performance, resilience to post-exercise muscle strength loss, and gastrointestinal health. Initially, we measured participants’ body composition, blood biochemical markers, exercise performance, and maximal oxygen consumption (VO2max) both prior to and following the probiotic intervention. For the assessment of muscle strength loss following exercise-induced muscle damage (EIMD), we conducted countermovement jump (CMJ) and isometric mid-thigh pull (IMTP) tests. Additionally, to measure the extent of muscle damage and inflammation, we analyzed specific biomarkers in the blood. To examine changes in the gut microbiota, we employed next-generation sequencing (NGS) technology pre- and post-intervention, providing a comprehensive analysis of the microbiota’s response to probiotic supplementation.
2. Materials and Methods
2.1. Experimental Test Samples
The probiotic strains of Lactococcus lactis subsp. lactis (LY-66) (BCRC 911055 = CGMCC 21838) and Lactobacillus plantarum (PL-02) (BCRC 911012 = CGMCC 20485) were isolated from the gut of a weightlifting Olympic gold medalist and obtained from Glac Biotech Co., Ltd (Tainan, Taiwan). [20]. The genetic sequences of these probiotic strains have been determined through sequencing techniques. Subsequently, the strains were encapsulated to produce a uniformly standardized dry beverage in appearance.
2.2. Participants
The Harvard calculator (http://hedwig.mgh.harvard.edu/sample_size/size.html, accessed on 12 February 2022) was used to determine the sample size, assuming a parallel design with a significance level of 0.05, a power of 0.9, and a minimal detectable difference (following our pilot study) of 0.36 for the difference. A total of 84 patients entered this study. To account for the possibility of subject dropout, we added one person to each group to ensure minimum sample size requirements. A total of 88 healthy non-athlete adults aged 20–40 were included in this study, among which 44 participants were males and 44 were females. The exclusion criteria were as follows: smokers, cardiovascular disease, high blood pressure, BMI > 27, metabolic disease, asthma or within 6 months, those who are unable to engage in sports due to physical or neuromuscular injuries, those who have taken anti-inflammatory and analgesic drugs or probiotic-related products in the past month, students or related stakeholders of the program investigator. The Institutional Review Board of Landseed International Hospital (Taoyuan, Taiwan; LSHIRB No. 21-042-A2) reviewed and approved the conduct of this clinical study. The trial was as registered at clinicaltrials.gov as NCT06092723 on 23 October 2023. Furthermore, this study was executed by following the principles of the Declaration of Helsinki.
2.3. Experimental Design and Content
A total of 88 healthy recruited participants were randomly and double-blindly divided into 4 groups of 22 people in each group (half male and half female), including (A) placebo group (2 sachets/day in 250 mL of water), (B) LY-66 probiotic group (7.5 × 109 CFU/sachet, 2 sachets/day in 250 mL of water), (C) PL-02 probiotic group (7.5 × 109 CFU/sachet, 2 sachets/day in 250 mL of water), (D) PL-02+LY-66 combined bacteria probiotic group 1:1 (7.5 × 109 CFU/sachet, 2 sachets/day in 250 mL of water). All participants included in the study were first numbered according to the order of registration, and then, randomly assigned to four groups using a computer-generated random sampling method. The numbers were then redefined for identification purposes. Additionally, the sample manufacturer randomly assigned the codes A, B, C, and D to the four samples: placebo, PL-02, LY-66, and PL-02+LY-66. These coded samples were then distributed to the researchers who assigned them to the corresponding groups of participants. After the test and data analysis were completed, the sample manufacturer performed unblinding to reveal which samples corresponded to each group code. The probiotics were stored in a 4 °C environment and administered in a daily dosage of two sachets per intake, dissolved in 250 milliliters of water to prepare the beverage. This formulation, presented as a probiotic drink, served not only as a means of rehydration post-exercise but also facilitated the intake of probiotics. The intervention period lasted 6 weeks. The placebo contained anhydrous glucose, sodium citrate, lemon flavor, citric acid, sodium chloride, potassium citrate, sodium ascorbate, acesulfame potassium, and vitamin B complex. Probiotic-related products, anti-oxidant, anti-inflammatory drugs, or nutritional supplements were not allowed during the test. Subjects were required to photograph their meals on the day before the exercise test. Each photograph included a scale to facilitate accurate determination of portion sizes and nutritional content by the nutritionist. Additionally, subjects were asked to avoid extra exercise training from 48 h before and after the exercise test (Figure 1). The muscle-training regimen consisted of Tabata workouts conducted twice a week. Each session included the following exercises: (1) squats, (2) push-ups, (3) lunges, (4) high knees, (5) spiderman planks, (6) leg raises, (7) plank jacks, and (8) crunches. Each exercise was performed for 20 s followed by a 10 s rest. The complete Tabata workout comprised one set of these eight exercises. All subjects were required to train Tabata twice a week for a total of six weeks, a total of 12 times. The participation rate of each group was 100%, and no one was absent.
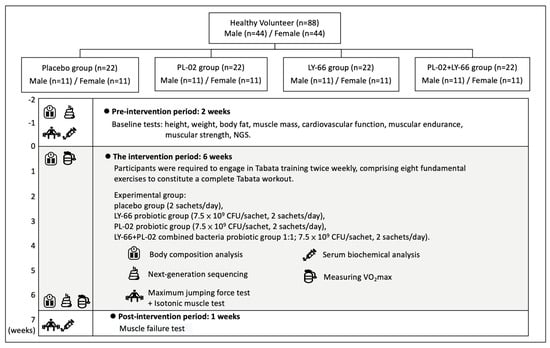
Figure 1.
Experimental procedure description.
The relevant details of the experiment are as follows: the blood biochemistry, exercise tests, feces collection, maximum oxygen uptake, and body composition were tested before and after the intervention. Blood samples were collected 24 and 48 h after the exercise-induced muscle exhaustion for analysis of the inflammation damage indicator. Body composition was measured every two weeks. During the exercise test process, sport protectors were on hand to assist and guide subjects for avoiding sports injuries.
2.4. Body Composition Analysis
Body composition was assessed utilizing the InBody 570 device (InBody, Seoul, Republic of Korea), assessing total body weight, body fat percentage, muscle mass distribution, BMI, and basal metabolic rate. All assessments were conducted after participants had fasted for eight hours, following the methodology of a prior study [18].
2.5. Exercise Program to Induce Muscle Fatigue and Soreness
An exercise regimen involving 100 repetitive jumps was designed to induce muscle fatigue and soreness. Participants performed sets of 10 consecutive jumps, each to be completed within 4 s, followed by a 90 s rest period before the next set. This cycle was repeated until 100 jumps were completed. During each jump, the participant’s knees were required to be bent to a 90-degree angle to standardize the movement across all participants.
2.6. Countermovement Jump Assessment (CMJ)
Participants performed the CMJ on a Kistler force-measuring platform (9260AA, Kistler GmbH, Winterthur, Switzerland). They began by standing with hands on hips, performed a squat to a 90-degree knee bend, and then, jumped with maximum effort. Each participant completed three trials, with the platform calibrated to their individual weight to ensure accuracy. Measured parameters comprised the rate of force development (RFD), relative peak force, and jump height, offering detailed insights into each participant’s lower body power and force generation capabilities during the jumps.
2.7. Isometric Mid-Thigh Pull (IMTP)
The IMTP was conducted to assess maximal force generation using a custom-built IMTP rack (Kairos Strength, Murphy, NC, USA) and a force plate (Type 9260AA, Kistler, Winterthur, Switzerland), as per the protocol outlined in a prior study [21]. Participants were required to exert their maximum strength on the bar for 3–5 s, with tests repeated at 2 min intervals. The primary metrics recorded were peak force (the maximum strength exerted) and relative peak force (the maximum strength relative to body weight).
2.8. Wingate Anaerobic Test (WAnT)
The Wingate Anaerobic Test was conducted using the 894E anaerobic power bicycle (Monark Exercise AB, Dalarnas Lan, Sweden). Participants initially ramped up their cycling speed to 120 rpm, at which point a resistance equivalent to 7.5% of the participant’s body weight was automatically applied to the wheel. This test required participants to sprint at full strength for 30 s. Metrics assessed included relative mean power (W/kg), relative peak power (W/kg), and the fatigue index, calculated as the percentage drop from peak power to the lowest power level sustained [22].
2.9. Measuring VO2max
VO2max was assessed using the Bruce maximal treadmill protocol, established in 1973 [23]. Participants wore a face mask connected to a gas analysis system and had electrocardiogram electrodes placed in appropriate locations. The test started at a velocity of 7.2 km/hr, progressively increasing by 1.8 km/hr every two minutes until exhaustion. Data collection commenced once the participant’s heart rate reached 170 beats per minute and continued until exhaustion, defined by meeting at least two of three criteria: maximal heart rate (220 minus age), a respiratory exchange ratio over 1.1, or a rating of perceived exertion (RPE) above 18.
2.10. Physiological Observation and Serum Biochemical Analysis
Venous blood samples were collected from participants following a minimum fasting period of 8 h, both before and after the intervention. Biochemical markers including AST, ALT, BUN, creatinine, uric acid, total protein, cholesterol profiles, and glucose levels were quantified utilizing a Hitachi 717 analyzer (Hitachi, Tokyo, Japan). Creatine kinase (CK) levels and IL-6 concentrations were determined using a Beckman Coulter AU5800 autoanalyzer (Beckman Coulter Inc., Brea, CA, USA) and an ELISA kit (R&D Systems, Inc., Minneapolis, MN, USA), respectively.
2.11. Fecal DNA Extraction and Next Generation Sequencing (NGS) Analysis
Participants provided a fecal sample before and after the experiment to examine alterations in the gut microbiota. DNA extraction was conducted from fecal samples following the experimental procedures outlined by the manufacturer of the QIAamp® DNA Mini Kit (QIAGEN Canada, Mississauga, ON, Canada). After undergoing purification and extraction processes, the DNA was employed as a template for polymerase chain reaction (PCR). The specific primer pair employed in our PCR analysis comprised the forward primer 314F (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG-3′) and the reverse primer 805R (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′). This primer set was devised for the selective amplification of the V3–V4 region within the bacterial 16S rRNA gene. The DNA amplification was conducted employing KAPA HiFi HotStart ReadyMix (Roche Sequencing Solutions, Pleasanton, CA, USA [KK2601]). Thermal cycling was performed using the following parameters: initially, the samples were denatured at 95 °C for 5 min. This was followed by 30 cycles, each consisting of denaturation at 95 °C for 30 s, annealing at 60 °C for 30 s, and extension at 72 °C for 30 s. Finally, an extension step was carried out at 72 °C for 5 min. To facilitate the construction of DNA libraries, the PCR products underwent processing with Nextera XT Index and Illumina sequencing adapters. The prepared DNA libraries underwent paired-end sequencing (2 × 300 bp) using an Illumina MiSeq platform (Illumina, San Diego, CA, USA), conducted by Majorbio Bio-Pharm Technology Co., Ltd. (Shanghai, China).
2.12. Bioinformatics Analysis and Statistics
We employed BaseSpace Sequence Hub (Illumina, San Diego, CA, USA) for the management of the sequenced data. The initial processing involved quality control and elimination of unpaired reads from the original paired-end sequences. These reads were subjected to clustering into operational taxonomic units (OTUs) using the May 2013 Greengenes taxonomic database for subsequent downstream analysis [24]. To investigate the diversity composition of intestinal microorganisms among distinct groups, alpha diversity and beta diversity were analyzed [25]. The diversity index was computed utilizing the ‘vegan’ package in the R statistical software environment; accessible at https://cran.r-project.org/, accessed on 20 December 2023. Alpha diversity pertains to the diversity within a particular geographical area or ecosystem, while beta diversity encompasses the diversity observed among different geographical areas or ecosystems. Shannon diversity has been employed as an assessment tool to gauge the richness of the community, wherein an elevated numerical value signifies increased abundance. The principal coordinate analysis (PCoA) figures depicting the beta diversity index were generated utilizing the ‘ggplot2’ R package. The Bray–Curtis matrix PERMANOVA testing was conducted employing the adonis function within the vegan package in R version 4.3.2. Pairwise comparisons were performed utilizing Student’s t-test, while analysis among multiple groups employed the same method. Permutational multivariate analysis of variance (PERMANOVA) was applied to assess statistical variances in beta diversity, utilizing QIIME2. A significance threshold of p < 0.05 was established for determining statistical significance.
2.13. Statistics
Statistical analyses were conducted using the SAS 9.0 software (SAS Institute, Cary, NC, USA). A one-way analysis of variance (ANOVA) was utilized to perform multi-group comparisons. Prior to conducting ANOVA, all data sets were assessed for normality. For data adhering to a normal distribution, the paired t-test was applied to evaluate differences between groups. In cases where data did not follow a normal distribution, the non-parametric Wilcoxon signed-rank test was employed. A p-value below 0.05 was considered statistically significant. All results are presented as mean ± standard deviation (SD).
3. Results
3.1. The Effects of Supplementing with PL-02, LY-66, or PL-02+LY-66 Probiotics Were Beneficial for Body Composition
Table 1 presents the body composition data of the subjects before and after six weeks of oral supplementation with PL-02, LY-66, and PL-02+LY-66 probiotics. In the placebo group, there were no significant differences observed in body weight (BW), body mass index (BMI), muscle mass, and fat body mass (FBM) values between the baseline and endpoint. Similarly, no significant differences were detected in either BW or BMI across all treatment groups before and after probiotic intervention. Regarding muscle mass, the initial mean muscle mass for the PL-02 group, LY-66 group, and PL-02+LY-66 group was 28.3 ± 7.1 kg, 27.7 ± 6.3 kg, and 30.0 ± 7.9 kg, respectively. After 6 weeks of intervention, the final mean muscle mass for the PL-02 group, LY-66 group, and PL-02+LY-66 group was 28.7 ± 7.0 kg, 27.9 ± 6.2 kg, and 30.0 ± 7.8 kg, respectively. Specifically, the PL-02 group showed a significant increase in muscle mass compared to baseline at 6 weeks of intervention (p < 0.05). Additionally, the initial mean body fat percentages of the PL-02 group, LY-66 group, and PL-02+LY-66 group were 21.2 ± 8.0%, 20.8 ± 8.1%, and 21.9 ± 6.5%, respectively. Following 6 weeks of intervention, the final mean body fat percentages for the PL-02 group, LY-66 group, and PL-02+LY-66 group were 20.4 ± 7.9%, 19.8 ± 8.0%, and 21.2 ± 6.2%, respectively. Notably, both the LY-66 group (p < 0.05) and PL-02+LY-66 group (p < 0.01) exhibited a significant reduction in body fat percentage compared to baseline at 6 weeks of intervention.

Table 1.
Body composition profiles.
3.2. Effects of Biochemical Characteristics of Subjects before and after 6-Week Probiotic Intervention with PL-02, LY-66, or PL-02+LY-66
Prior to the intervention, all participants underwent baseline blood parameter analysis to assess their physiological state and to identify any potential adverse effects or side effects following the 6-week PL-02, LY-66, or PL-02+LY-66 intervention. The results, as presented in Table 2, indicate that liver function markers (AST, ALT), renal function markers (BUN, CREA, UA, TP), blood lipid profile (TC, TG, HDL-C, LDL-C), and blood glucose levels were all within the normal range before and after the intervention. No significant differences were observed among the groups pre- and post-intervention. Furthermore, there were no significant changes within each group after the intervention compared to before the intervention. Importantly, no adverse events associated with the intervention were reported throughout the study period.

Table 2.
Routine blood biochemical parameters of subjects before (Week 0) and after (Week 6) probiotic intervention.
3.3. Effect of PL-02, LY-66, or PL-02+LY-66 Probiotic Supplementation on Maximal Oxygen Consumption
Prior to the intervention, the baseline VO2max values were recorded for the placebo group, PL-02 group, LY-66 group, and PL-02+LY-66 group as 44.1 ± 4.4, 43.9 ± 4.3, 43.8 ± 5.2, and 44.2 ± 4.3 mL/min/kg, respectively (Figure 2). After a 6-week intervention period, the placebo group exhibited a slight increase to 45.1 ± 6.3 mL/min/kg. In contrast, the PL-02 group, LY-66 group, and PL-02+LY-66 group demonstrated significant increases to 47.8 ± 6.9, 46.3 ± 5.3, and 47.3 ± 5.6 mL/min/kg for each respective group (Figure 2). Statistical analysis revealed significant improvements in VO2max within the PL-02 group (increase of 1.09-fold, p < 0.001), LY-66 group (increase of 1.06-fold, p < 0.001), and PL-02+LY-66 group (increase of 1.07-fold, p < 0.001) post-intervention. Conversely, no significant change was observed in the placebo group’s VO2max pre- and post-intervention.
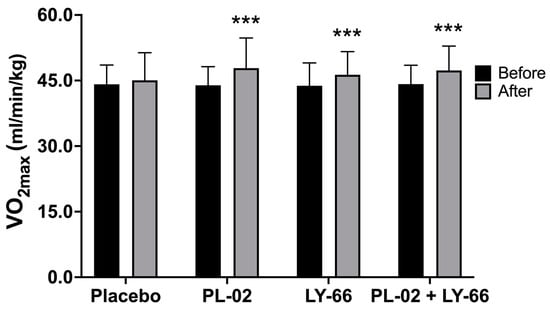
Figure 2.
Comparing the effects of PL-02, LY-66, and PL-02+LY-66 on maximal oxygen uptake (VO2max) before and after 6 weeks of intervention. All values are expressed as mean ± SD. * indicates significant differences compared to the baseline within each group (*** p < 0.001).
3.4. Effect of PL-02, LY-66, or PL-02+LY-66 Probiotic Supplementation on Enhancing Strength Performance, Explosive Power, and Anaerobic Power
To investigate the effects of supplementing with PL-02, LY-66, or PL-02+LY-66 probiotics on strength performance, explosive power, and anaerobic power, participants from different treatment groups underwent 6 weeks of oral probiotic supplementation before testing. Firstly, we investigated the effects of probiotic supplementation on strength performance and explosive power in a countermovement jump (CMJ) test. The baseline rate of force development (RFD) values were recorded for the placebo group, PL-02 group, LY-66 group, and PL-02+LY-66 group as 8.9 ± 2.0, 8.8 ± 1.3, 8.5 ± 0.8, and 8.7 ± 1.3 N/kg/sec, respectively (Figure 3A). Following a 6-week intervention period, the placebo group exhibited a slight increase to 9.0 ± 2.0 N/kg/sec. In contrast, the PL-02 group, LY-66 group, and PL-02+LY-66 group demonstrated significant increases, with values rising to 9.1 ± 1.4, 8.8 ± 0.8, and 9.3 ± 1.2 N/kg/sec, respectively (Figure 3A). Notably, the RFD increased significantly, by 1.04-fold in the PL-02 group (p < 0.01), 1.04-fold in the LY-66 group (p < 0.001), and 1.07-fold in the PL-02+LY-66 group (p < 0.001) (Figure 3A). Baseline relative peak force (RPF) values were initially recorded for the placebo group, PL-02 group, LY-66 group, and PL-02+LY-66 group, yielding values of 16.1 ± 2.0, 16.0 ± 2.1, 15.9 ± 2.8, and 15.7 ± 2.4 N/kg, respectively (Figure 3B). Following a 6-week intervention period, the placebo group exhibited a marginal increase to 16.2 ± 2.2 N/kg. In contrast, the PL-02 group, LY-66 group, and PL-02+LY-66 group demonstrated significant enhancements to 16.9 ± 2.0, 16.5 ± 2.8, and 16.3 ± 2.2 N/kg, respectively (Figure 3B). Remarkably, the PL-02 group, LY-66 group, and PL-02+LY-66 group exhibited noteworthy increases in RPF, demonstrating respective increases of 1.06-fold (p < 0.001), 1.04-fold (p < 0.001), and 1.07-fold (p < 0.001) (Figure 3B).
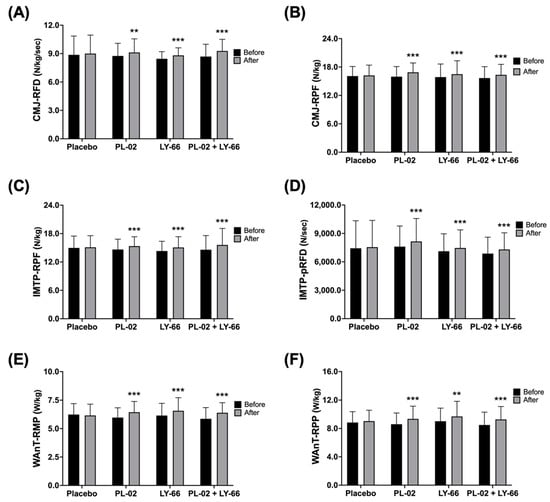
Figure 3.
The effect of supplementation with PL-02, LY-66, or PL-02+LY-66 on athletic performance was evaluated using CMJ, IMTP, and WAnT tests. The CMJ test assessed performance in (A) RFD and (B) RPF before and after the intervention. The IMTP test assessed performance in (C) RPF and (D) pRFD before and after the intervention. Additionally, the WAnT test assessed performance in (E) RMP and (F) RPP before and after the intervention. All values are expressed as mean ± SD with a sample size of n = 22 for each group. * indicates a significant difference compared to before and after intervention within each group (** p < 0.01 and *** p < 0.001).
Next, the impact on IMTP performance and explosiveness was assessed. The baseline RPF values were documented for the placebo group, PL-02 group, LY-66 group, and PL-02+LY-66 group as 15.0 ± 2.5, 14.6 ± 2.2, 14.3 ± 2.1, and 14.6 ± 3.0 N/kg, correspondingly (Figure 3C). Subsequent to a 6-week intervention period, the placebo group displayed a slight increase to 15.1 ± 2.5 N/kg. Conversely, the PL-02 group, LY-66 group, and PL-02+LY-66 group demonstrated notable increases, with values increasing to 15.4 ± 2.0, 15.1 ± 2.3, and 15.6 ± 3.5 N/kg, respectively (Figure 3C). Particularly, the PL-02 group, LY-66 group, and PL-02+LY-66 group exhibited respective increases of 1.05-fold (p < 0.001), 1.05-fold (p < 0.001), and 1.07-fold (p < 0.001) in RPF (Figure 3C). The baseline peak rate of force development (pRFD) values were documented for the placebo group, PL-02 group, LY-66 group, and PL-02+LY-66 group as follows: 7435.8 ± 2903.4, 7605.8 ± 2184.0, 7120.6 ± 1845.4, and 6870.8 ± 1735.2 N/s, correspondingly (Figure 3D). Subsequent to a 6-week intervention period, the placebo group displayed a modest increase to 7554.1 ± 2823.3 N/s. Conversely, the PL-02 group, LY-66 group, and PL-02+LY-66 group demonstrated notable increases, with values rising to 8156.9 ± 2421.6, 7466.4 ± 1903.9, and 7307.3 ± 1760.1 N/s, respectively (Figure 3D). Significant enhancements in pRFD were observed in the PL-02 group, LY-66 group, and PL-02+LY-66 group, with increases of 1.07-fold (p < 0.001), 1.05-fold (p < 0.001), and 1.06-fold (p < 0.001), respectively (Figure 3D).
Regarding anaerobic power performance, baseline body weight relative mean power (RMP) values were documented for the placebo group, PL-02 group, LY-66 group, and PL-02+LY-66 group at 6.2 ± 1.0, 6.0 ± 0.9, 6.2 ± 1.1, and 5.9 ± 1.0 W/kg, respectively (Figure 3E). Following a 6-week intervention period, the placebo group exhibited a sustained mean value of 6.2 ± 1.0 W/kg. In contrast, the PL-02 group, LY-66 group, and PL-02+LY-66 group exhibited notable increases, with mean values rising to 6.4 ± 1.0, 6.6 ± 1.2, and 6.4 ± 0.9 W/kg, respectively (Figure 3E). Specifically, the PL-02 group, LY-66 group, and PL-02+LY-66 group demonstrated respective increases of 1.08-fold (p < 0.001), 1.07-fold (p < 0.001), and 1.09-fold (p < 0.001) in RMP performance (Figure 3E). Baseline relative peak power (RPP) values were recorded for the placebo group, PL-02 group, LY-66 group, and PL-02+LY-66 group, yielding measurements of 8.8 ± 1.5, 8.6 ± 1.6, 9.0 ± 1.9, and 8.5 ± 1.8 W/kg, respectively (Figure 3F). Following a 6-week intervention period, the placebo group demonstrated a modest increase to 9.0 ± 1.6 W/kg. In contrast, the PL-02 group, LY-66 group, and PL-02+LY-66 group exhibited notable increases, with values increasing to 9.4 ± 1.8, 9.7 ± 2.1, and 9.3 ± 1.8 W/kg, respectively (Figure 3F). Correspondingly, RPP performance improved by 1.09-fold (p < 0.001), 1.08-fold (p < 0.01), and 1.09-fold (p < 0.001) for the PL-02 group, LY-66 group, and PL-02+LY-66 group, respectively (Figure 3F).
3.5. Effect of PL-02, LY-66, or PL-02+LY-66 Probiotic Supplementation on Restoring Muscle Performance and Explosive Strength after EIMD
To assess the benefits of PL-02, LY-66, or PL-02+LY-66 probiotic supplementation on CMJ and IMTP after EIMD, we investigated the extent of strength performance and explosive force reduction, as well as the recovery profiles at 24 and 48 h post-EIMD for different treatment groups following continuous 6-week probiotic intake prior to EIMD. We analyzed the percentage changes in RFD and RPF during CMJ observed at 24 and 48 h after the exhaustive exercise (Figure 4A,B). Upon comparing the post-EIMD measurements at 24 and 48 h between the placebo and probiotic groups, it was observed that probiotic groups exhibited a trend of acceleration in the recovery rate of RFD (Figure 4A). Regarding the RPF at 24 and 48 h post-EIMD, it was observed that the PL-02, LY-66, and PL-02+LY-66 groups demonstrated a significantly smaller decrease in RPF in comparison to the placebo group (p < 0.05) (Figure 4B).
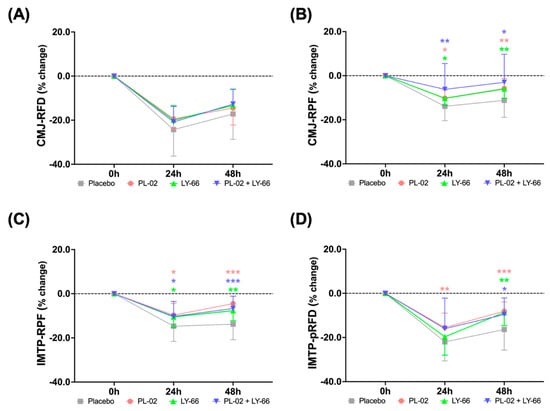
Figure 4.
The efficacy of supplementation with PL-02, LY-66, or PL-02+LY-66 in mitigating strength loss was evaluated at 24 and 48 h post-EIMD using CMJ and IMTP tests. The CMJ test assessed performance in (A) RFD and (B) RPF at 24 and 48 h post-EIMD. The IMTP test assessed performance in (C) RPF and (D) pRFD at 24 and 48 h post-EIMD. All values are expressed as mean ± SD with a sample size of n = 22 for each group. * indicates a significant difference compared to placebo and treatment groups at same time points (* p < 0.05, ** p < 0.01, and *** p < 0.001).
We further investigated the percentage changes in IMTP and observed its RPF and pRFD at 24 and 48 h post-EIMD (Figure 4C,D). The PL-02, LY-66, and PL-02+LY-66 groups displayed a notable attenuation in the decline in RPF at 24 and 48 h post-EIMD compared to the control group (p < 0.05) (Figure 4C). At 48 h post-EIMD, these same treatment groups exhibited a significant increase in the recovery of RPF compared to the placebo group (p < 0.01). On the other hand, the PL-02, LY-66, and PL-02+LY-66 groups showed markedly lower levels of pRFD loss than the placebo group at 48 h post-EIMD (p < 0.05) (Figure 4D). Notably, the PL-02 group demonstrated significant protective effects in reducing the decline in pRFD compared to the placebo group at all post-EIMD time points (p < 0.01).
3.6. Effect of PL-02, LY-66, or PL-02+LY-66 Probiotic Supplementation on Blood Markers of Muscle Damage and Inflammation
We proceeded to assess the effects of PL-02, LY-66, or PL-02+LY-66 probiotic supplementation on muscle damage and inflammatory markers following EIMD. Blood samples were collected from participants in each group, pre-EIMD and 24 and 48 h post-EIMD, after a 6-week probiotic supplementation period, for subsequent analysis. The activity percentage of the muscle damage marker CK increased in the placebo, PL-02, LY-66, and PL-02+LY-66 groups at 24 and 48 h after EIMD (Figure 5A). At 24 h post-EIMD, the PL-02 group exhibited a significant reduction in the CK activity compared to the placebo group (p < 0.05). Upon reaching the 48 h post-EIMD time point, the PL-02 group (p < 0.01), LY-66 group (p < 0.01), and PL-02+LY-66 group (p < 0.01) all demonstrated significant reductions in CK activity increase and accelerated recovery compared with the placebo group. The percentage changes in IL-6 concentration were significantly reduced at 24 h post-EIMD in the PL-02 group (p < 0.01), LY-66 group (p < 0.001), and PL-02+LY-66 group (p < 0.001) compared to the placebo group (Figure 5B). Additionally, at 48 h post-EIMD, the LY-66 group demonstrated a notable reduction in IL-6 concentration compared to the placebo group (p < 0.05). At all post-EIMD time points, the PL-02 group showed a notable decrease in CK concentration (p < 0.05), while the LY-66 group displayed significant efficacy in attenuating IL-6 levels (p < 0.05).
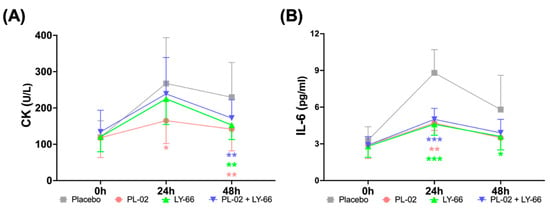
Figure 5.
The effect of supplementation with PL-02, LY-66, or PL-02+LY-66 on blood markers were evaluated at 24 and 48 h post-EIMD, focusing on (A) creatine kinase (CK) levels, and (B) interleukin-6 (IL-6) concentrations. All values are expressed as mean ± SD with a sample size of n = 22 for each group. * indicates a significant difference compared to placebo and treatment groups at same time points (* p < 0.05, ** p < 0.01, and *** p < 0.001).
3.7. Effect of PL-02, LY-66, or PL-02+LY-66 Probiotic Supplementation on Modulation of Gut Microbiota
To delve deeper into alterations in intestinal microbiota, we utilized next-generation sequencing (NGS) technology to analyze stool samples collected before and after a six-week period of probiotic intervention. Alpha diversity analysis revealed no significant differences across all groups (Figure 6A). Regarding beta diversity, while no significant alterations were observed in the placebo, LY-66, and PL-02+LY-66 groups, notable changes were detected in the PL-02 group (p < 0.05) (Figure 6B). Examination of gut microbiota composition at the phylum level revealed that Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria comprised over 90% of the relative abundance in each group (Figure 6C). At the genus level, predominant taxa such as Bacteroides, Blautia, Faecalibacterium, Prevotella, and Ruminococcus collectively represented approximately 80% of the relative abundance across all groups (Figure 6D).
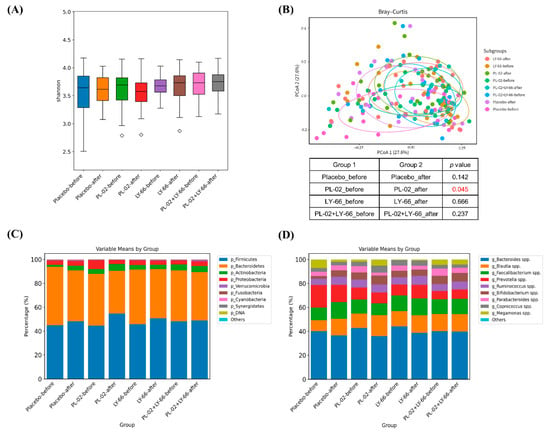
Figure 6.
The alterations in the gut microbiota composition were evaluated both before and after a six-week period of supplementation with either PL-02, LY-66, or PL-02+LY-66 intervention. This assessment included (A) alpha diversity, (B) beta diversity, (C) the composition of the 10 most abundant phyla, and (D) genera. The red number in the (B) indicates a significant difference compared to before and after intervention within each group (p < 0.05).
Further analysis focused on specific bacterial populations within the intestinal microbiota. In the PL-02 group, there was a significant increase in the relative abundance of Lactobacillus plantarum (p < 0.01) and butyrate-producing Lachnospiraceae (p < 0.05) (Figure 7A). Additionally, a notable decrease in the pathobiont Sutterella was observed (p < 0.01) (Figure 7A). In the LY-66 group, trends toward an increase in Ruminococcus and Prevotella copri were noted, with a significant rise in the relative abundance of Lactococcus lactis (p < 0.05) (Figure 7B). The PL-02+LY-66 group showed a significant enhancement in the relative abundance of Akkermansia muciniphila (p < 0.05) (Figure 7C).
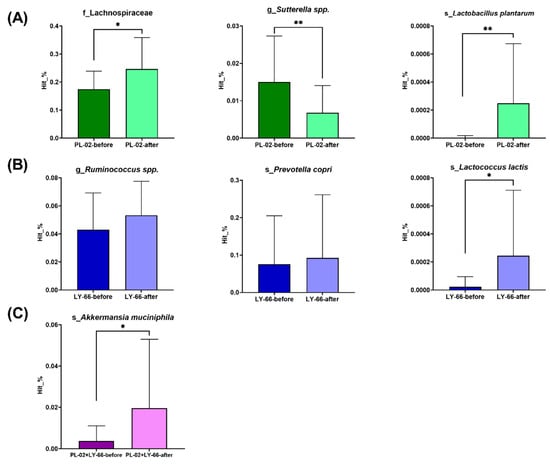
Figure 7.
Following six weeks of supplementation with (A) PL-02, (B) LY-66, or (C) PL-02+LY-66, alterations in the microbiota were observed, specifically regarding changes in bacterial families, genera, and species. * The paired t-test was utilized to evaluate the differences in abundance within each group pre- and post-intervention: * p < 0.05 and ** p < 0.01.
4. Discussion
Regular exercise is advantageous for enhancing human health by inducing increased muscle tension, enhancing posture, augmenting cardiovascular capacity, and elevating metabolic rates [26,27,28]. In recent years, the emphasis placed by health-conscious individuals on the potential applicability of probiotics in the development of sports nutrition supplements has witnessed a steady escalation. In this study, our objective was to investigate the potential benefits of probiotic supplementation in enhancing muscular performance. We enrolled a cohort of healthy non-athletic individuals and randomly assigned them into four groups, placebo, PL-02, LY-66, and PL-02+LY-66 supplementation, followed by a six-week intervention period. The results suggested that probiotic supplementation may contribute to improvement in exercise endurance and explosive strength, modulation of gut microbiota composition, reduction in circulating inflammatory biomarkers, mitigation of muscle damage, and acceleration of muscular recovery.
The public has consistently prioritized maintaining health, with recent research frequently linking changes in gut microbiota composition to various health conditions. As individuals age, a discernible reduction in the diversity of gut microbiota is often observed, typically coinciding with declines in skeletal muscle mass and function [29,30]. The importance of the gut microbiota in the growth and development of skeletal muscles has been validated in previous animal investigations. These investigations have delineated a correlation between gut microbiota and muscles, suggesting that a deficiency in gut microbiota may lead to loss in muscle mass [30,31]. For example, research conducted on rodents has demonstrated that germ-free (GF) mice, devoid of gut microbiota, exhibited muscle atrophy and reduced muscle mass compared to pathogen-free (PF) mice [32]. Similarly, GF piglets displayed a 40% decrease in lean body mass relative to conventional piglets [33]. Utilizing fecal microbiota transplantation, the introduction of gut microbiota into GF piglets not only successfully colonized the intestines but also partially restored muscle growth and development [33]. It has been hypothesized that the gut microbiota may influence the growth and function of host muscle tissue through two primary mechanisms: (1) by modulating the secretion of muscle-related hormones (such as insulin and IGF1) via activation of the gut–brain axis, and/or (2) by producing functional metabolic byproducts (such as SCFAs) that serve as signaling molecules in muscle cells [33,34,35,36,37].
This study noted an increase in beneficial bacteria such as Lactobacillus, Akkermansia muciniphila, and short-chain fatty acid (SCFA)-producing microbes following interventions with PL-02, LY-66, and PL-02+LY-66. Notably, in the PL-02 group, there was a significant increase in Lactobacillus plantarum and butyric acid-producing Lachnospiraceae, alongside a significant reduction in Sutterella. Butyrate, produced by microbial fermentation in the colon, offers numerous health benefits, including promoting bacterial equilibrium, attenuating inflammatory responses, and maintaining intestinal barrier integrity [38,39]. Moreover, SCFAs can activate AMP-activated protein kinase (AMPK) in muscle tissue by increasing the AMP/ATP ratio or through the Ffar2-leptin signaling pathway. This activation subsequently enhances protein synthesis in skeletal muscle [16,40]. In patients with inflammatory muscle diseases, a decrease in butyrate-producing bacteria has been observed [41], while butyrate synthesis by gut microbes positively influences skeletal muscle mass in healthy menopausal women [37].
The increased abundance of Sutterella within the intestinal microbiota has been identified as correlating with various human diseases, including inflammatory bowel disease (IBD) and asthma [42,43]. Asthma induces bronchial inflammation, triggering the accumulation of inflammatory cytokines (such as IL-4, IL-5, and IL-13) and free radicals [44,45]. This cascade ultimately results in the destruction of bronchial epithelial cells. Within the LY-66 group, L. lactis exhibited a significant increase, whereas Ruminococcus and P. copri demonstrated an upward trend. Ruminococcus is known for its role as a producer of SCFAs [46,47]. The increase in its relative abundance has been associated with a reduction in cardiovascular risk among obese individuals [47]. Elevating the abundance of P. copri in the human gastrointestinal tract is not only associated with a high-fiber diet but also with a reduction in visceral fat [48,49]. An animal study has shown that obese mice receiving prebiotic supplementation experienced an increase in Prevotella abundance alongside a decrease in fat mass [50]. Moreover, A. muciniphila was significantly increased in the PL-02+LY-66 group. Extensive research has consistently indicated an association between the depletion or decreased abundance of A. muciniphila and various diseases, including obesity, diabetes, hepatic steatosis, inflammation, and immune response to cancer therapy [51]. Briefly, our findings indicate that PL-02, LY-66, and PL-02+LY-66 each exhibit distinct efficacy in enhancing physical fitness. PL-02 demonstrated benefits in promoting muscle health, increasing muscle mass, and preventing conditions such as asthma. LY-66, on the other hand, primarily contributed to reducing body fat accumulation. PL-02+LY-66 effectively enhanced the colonization of A. muciniphila in the gut, indicating a comprehensive improvement in physical fitness. These probiotics all demonstrated efficacy in improving gut microbiota composition, with daily supplementation leading to a favorable shift in gut microbiota composition. Specifically, supplementation with the combination of PL-02 and LY-66 accelerated the improvement in gut microbiota composition.
The composition of the gut microbiota substantially influences systemic health. Observations from this study of improvements in body composition provide insight into the health benefits of probiotic supplementation. Following the probiotic intervention, significant enhancements were observed: the PL-02 group showed an increase in muscle mass (p < 0.05), while both the LY-66 and PL-02+LY-66 groups experienced notable reductions in body fat accumulation (p < 0.05). Furthermore, previous research has demonstrated that PL-02 supplementation significantly enhances muscle mass, strength, and endurance performance in mice (p = 0.0014) [52]. A systematic review by Prokopidis et al., covering 24 studies, indicated that probiotic supplementation could improve muscle mass relative to a placebo [53]. Additional studies have shown that probiotics, such as L. sakei CJLS03 and the MED-02 probiotic complex, effectively reduce body fat accumulation [54,55]. These findings suggest that long-term and moderate probiotic supplementation may be beneficial for increasing muscle mass and decreasing body fat. Importantly, unlike pharmaceutical interventions, probiotics are generally considered safe and devoid of adverse reactions.
VO2max is measured through gas exchange analysis and sets the physiological upper limit for aerobic performance [4,56]. Currently, an effective training approach to enhance VO2max involves the utilization of high-intensity interval training (HIIT), a method proven to rapidly improve cardiopulmonary fitness [57,58,59]. When the body can efficiently consume and utilize oxygen, physical activity becomes more achievable at a given intensity. Our findings indicate that supplementation with PL-02, LY-66, and PL-02+LY-66 leads to a significant increase of approximately 1-fold in VO2max after 6 weeks of probiotic intervention compared to baseline (p < 0.001). Animal experimentation has confirmed that supplementation with Saccharomyces boulardii can increase their VO2max and aerobic exercise capacity (p < 0.05), while a human trial has demonstrated a similar enhancement with the intake of the multi-strain probiotic SANPROBI BARRIER (Sanprobi Ltd. Szczecin, Poland) [60,61]. These investigations demonstrated that probiotics had positive effects on the participants’ body composition and cardiopulmonary health.
Various strategies have been proposed to enhance athletic performance, with probiotic supplementation emerging as a promising approach [62]. Athletic performance is influenced by a complex interplay of biological, psychological, and environmental factors [63]. Among these, muscular explosiveness is crucial for athletic success. In our study, we utilized three distinct assessment methods—CMJ, IMTP, and WAnT—to evaluate exercise performance before and after probiotic supplementation. Maximal rate of force development (RFD) and relative peak force are widely recognized as critical indicators for assessing muscle strength and power [11,64,65]. Our findings indicate that supplementation with PL-02, LY-66, and PL-02+LY-66 enhances muscular explosiveness, leading to significant improvements in athletic performance across all tests. These observations align with prior research findings, wherein male individuals participating in resistance training and soldiers enrolled in soldier self-defense courses demonstrated enhanced vertical jump performance following B. coagulans supplementation [66,67]. Similarly, athletes who received supplementation with yogurt containing Streptococcus thermophilus, L. delbrueckii subsp. bulgaricus, and L. casei CNCM-15182 showed improvements in vertical jump performance [68].
Additionally, we observed the effects of probiotic supplementation on mitigating EIMD and enhancing recovery. Research shows that muscle strength typically decreases within 24 to 48 h post-exercise and gradually returns to baseline after about 72 h [69,70]. Our results demonstrated that supplementation with PL-02, LY-66, and PL-02+LY-66 mitigated reductions in RPF and pRFD post-exercise, while also accelerating recovery of these parameters, thus supporting muscle protection and efficient recovery after physical exertion. Prior research has found that the administration of 1 billion CFU of B. coagulans GBI-30 6086 with 20 g of casein for two weeks significantly improved perceived recovery at 24 and 72 h after resistance exercise, as well as reducing muscle soreness at the 72 h mark [17]. In another investigation, the intake of Ultrabiotic 60™ and S. boulardii was observed to contribute to the alleviation of muscle soreness in elite male rugby players over a 17-week training and competition period [71].
The beneficial effects of probiotics on muscle health may stem from the influence of gut microbiota, potentially mediated through metabolic products such as SCFAs, secondary bile acids (BAs), and specific amino acids that modulate muscle function [29,62]. Protein or amino acid intake can activate the mammalian target of rapamycin (mTOR) pathway, enhancing muscle protein synthesis and supporting recovery after exercise [16]. Some studies have combined probiotics with pea protein, enhancing amino acid absorption [72,73]. Furthermore, research into the enhancement in muscle explosiveness through probiotics has also attracted attention. Over a 12-week period, the administration of L. casei Shirota (1 × 109 CFU/mouse/day) to SAMP8 mice elicited alterations in gene expression associated with mitochondrial biogenesis in muscle, notably impacting PGC1α, SIRT1, NRF1, and TFAM [74]. This modulation served to mitigate mitochondrial alterations associated with aging. The upregulation of the AMPK/SIRT1/PGC1α signaling pathway, as evidenced by previous research, has been found to enhance mitochondrial functionality and increase ATP production [75]. PGC-1α4, an isoform of PGC-1α, formed a complex with nuclear receptor PPARβ after resistance exercise training (RET), resulting in subsequent effects on the expression of key glycolytic genes [76]. This cascade facilitated glycolysis and glucose uptake within muscle tissue. Hence, we postulate that supplementation with PL-02, LY-66, and PL-02+LY-66 may facilitate muscle recovery by improving the efficiency of protein absorption and utilization. However, further research is needed to validate this hypothesis.
Biological markers such as CK and IL-6 are essential for evaluating muscle damage and inflammation. CK levels typically rise after intense physical exertion, serving as an indirect indicator of skeletal muscle damage, while IL-6 is often used to assess inflammatory responses triggered by exercise [62,77,78,79]. Probiotic supplementation has shown promise in reducing the production of inflammatory biomarkers and mitigating unnecessary immune system activation [80]. Previous studies have demonstrated that probiotics, including PL-02, LY-66, and PL-02+LY-66, can mitigate the elevation of CK levels post-exercise and decrease IL-6 concentrations [52,81]. These findings suggest that probiotic supplementation offers protective effects against muscle damage and reduces the inflammatory response following EIMD. Moreover, research on PL-02 has shown reductions in lactate, BUN, blood ammonia, and CK levels following exercise in animal models. Additionally, studies involving Lactobacillus plantarum PS128 and a high-dose combination probiotic capsule have shown decreases in exercise-induced IL-6 concentrations [82,83]. Our study’s six-week supplementation period highlighted improvements in gut microbiota composition and overall gut health. In this study, we found that probiotic supplementation significantly improved markers related to muscle damage and inflammation, specifically CK and IL-6 levels. Although there may be deviations due to individual physiological differences among a small number of subjects, all participants in this study were students from the same university, of similar age groups, with the same daily routines and living habits. Additionally, the experimental intervention included the same exercise training for all subjects, and participants were required to refrain from exercise for two days before the exercise test. Therefore, despite some individual differences within the group leading to larger standard deviations, this does not affect the overall trend of improvement observed with probiotic supplementation. Looking ahead, further research should explore the potential benefits of prolonged probiotic supplementation and conduct in-depth studies on specific changes in gut microbial ratios. These investigations are crucial for understanding the cellular and molecular mechanisms underlying probiotics’ influence on muscle health and fatigue recovery.
This study has several limitations: First, all participants were non-athletic majors from the same university. Although their living conditions were similar, reducing variability, the results may not be generalizable to a broader population. Secondly, the primary focus of this study was to compare the effects of different probiotics on muscle mass and exercise performance, necessitating the inclusion of both male and female subjects in the same group for comparison. Therefore, future trials should include a larger sample size to more thoroughly compare the effects and mechanisms in both sexes. Third, our study subjects were non-professional athletes whose physical activity levels were relatively consistent. Given that all subjects received Tabata training during the study period, we considered it feasible to disregard their individual athletic background. Furthermore, due to the lack of professional nutritionists in our research team, we were unable to conduct a comprehensive analysis of the dietary intake of our subjects. However, we recognize the importance of collecting comprehensive data on participants’ physical fitness levels, diet, and other lifestyle factors, as these variables can significantly influence our study results. In future research, we aim to incorporate these factors to better understand their impact and increase the robustness of our findings. Fourth, currently, there are no studies that clearly delineate the effectiveness of the probiotic supplementation period in improving sports performance. Despite this, we referenced the past literature and provided probiotic supplementation for over six weeks. However, further research is needed to compare whether longer-term interventions yield greater benefits. Fifth, additionally, given the extensive testing in this experiment, we limited data collection to 24 and 48 h after EIMD to consider the burden on participants. However, more significant changes might be observed over extended periods, including both the elevation and recovery phases. For instance, CK levels peak at 24–48 h, and numerous studies have documented recovery conditions beyond 72 and 96 h [84]. Therefore, more in-depth investigations into key parameters over longer durations are necessary for future research.
5. Conclusions
Our study demonstrates that six weeks of a continuous combination of exercise training and supplementation with PL-02, LY-66, and PL-02+LY-66 significantly enhances muscle strength, explosive power, and endurance performance. Probiotic supplementation was also found to effectively attenuate the decline in muscle strength and explosive power following EIMD, reduce the elevation of inflammatory markers in the blood, and accelerate the recovery process. Notably, the intake of PL-02 significantly increased muscle mass, while the consumption of LY-66 and the combined PL-02+LY-66 formulation led to a substantial reduction in body fat percentage. Additionally, supplementation with these probiotics resulted in the modulation of gut microbiota, increasing the prevalence of beneficial bacteria. These findings highlight that the combination of exercise training and supplementation with probiotics not only enhances exercise performance but also improves the management of exercise-induced muscle damage and recovery. Future research should delve deeper into the mechanisms by which PL-02, LY-66, and PL-02+LY-66 aid in muscle damage recovery, to further elucidate the role of probiotics in enhancing athletic performance. This will expand our understanding of how probiotics can be effectively integrated into sports nutrition strategies.
Author Contributions
M.-C.L., Y.-J.H., Y.-W.K., Y.-C.H., H.-H.H. and C.-C.H. designed the experiments; M.-C.L., Y.-J.H., M.-T.C., J.-H.L., Y.-Y.H. and C.-M.L. performed the laboratory experiments; M.-T.C., Y.-W.K., S.-Y.T., K.-C.H., Y.-J.H. and C.-C.H. analyzed the data and interpreted the results; M.-C.L., M.-T.C., Y.-W.K., Y.-J.H. and C.-C.H. prepared the figures and wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the University–Industry Cooperation Fund, National Taiwan Sport University, Taoyuan, Taiwan (NTSU No. 1101090).
Institutional Review Board Statement
The study was performed in accordance with the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Landseed International Hospital (Taoyuan, Taiwan; LSHIRB No. 21-042-A2). Approval Date: 28 January 2022.
Informed Consent Statement
Before the experiment, the researchers explained the experimental process in detail and obtained informed consent from all subjects participating in the study, and the experiment started after the subjects signed the consent form.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
The authors are grateful to the graduate students at the Sport Nutrition Laboratory, National Taiwan Sport University, for their technical assistance in conducting the analysis experiments.
Conflicts of Interest
Authors Yi-Wei Kuo, Jia-Hung Lin, Yu-Chieh Hsu, Yen-Yu Huang, Ching-Min Li, Shin-Yu Tsai, Ko-Chiang Hsia and Hsieh-Hsun Ho were employed by the company Glac Biotech Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Penedo, F.J.; Dahn, J.R. Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Curr. Opin. Psychiatry 2005, 18, 189–193. [Google Scholar] [CrossRef]
- Paulsen, G.; Ramer Mikkelsen, U.; Raastad, T.; Peake, J.M. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar]
- Sheridan, S.; McCarren, A.; Gray, C.; Murphy, R.P.; Harrison, M.; Wong, S.H.; Moyna, N.M. Maximal oxygen consumption and oxygen uptake efficiency in adolescent males. J. Exerc. Sci. Fit. 2021, 19, 75–80. [Google Scholar] [CrossRef]
- Denham, J.; Scott-Hamilton, J.; Hagstrom, A.D.; Gray, A.J. Cycling power outputs predict functional threshold power and maximum oxygen uptake. J. Strength. Cond. Res. 2020, 34, 3489–3497. [Google Scholar] [CrossRef]
- Daly, R.M. Independent and combined effects of exercise and vitamin D on muscle morphology, function and falls in the elderly. Nutrients 2010, 2, 1005–1017. [Google Scholar] [CrossRef]
- Kell, R.T.; Bell, G.; Quinney, A. Musculoskeletal fitness, health outcomes and quality of life. Sports Med. 2001, 31, 863–873. [Google Scholar] [CrossRef]
- Gremeaux, V.; Gayda, M.; Lepers, R.; Sosner, P.; Juneau, M.; Nigam, A. Exercise and longevity. Maturitas 2012, 73, 312–317. [Google Scholar] [CrossRef]
- Ekeland, E.; Heian, F.; Hagen, K.B.; Abbott, J.; Nordheim, L. Exercise to improve self-esteem in children and young people. Cochrane Database Syst. Rev. 2004, CD003683. [Google Scholar] [CrossRef]
- Larun, L.; Nordheim, L.V.; Ekeland, E.; Hagen, K.B.; Heian, F. Exercise in prevention and treatment of anxiety and depression among children and young people. Cochrane Database Syst. Rev. 2006, CD004691. [Google Scholar] [CrossRef]
- Fatouros, I.G.; Jamurtas, A.Z. Insights into the molecular etiology of exercise-induced inflammation: Opportunities for optimizing performance. J. Inflamm. Res. 2016, 9, 175–186. [Google Scholar] [CrossRef]
- Lee, M.-C.; Ho, C.-S.; Hsu, Y.-J.; Huang, C.-C. Live and heat-killed probiotic Lactobacillus paracasei PS23 accelerated the improvement and recovery of strength and damage biomarkers after exercise-induced muscle damage. Nutrients 2022, 14, 4563. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Fernandez-Lazaro, C.I.; Mielgo-Ayuso, J.; Navascués, L.J.; Córdova Martínez, A.; Seco-Calvo, J. The role of selenium mineral trace element in exercise: Antioxidant defense system, muscle performance, hormone response, and athletic performance. A systematic review. Nutrients 2020, 12, 1790. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Sanchez-Serrano, N.; Rabail, R.; Aadil, R.M.; Mielgo-Ayuso, J.; Radesca Fabiano, K.; Garrosa, E. Is Probiotics Supplementation an Appropriate Strategy to Modulate Inflammation in Physically Active Healthy Adults or Athletes? A Systematic Review. Appl. Sci. 2023, 13, 3448. [Google Scholar] [CrossRef]
- Ticinesi, A.; Lauretani, F.; Milani, C.; Nouvenne, A.; Tana, C.; Del Rio, D.; Maggio, M.; Ventura, M.; Meschi, T. Aging gut microbiota at the cross-road between nutrition, physical frailty, and sarcopenia: Is there a gut–muscle axis? Nutrients 2017, 9, 1303. [Google Scholar] [CrossRef]
- Scheiman, J.; Luber, J.M.; Chavkin, T.A.; MacDonald, T.; Tung, A.; Pham, L.-D.; Wibowo, M.C.; Wurth, R.C.; Punthambaker, S.; Tierney, B.T. Meta-omics analysis of elite athletes identifies a performance-enhancing microbe that functions via lactate metabolism. Nat. Med. 2019, 25, 1104–1109. [Google Scholar] [CrossRef]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J.J. Gut-muscle axis exists and may affect skeletal muscle adaptation to training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef]
- Jager, R.; Shields, K.A.; Lowery, R.P.; De Souza, E.O.; Partl, J.M.; Hollmer, C.; Purpura, M.; Wilson, J.M. Probiotic Bacillus coagulans GBI-30, 6086 reduces exercise-induced muscle damage and increases recovery. PeerJ 2016, 4, e2276. [Google Scholar] [CrossRef]
- Lin, C.-L.; Hsu, Y.-J.; Ho, H.-H.; Chang, Y.-C.; Kuo, Y.-W.; Yeh, Y.-T.; Tsai, S.-Y.; Chen, C.-W.; Chen, J.-F.; Huang, C.-C. Bifidobacterium longum subsp. longum OLP-01 supplementation during endurance running training improves exercise performance in middle-and long-distance runners: A double-blind controlled trial. Nutrients 2020, 12, 1972. [Google Scholar] [CrossRef]
- Huang, W.C.; Hsu, Y.J.; Huang, C.C.; Liu, H.C.; Lee, M.C. Exercise Training Combined with Bifidobacterium longum OLP-01 Supplementation Improves Exercise Physiological Adaption and Performance. Nutrients 2020, 12, 1145. [Google Scholar] [CrossRef]
- Lin, W.Y.; Kuo, Y.W.; Lin, J.H.; Lin, C.H.; Chen, J.F.; Tsai, S.Y.; Lee, M.C.; Hsu, Y.J.; Huang, C.C.; Tsou, Y.A.; et al. Probiotic Strains Isolated from an Olympic Woman’s Weightlifting Gold Medalist Increase Weight Loss and Exercise Performance in a Mouse Model. Nutrients 2022, 14, 1270. [Google Scholar] [CrossRef]
- Dos’Santos, T.; Thomas, C.; Comfort, P.; McMahon, J.J.; Jones, P.A.; Oakley, N.P.; Young, A.L. Between-Session Reliability of Isometric Midthigh Pull Kinetics and Maximal Power Clean Performance in Male Youth Soccer Players. J. Strength. Cond. Res. 2018, 32, 3364–3372. [Google Scholar] [CrossRef]
- Huang, C.C.; Lee, M.C.; Ho, C.S.; Hsu, Y.J.; Ho, C.C.; Kan, N.W. Protective and Recovery Effects of Resveratrol Supplementation on Exercise Performance and Muscle Damage following Acute Plyometric Exercise. Nutrients 2021, 13, 3217. [Google Scholar] [CrossRef]
- Bruce, R.A.; Kusumi, F.; Hosmer, D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am. Heart J. 1973, 85, 546–562. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; DeSantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Kramer, A. An Overview of the Beneficial Effects of Exercise on Health and Performance. Adv. Exp. Med. Biol. 2020, 1228, 3–22. [Google Scholar]
- Moreira, J.B.; Wohlwend, M.; Wisløff, U. Exercise and cardiac health: Physiological and molecular insights. Nat. Metab. 2020, 2, 829–839. [Google Scholar] [CrossRef]
- Vechetti, I.J., Jr.; Valentino, T.; Mobley, C.B.; McCarthy, J.J. The role of extracellular vesicles in skeletal muscle and systematic adaptation to exercise. J. Physiol. 2021, 599, 845–861. [Google Scholar] [CrossRef]
- Giron, M.; Thomas, M.; Dardevet, D.; Chassard, C.; Savary-Auzeloux, I. Gut microbes and muscle function: Can probiotics make our muscles stronger? J. Cachexia Sarcopenia Muscle 2022, 13, 1460–1476. [Google Scholar] [CrossRef]
- Chew, W.; Lim, Y.P.; Lim, W.S.; Chambers, E.S.; Frost, G.; Wong, S.H.; Ali, Y. Gut-muscle crosstalk. A perspective on influence of microbes on muscle function. Front. Med. 2023, 9, 1065365. [Google Scholar] [CrossRef]
- Li, G.; Jin, B.; Fan, Z. Mechanisms involved in gut microbiota regulation of skeletal muscle. Oxidative Med. Cell. Longev. 2022, 2022, 2151191. [Google Scholar] [CrossRef]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef]
- Qi, R.; Sun, J.; Qiu, X.; Zhang, Y.; Wang, J.; Wang, Q.; Huang, J.; Ge, L.; Liu, Z. The intestinal microbiota contributes to the growth and physiological state of muscle tissue in piglets. Sci. Rep. 2021, 11, 11237. [Google Scholar] [CrossRef]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Bhardwaj, G.; Penniman, C.M.; Jena, J.; Beltran, P.A.S.; Foster, C.; Poro, K.; Junck, T.L.; Hinton, A.O.; Souvenir, R.; Fuqua, J.D. Insulin and IGF-1 receptors regulate complex I–dependent mitochondrial bioenergetics and supercomplexes via FoxOs in muscle. J. Clin. Investig. 2021, 131, e146415. [Google Scholar] [CrossRef]
- Lv, W.Q.; Lin, X.; Shen, H.; Liu, H.M.; Qiu, X.; Li, B.Y.; Shen, W.D.; Ge, C.L.; Lv, F.Y.; Shen, J. Human gut microbiome impacts skeletal muscle mass via gut microbial synthesis of the short-chain fatty acid butyrate among healthy menopausal women. J. Cachexia Sarcopenia Muscle 2021, 12, 1860–1870. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, S.; Wu, J.; Ye, T.; Wang, S.; Wang, P.; Xing, D. Butyrate-producing bacteria and the gut-heart axis in atherosclerosis. Clin. Chim. Acta 2020, 507, 236–241. [Google Scholar] [CrossRef]
- Pant, K.; Venugopal, S.K.; Pisarello, M.J.L.; Gradilone, S.A. The role of gut microbiome-derived short chain fatty acid butyrate in hepatobiliary diseases. Am. J. Pathol. 2023, 193, 1455–1467. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Liu, H.; Xi, Q.; Tan, S.; Qu, Y.; Meng, Q.; Zhang, Y.; Cheng, Y.; Wu, G. The metabolite butyrate produced by gut microbiota inhibits cachexia-associated skeletal muscle atrophy by regulating intestinal barrier function and macrophage polarization. Int. Immunopharmacol. 2023, 124, 111001. [Google Scholar] [CrossRef] [PubMed]
- Derqaoui, S.; Oukessou, M.; Attrassi, K.; Elftouhy, F.Z.; Nassik, S. Detection of Sutterella spp. in Broiler Liver and Breast. Front. Vet. Sci. 2022, 9, 859902. [Google Scholar] [CrossRef] [PubMed]
- Salameh, M.; Burney, Z.; Mhaimeed, N.; Laswi, I.; Yousri, N.A.; Bendriss, G.; Zakaria, D. The role of gut microbiota in atopic asthma and allergy, implications in the understanding of disease pathogenesis. Scand. J. Immunol. 2020, 91, e12855. [Google Scholar] [CrossRef]
- Mabalirajan, U.; Ahmad, T.; Rehman, R.; Leishangthem, G.D.; Dinda, A.K.; Agrawal, A.; Ghosh, B.; Sharma, S.K. Baicalein reduces airway injury in allergen and IL-13 induced airway inflammation. PLoS ONE 2013, 8, e62916. [Google Scholar] [CrossRef]
- Ma, J.-X.; Xiao, X.; Zhou, K.-F.; Huang, G.; Ao, B.; Zhang, Y.; Gao, W.-J.; Lei, T.; Yang, L.; Fan, X.-C. Herb pair of Ephedrae Herba-Armeniacae Semen Amarum alleviates airway injury in asthmatic rats. J. Ethnopharmacol. 2021, 269, 113745. [Google Scholar] [CrossRef]
- Chai, L.; Luo, Q.; Cai, K.; Wang, K.; Xu, B. Reduced fecal short-chain fatty acids levels and the relationship with gut microbiota in IgA nephropathy. BMC Nephrol. 2021, 22, 209. [Google Scholar] [CrossRef]
- Lakshmanan, A.P.; Al Zaidan, S.; Bangarusamy, D.K.; Al-Shamari, S.; Elhag, W.; Terranegra, A. Increased relative abundance of ruminoccocus is associated with reduced cardiovascular risk in an obese population. Front. Nutr. 2022, 9, 849005. [Google Scholar] [CrossRef]
- Asnicar, F.; Berry, S.E.; Valdes, A.M.; Nguyen, L.H.; Piccinno, G.; Drew, D.A.; Leeming, E.; Gibson, R.; Le Roy, C.; Khatib, H.A. Microbiome connections with host metabolism and habitual diet from 1098 deeply phenotyped individuals. Nat. Med. 2021, 27, 321–332. [Google Scholar] [CrossRef]
- Yeoh, Y.K.; Sun, Y.; Ip, L.Y.T.; Wang, L.; Chan, F.K.; Miao, Y.; Ng, S.C. Prevotella species in the human gut is primarily comprised of Prevotella copri, Prevotella stercorea and related lineages. Sci. Rep. 2022, 12, 9055. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; de Vos, W.M. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef]
- Cani, P.D.; Depommier, C.; Derrien, M.; Everard, A.; de Vos, W.M. Akkermansia muciniphila: Paradigm for next-generation beneficial microorganisms. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 625–637. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.-C.; Hsu, Y.-J.; Ho, H.H.; Kuo, Y.W.; Lin, W.-Y.; Tsai, S.-Y.; Chen, W.-L.; Lin, C.-L.; Huang, C.-C. Effectiveness of human-origin Lactobacillus plantarum PL-02 in improving muscle mass, exercise performance and anti-fatigue. Sci. Rep. 2021, 11, 19469. [Google Scholar] [CrossRef]
- Prokopidis, K.; Giannos, P.; Kirwan, R.; Ispoglou, T.; Galli, F.; Witard, O.C.; Triantafyllidis, K.K.; Kechagias, K.S.; Morwani-Mangnani, J.; Ticinesi, A. Impact of probiotics on muscle mass, muscle strength and lean mass: A systematic review and meta-analysis of randomized controlled trials. J. Cachexia Sarcopenia Muscle 2023, 14, 30–44. [Google Scholar] [CrossRef]
- Lim, S.; Moon, J.H.; Shin, C.M.; Jeong, D.; Kim, B. Effect of Lactobacillus sakei, a probiotic derived from kimchi, on body fat in Koreans with obesity: A randomized controlled study. Endocrinol. Metab. 2020, 35, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.G.; Yang, Y.J.; Yoon, Y.S.; Lee, E.S.; Lee, J.H.; Jeong, Y.; Kang, C.H. Effect of MED-02 Containing Two Probiotic Strains, Limosilactobacillus fermentum MG4231 and MG4244, on Body Fat Reduction in Overweight or Obese Subjects: A Randomized, Multicenter, Double-Blind, Placebo-Controlled Study. Nutrients 2022, 14, 3583. [Google Scholar] [CrossRef] [PubMed]
- Noakes, T.D. Testing for maximum oxygen consumption has produced a brainless model of human exercise performance. Br. J. Sports Med. 2008, 42, 551–555. [Google Scholar]
- Astorino, T.A.; Allen, R.P.; Roberson, D.W.; Jurancich, M. Effect of high-intensity interval training on cardiovascular function, VO2max, and muscular force. J. Strength Cond. Res. 2012, 26, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Gist, N.H.; Freese, E.C.; Cureton, K.J. Comparison of responses to two high-intensity intermittent exercise protocols. J. Strength Cond. Res. 2014, 28, 3033–3040. [Google Scholar] [CrossRef]
- Menz, V.; Marterer, N.; Amin, S.B.; Faulhaber, M.; Hansen, A.B.; Lawley, J.S. Functional vs. Running low-volume high-intensity interval training: Effects on vo2max and muscular endurance. J. Sports Sci. Med. 2019, 18, 497. [Google Scholar]
- Soares, A.D.N.; Wanner, S.P.; Morais, E.S.S.; Hudson, A.S.R.; Martins, F.S.; Cardoso, V.N. Supplementation with Saccharomyces boulardii increases the maximal oxygen consumption and maximal aerobic speed attained by rats subjected to an incremental-speed exercise. Nutrients 2019, 11, 2352. [Google Scholar] [CrossRef]
- Smarkusz-Zarzecka, J.; Ostrowska, L.; Leszczyńska, J.; Orywal, K.; Cwalina, U.; Pogodziński, D. Analysis of the impact of a multi-strain probiotic on body composition and cardiorespiratory fitness in long-distance runners. Nutrients 2020, 12, 3758. [Google Scholar] [CrossRef]
- Marttinen, M.; Ala-Jaakkola, R.; Laitila, A.; Lehtinen, M.J. Gut microbiota, probiotics and physical performance in athletes and physically active individuals. Nutrients 2020, 12, 2936. [Google Scholar] [CrossRef]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Hermans, E.; Saeterbakken, A.H.; Vereide, V.; Nord, I.S.; Stien, N.; Andersen, V. The Effects of 10 Weeks Hangboard Training on Climbing Specific Maximal Strength, Explosive Strength, and Finger Endurance. Front. Sports Act. Living 2022, 4, 888158. [Google Scholar] [CrossRef] [PubMed]
- Stien, N.; Saeterbakken, A.H.; Hermans, E.; Vereide, V.A.; Olsen, E.; Andersen, V. Comparison of climbing-specific strength and endurance between lead and boulder climbers. PLoS ONE 2019, 14, e0222529. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Hoffman, M.W.; Zelicha, H.; Gepner, Y.; Willoughby, D.S.; Feinstein, U.; Ostfeld, I. The effect of 2 weeks of inactivated probiotic Bacillus coagulans on endocrine, inflammatory, and performance responses during self-defense training in soldiers. J. Strength Cond. Res. 2019, 33, 2330–2337. [Google Scholar] [CrossRef] [PubMed]
- Tarik, M.; Ramakrishnan, L.; Bhatia, N.; Goswami, R.; Kandasamy, D.; Roy, A.; Chandran, D.S.; Singh, A.; Upadhyay, A.D.; Kalaivani, M. The effect of Bacillus coagulans Unique IS-2 supplementation on plasma amino acid levels and muscle strength in resistance trained males consuming whey protein: A double-blind, placebo-controlled study. Eur. J. Nutr. 2022, 61, 2673–2685. [Google Scholar] [CrossRef]
- Gomaa, M.A.; Allam, M.G.; Haridi, A.A.; Eliwa, A.-E.M.; Darwish, A.M. High-protein concentrated pro-yogurt (Pro-WPI) enriched with whey protein isolate improved athletic anemia and performance in a placebo-controlled study. Front. Nutr. 2022, 8, 788446. [Google Scholar] [CrossRef]
- Naderi, A.; Rezvani, M.H.; Degens, H. Foam rolling and muscle and joint proprioception after exercise-induced muscle damage. J. Athl. Train. 2020, 55, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Fakhro, M.A.; AlAmeen, F.; Fayad, R. Comparison of total cold-water immersion’s effects to ice massage on recovery from exercise-induced muscle damage. J. Exp. Orthop. 2022, 9, 59. [Google Scholar] [CrossRef]
- Harnett, J.E.; Pyne, D.B.; McKune, A.J.; Penm, J.; Pumpa, K.L. Probiotic supplementation elicits favourable changes in muscle soreness and sleep quality in rugby players. J. Sci. Med. Sport 2021, 24, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Zaragoza, J.; Purpura, M.; Iametti, S.; Marengo, M.; Tinsley, G.M.; Anzalone, A.J.; Oliver, J.M.; Fiore, W.; Biffi, A. Probiotic administration increases amino acid absorption from plant protein: A placebo-controlled, randomized, double-blind, multicenter, crossover study. Probiotics Antimicrob. Proteins 2020, 12, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Walden, K.E.; Hagele, A.M.; Orr, L.S.; Gross, K.N.; Krieger, J.M.; Jäger, R.; Kerksick, C.M. Probiotic BC30 Improves Amino Acid Absorption from Plant Protein Concentrate in Older Women. Probiotics Antimicrob. Proteins 2022, 16, 125–137. [Google Scholar] [CrossRef]
- Chen, L.H.; Chang, S.S.; Chang, H.Y.; Wu, C.H.; Pan, C.H.; Chang, C.C.; Chan, C.H.; Huang, H.Y. Probiotic supplementation attenuates age-related sarcopenia via the gut–muscle axis in SAMP8 mice. J. Cachexia Sarcopenia Muscle 2022, 13, 515–531. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Wei, Z.; Wang, X.; Gao, Y.; Wang, D.; Liu, X.; Bai, C.; Su, G.; Yang, L.; Li, G. Myostatin Knockout Affects Mitochondrial Function by Inhibiting the AMPK/SIRT1/PGC1α Pathway in Skeletal Muscle. Int. J. Mol. Sci. 2022, 23, 13703. [Google Scholar] [CrossRef]
- Koh, J.-H.; Pataky, M.W.; Dasari, S.; Klaus, K.A.; Vuckovic, I.; Ruegsegger, G.N.; Kumar, A.P.; Robinson, M.M.; Nair, K.S. Enhancement of anaerobic glycolysis–a role of PGC-1α4 in resistance exercise. Nat. Commun. 2022, 13, 2324. [Google Scholar] [CrossRef]
- Fischer, C.P. Interleukin-6 in acute exercise and training: What is the biological relevance. Exerc. Immunol. Rev. 2006, 12, 41. [Google Scholar]
- Isaacs, A.W.; Macaluso, F.; Smith, C.; Myburgh, K.H. C-reactive protein is elevated only in high creatine kinase responders to muscle damaging exercise. Front. Physiol. 2019, 10, 86. [Google Scholar] [CrossRef]
- Walzik, D.; Joisten, N.; Zacher, J.; Zimmer, P. Transferring clinically established immune inflammation markers into exercise physiology: Focus on neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index. Eur. J. Appl. Physiol. 2021, 121, 1803–1814. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: A door to the body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Yeh, W.-L.; Hsu, Y.-J.; Ho, C.-S.; Ho, H.-H.; Kuo, Y.-W.; Tsai, S.-Y.; Huang, C.-C.; Lee, M.-C. Lactobacillus plantarum Pl-02 supplementation combined with resistance training improved muscle mass, force, and exercise performance in mice. Front. Nutr. 2022, 9, 896503. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-C.; Wei, C.-C.; Huang, C.-C.; Chen, W.-L.; Huang, H.-Y. The beneficial effects of Lactobacillus plantarum PS128 on high-intensity, exercise-induced oxidative stress, inflammation, and performance in triathletes. Nutrients 2019, 11, 353. [Google Scholar] [CrossRef] [PubMed]
- Pugh, J.N.; Wagenmakers, A.J.; Doran, D.A.; Fleming, S.C.; Fielding, B.A.; Morton, J.P.; Close, G.L. Probiotic supplementation increases carbohydrate metabolism in trained male cyclists: A randomized, double-blind, placebo-controlled crossover trial. Am. J. Physiol. Endocrinol. Metab. 2020, 318, E504–E513. [Google Scholar] [CrossRef] [PubMed]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-kinase-and exercise-related muscle damage implications for muscle performance and recovery. J. Nutr. Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).