Bioactive Compounds in Citrus reticulata Peel Are Potential Candidates for Alleviating Physical Fatigue through a Triad Approach of Network Pharmacology, Molecular Docking, and Molecular Dynamics Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Establishing Database for Bioactive Compounds of Citrus reticulata Peel
2.2. Identification of Bioactive Compounds of CR-P and Related Targets
2.3. Identification of Physical Fatigue-Related Targets
2.4. Key Overlapping Targets of CR-P and Physical Fatigue
2.5. Network Construction of Protein–Protein Interaction (PPI)
2.6. GO and KEGG Pathway Enrichment Analysis
2.7. Network Construction of Target-Pathway Interaction
2.8. Molecular Docking
2.9. Molecular Dynamics Simulation
3. Results
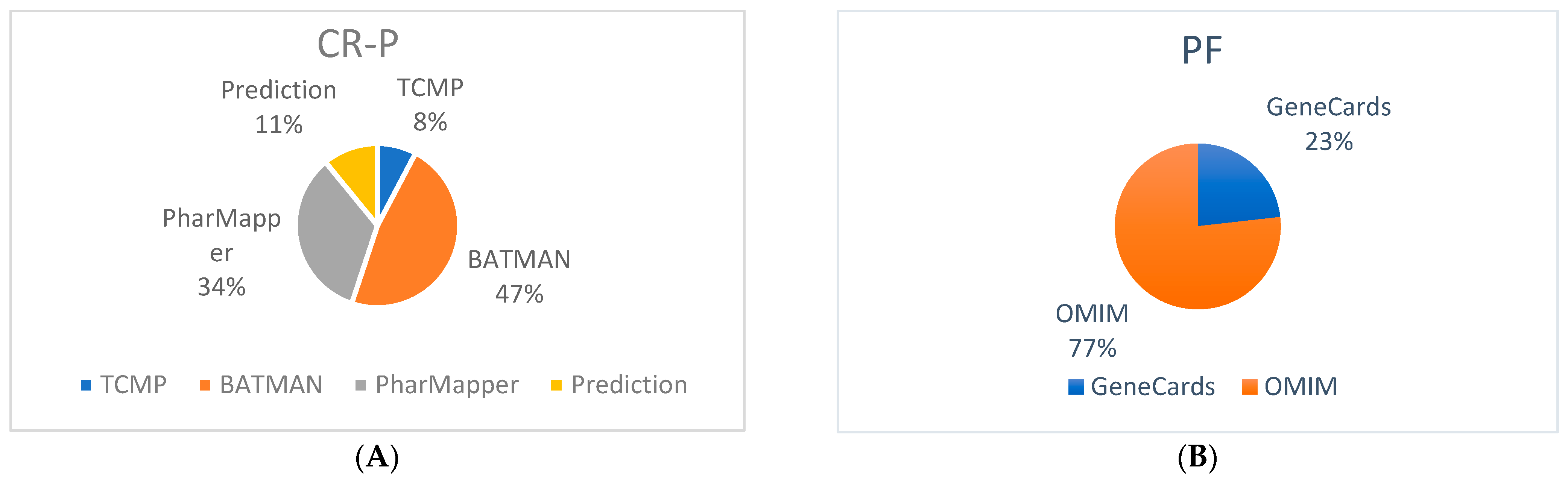
3.1. Bioactive Compounds of CR-P and Related Targets
3.2. Potential Targets of Physical Fatigue
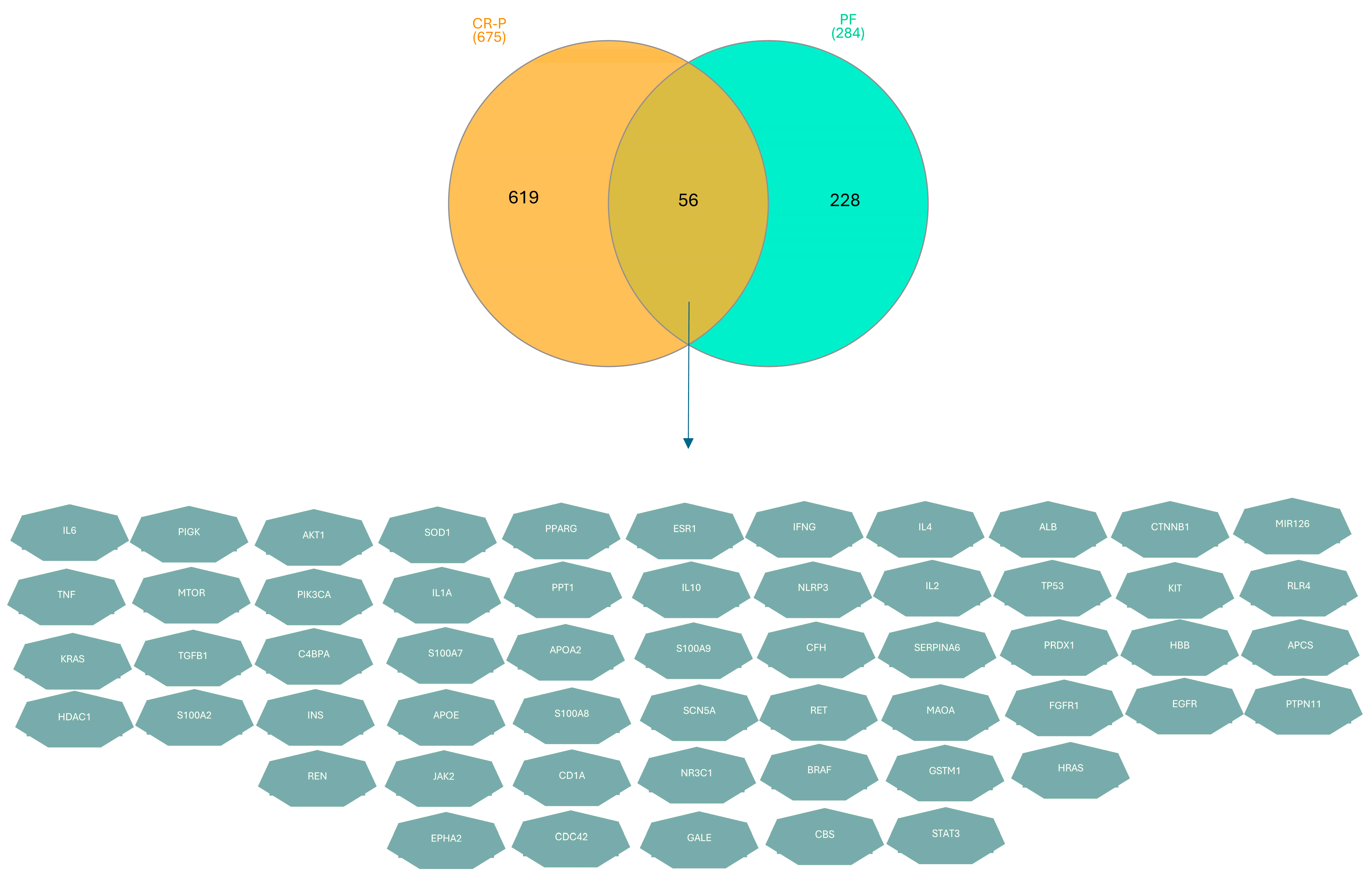
3.3. Key Overlapping Targets of CR-P and Physical Fatigue
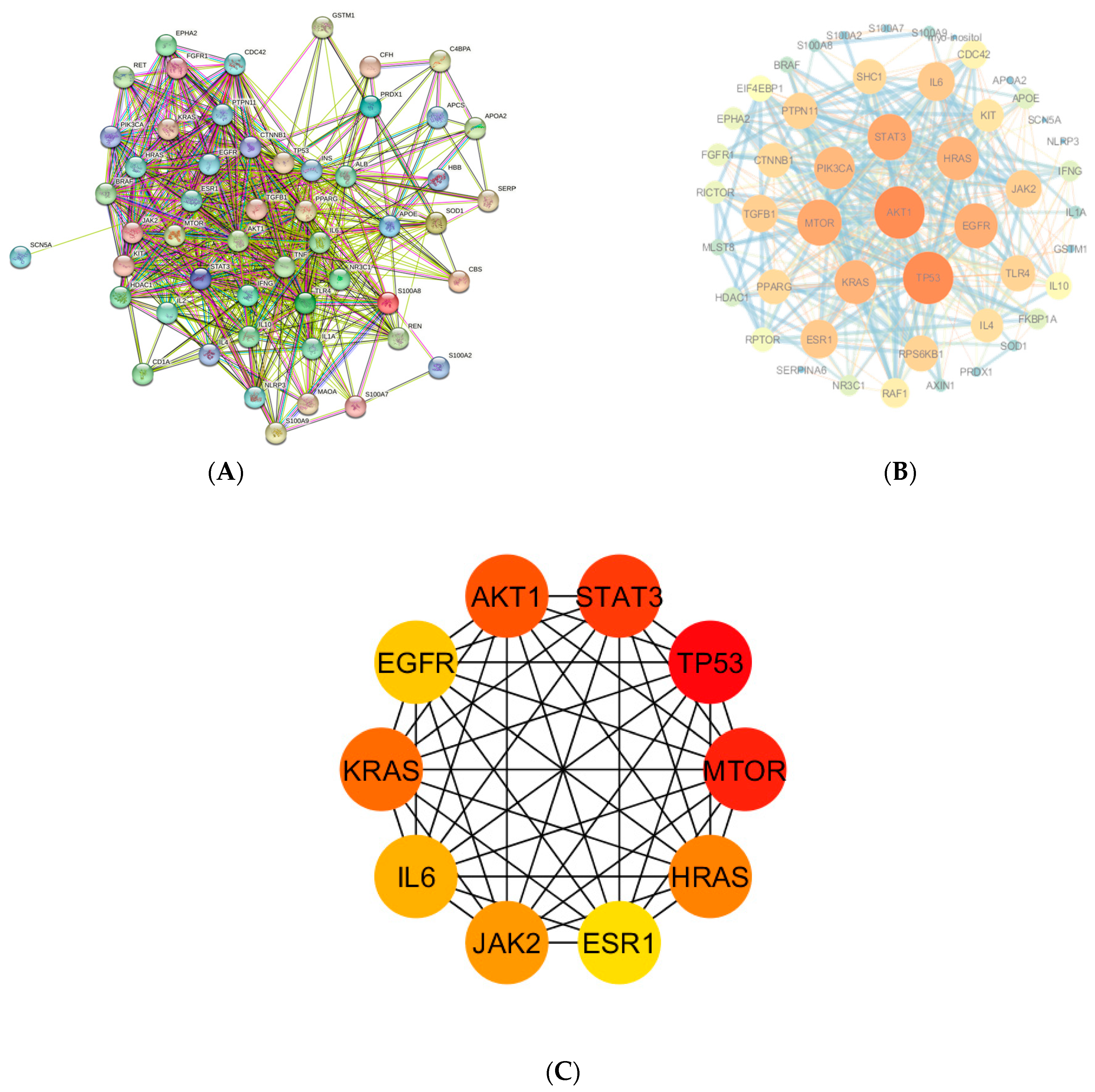
3.4. Key Overlapping Targets and PPI Network Analysis
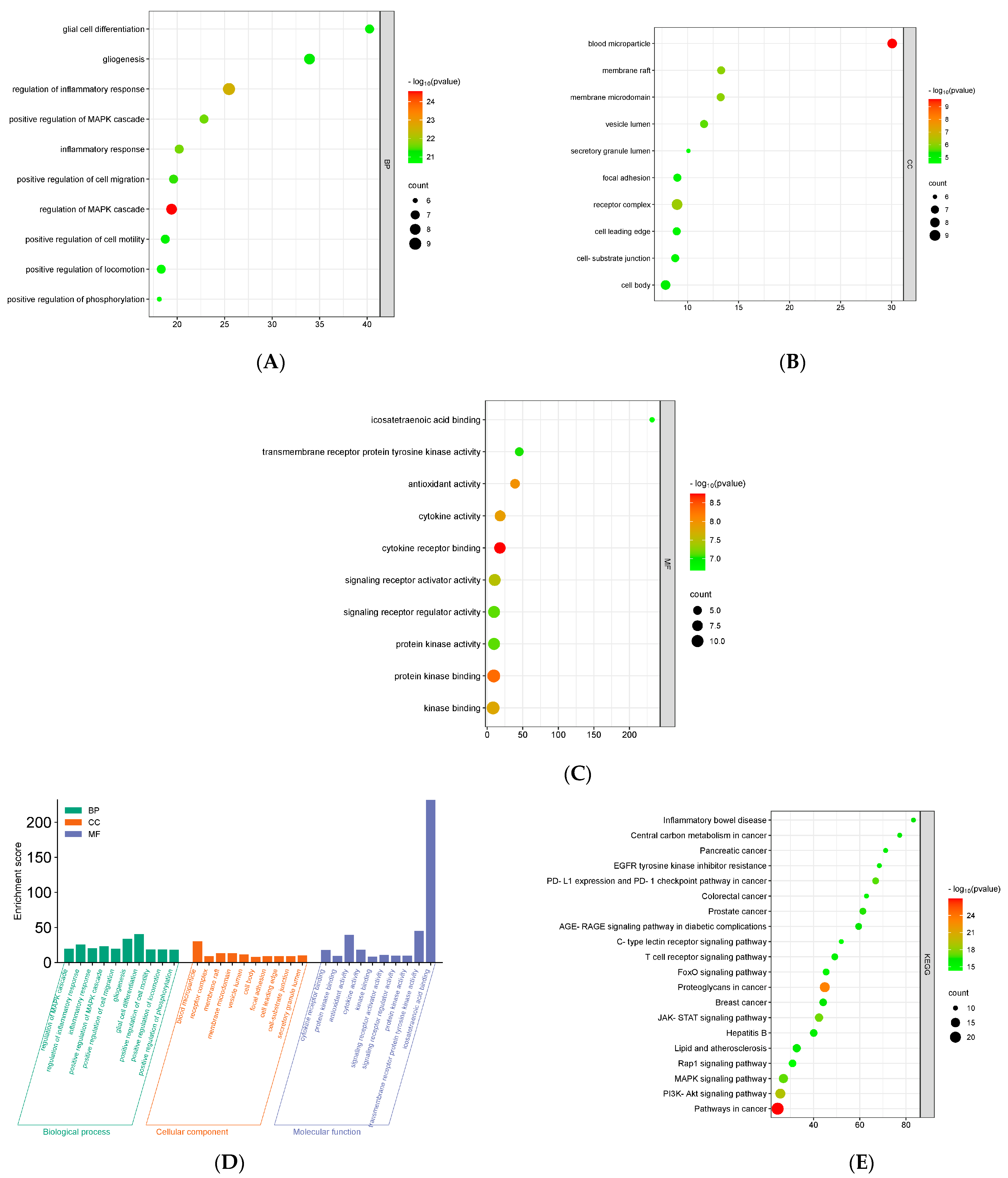
GO and KEGG Analysis of Key Overlapping Targets
3.5. Molecular Docking
3.6. Molecular Dynamics Modeling
3.7. MM-GBSA Binding Free Energy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ullah, H.; Khan, A.; Riccioni, C.; Di Minno, A.; Tantipongpiradet, A.; Buccato, D.G.; De Lellis, L.F.; Khan, H.; Xiao, J.; Daglia, M. Polyphenols as possible alternative agents in chronic fatigue: A review. Phytochem. Rev. 2023, 22, 1637–1661. [Google Scholar] [CrossRef]
- Yang, Z.; Sunil, C.; Jayachandran, M.; Zheng, X.; Cui, K.; Su, Y.; Xu, B. Anti-fatigue effect of aqueous extract of Hechong (Tylorrhynchus heterochaetus) via AMPK linked pathway. Food. Chem. Toxicol. 2020, 135, 111043. [Google Scholar] [CrossRef] [PubMed]
- Tornero-Aguilera, J.F.; Jimenez-Morcillo, J.; Rubio-Zarapuz, A.; Clemente-Suárez, V.J. Central and peripheral fatigue in physical exercise explained: A narrative review. Int. J. Environ. Res. Public Health 2022, 19, 3909. [Google Scholar] [CrossRef] [PubMed]
- Kunasegaran, K.; Ismail, A.M.H.; Ramasamy, S.; Gnanou, J.V.; Caszo, B.A.; Chen, P.L. Understanding mental fatigue and its detection: A comparative analysis of assessments and tools. PeerJ 2023, 11, e15744. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Yin, H.; Du, B.; Niu, K.; Ren, X.; Yang, Y. Anti-fatigue activity of purified flavonoids prepared from chestnut (Castanea mollissima) flower. J. Funct. Foods 2021, 79, 104365. [Google Scholar] [CrossRef]
- Wan, J.J.; Qin, Z.; Wang, P.Y.; Sun, Y.; Liu, X. Muscle fatigue: General understanding and treatment. Exp. Mol. Med. 2017, 49, e384. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chen, X.; Yu, Z.; Du, R.; Chen, B.; Wang, Y.; Cai, X.; Xu, J.; Chen, J.; Duan, H.; et al. Progress of research on the role of active ingredients of Citri Reticulatae Pericarpium in liver injury. Phytomedicine 2023, 115, 154836. [Google Scholar] [CrossRef] [PubMed]
- Rahib, A.; Karhib, M.M.; Nasr, H.M.; El-Sayed, R.A.; Abdel-Daim, M.M.; Jebur, A.B.; El-Demerdash, F.M. Citrus reticulata peel extract mitigates oxidative stress and liver injury induced by abamectin in rats. Tissue Cell 2024, 87, 102321. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Wang, J.; Ye, W.; Lu, J.; Li, C.; Zhang, D.; Ye, W.; Xu, S.; Chen, C.; Liu, P.; et al. Citri Reticulatae Pericarpium (Chenpi): A multi-efficacy pericarp in treating cardiovascular diseases. Biomed. Pharmacother. 2022, 154, 113626. [Google Scholar] [CrossRef]
- Lan, H.C.; Liang, Y.; Hsu, H.C.; Shu, J.H.; Su, C.W.; Hung, H.H.; Hou, M.C.; Lin, H.C.; Lee, S.D.; Wang, Y.J. Citrus reticulata peel improves patient tolerance of low-volume polyethylene glycol for colonoscopy preparation. J. Chin. Med. Assoc. 2012, 75, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.; Yu, Z.; Wang, J.; Deng, Q.; Deng, J.; Sun, Z.; Ye, Q.; Ye, Z.; Qin, K.; Peng, X. Inhalation of Citrus reticulata essential oil alleviates airway inflammation and emphysema in COPD rats through regulation of macrophages. J. Ethnopharmacol. 2024, 320, 117407. [Google Scholar] [CrossRef] [PubMed]
- Mamy, D.; Huang, Y.; Akpabli-Tsigbe, N.D.K.; Battino, M.; Chen, X. Valorization of Citrus reticulata Peels for Flavonoids and Antioxidant Enhancement by Solid-State Fermentation Using Aspergillus niger CGMCC 3.6189. Molecules 2022, 27, 8949. [Google Scholar] [CrossRef]
- Falduto, M.; Smedile, F.; Zhang, M.; Zheng, T.; Zhu, J.; Huang, Q.; Weeks, R.; Ermakov, A.M.; Chikindas, M.L. Anti-obesity effects of Chenpi: An artificial gastrointestinal system study. Microb. Biotechnol. 2022, 15, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Xu, X.; Wei, X.; Feng, W.; Huang, H.; Liu, H.; Xu, R.; Lin, J.; Han, L.; Zhang, D. Natural medicines for the treatment of fatigue: Bioactive components, pharmacology, and mechanisms. Pharmacol. Res. 2019, 148, 104409. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Huang, R.; Shi, X.; Xu, Z.; Mang, J. Exploration of the mechanism of luteolin against ischemic stroke based on network pharmacology, molecular docking and experimental verification. Bioengineered 2021, 12, 12274–12293. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Xiao, Y.; Wang, Z.; Xiao, H.; Liu, H. The Mechanism Study of Common Flavonoids on Antiglioma Based on Network Pharmacology and Molecular Docking. Evid. Based Complement. Alternat. Med. 2022, 2022, 2198722. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Xia, G.Q.; Cai, J.N.; Lv, X.; Dai, M. Hesperitin attenuates alcoholic steatohepatitis by regulating TLR4/NF-kappaB signaling in mice. Anal. Biochem. 2023, 682, 115339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, Q.; Yu, W.; Ye, F.; Cao, Y.; Akan, O.D.; Wu, X.; Xie, T.; Lu, H.; Cao, F. Gastrodin ameliorates exercise-induced fatigue via modulating Nrf2 pathway and inhibiting inflammation in mice. Food Biosci. 2023, 51, 102262. [Google Scholar] [CrossRef]
- Wang, N.; An, W.B.; Zhou, N.; Fan, J.C.; Feng, X.; Yang, W.J. Exploring the Active Ingredients and Mechanism of Action of Huanglian Huazhuo Capsule for the Treatment of Obese Type-2 Diabetes Mellitus Based on Using Network Pharmacology and Molecular Docking. Evid. Based Complement. Alternat. Med. 2022, 2022, 2780647. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Wang, Y.; Li, J.; Zhou, F.; Xiao, K.; Liu, Y.; Zhang, M.; Wang, S.; Yang, S. Mechanism of Sijunzi Decoction in the treatment of colorectal cancer based on network pharmacology and experimental validation. J. Ethnopharmacol. 2023, 302, 115876. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Huang, C.; Wang, S.; Huang, B.; Wu, D.; Zheng, G.; Cai, Y. Network Pharmacology-Based Strategy for Predicting Therapy Targets of Citri Reticulatae Pericarpium on Myocardial Hypertrophy. BioMed Res. Int. 2022, 2022, 4293265. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ye, X.; Yang, S.; Yu, H.; Zhong, L.; Gong, Q. Systems Pharmacology Study of the Anti-Liver Injury Mechanism of Citri Reticulatae Pericarpium. Front. Pharmacol. 2021, 12, 618846. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Ullah, A.; Zhang, R.; Sun, Y.; Li, J.; Kou, G. Network Pharmacology and Molecular Modeling Techniques in Unraveling the Underlying Mechanism of Citri Reticulatae Pericarpium aganist Type 2 Diabetic Osteoporosis. Nutrients 2024, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Liu, Z.; Deng, L.; Xia, S.; Mu, Q.; Xiao, B.; Xiu, Y.; Liu, Z. Hesperetin promotes bladder cancer cells death via the PI3K/AKT pathway by network pharmacology and molecular docking. Sci. Rep. 2024, 14, 1009. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ouyang, S.; Yu, B.; Liu, Y.; Huang, K.; Gong, J.; Zheng, S.; Li, Z.; Li, H.; Jiang, H. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 2010, 38, W609–W614. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.Y.; Jiao, X.; Song, W.X.; Wu, P.; Xiao, P.Q.; Huang, X.F.; Wang, K.; Zhan, S.F. Network pharmacology and bioinformatics analysis identifies potential therapeutic targets of Naringenin against COVID-19/LUSC. Front. Endocrinol. 2023, 14, 1187882. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ma, Y.; Cao, J.; Xie, R.; Chen, F.; Hu, W.; Huang, Y. Feasibility study on the use of “Qi-tonifying medicine compound” as an anti-fatigue functional food ingredient based on network pharmacology and molecular docking. Front. Nutr. 2023, 10, 1131972. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, R.; Hua, H.; Cheng, Y.; Guo, Y.; Qian, H.; Du, P. Network Pharmacology Exploration Reveals Gut Microbiota Modulation as a Common Therapeutic Mechanism for Anti-Fatigue Effect Treated with Maca Compounds Prescription. Nutrients 2022, 14, 1533. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Deng, S.; Li, J.; Gong, S.; Song, T.; Ge, J.; Zhao, Y.; Zhang, J.; Ma, L.; Zheng, Y.; et al. UPLC-MS analysis and network pharmacology-based investigation into the active ingredients and molecular mechanisms of anti-fatigue of male flowers with Eucommia ulmoides Oliv. Fundam. Clin. Pharmacol. 2022, 36, 1083–1098. [Google Scholar] [CrossRef]
- Hermawan, A.; Putri, H.; Utomo, R.Y. Comprehensive bioinformatics study reveals targets and molecular mechanism of hesperetin in overcoming breast cancer chemoresistance. Mol. Divers. 2020, 24, 933–947. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liu, F.; Yao, Y.; Zhang, Q.; Lu, Z.; Zhang, R.; Liu, C.; Lin, C.; Zhu, C. Corrigendum to “Network pharmacology-based mechanism prediction and pharmacological validation of Xiaoyan Lidan formula on attenuating alpha-naphthylisothiocyanate induced cholestatic hepatic injury in rats” [J. Ethnopharmacol. 270 (2021) 113816]. J. Ethnopharmacol. 2023, 303, 115976. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xing, Y.; Wang, R.; Jin, Z. Jianpi Huayu Decoction suppresses cellular senescence in colorectal cancer via p53-p21-Rb pathway: Network pharmacology and in vivo validation. J. Ethnopharmacol. 2024, 319, 117347. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zeng, Z.; Zhang, J.; Wu, D.; Li, H.; Geng, F. Molecular dynamics simulation of the interaction of food proteins with small molecules. Food Chem. 2023, 405, 134824. [Google Scholar] [CrossRef] [PubMed]
- Salomon-Ferrer, R.; Case, D.A.; Walker, R.C. An overview of the Amber biomolecular simulation package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3, 198–210. [Google Scholar] [CrossRef]
- Frisch, M. Gaussian 09, Revision d. 01; Gaussian. Inc.: Wallingford, CT, USA, 2009; Volume 201. [Google Scholar]
- Wang, J.; Wang, W.; Kollman, P.A.; Case, D.A. Antechamber: An accessory software package for molecular mechanical calculations. J. Am. Chem. Soc. 2001, 222, 2001. [Google Scholar]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. ff14SB: Improving the Accuracy of Protein Side Chain and Backbone Parameters from ff99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Mark, P.; Nilsson, L. Structure and dynamics of the TIP3P, SPC, and SPC/E water models at 298 K. J. Phys. Chem. A 2001, 105, 9954–9960. [Google Scholar] [CrossRef]
- Kräutler, V.; Van Gunsteren, W.F.; Hünenberger, P.H. A fast SHAKE algorithm to solve distance constraint equations for small molecules in molecular dynamics simulations. J. Comput. Chem. 2001, 22, 501–508. [Google Scholar] [CrossRef]
- Sagui, C.; Darden, T.A. Molecular dynamics simulations of biomolecules: Long-range electrostatic effects. Annu. Rev. Biophys. Biomol. Struct. 1999, 28, 155–179. [Google Scholar] [CrossRef]
- Larini, L.; Mannella, R.; Leporini, D. Langevin stabilization of molecular-dynamics simulations of polymers by means of quasisymplectic algorithms. J. Chem. Phys. 2007, 126, 104101. [Google Scholar] [CrossRef] [PubMed]
- Hou, T.; Wang, J.; Li, Y.; Wang, W. Assessing the performance of the MM/PBSA and MM/GBSA methods. 1. The accuracy of binding free energy calculations based on molecular dynamics simulations. J. Chem. Inf. Model. 2011, 51, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Rastelli, G.; Rio, A.D.; Degliesposti, G.; Sgobba, M. Fast and accurate predictions of binding free energies using MM-PBSA and MM-GBSA. J. Comput. Chem. 2010, 31, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Guo, L.; Ma, H.; Wu, H.; Fan, X. Network Pharmacology and Molecular Docking Suggest the Mechanism for Biological Activity of Rosmarinic Acid. Evid. Based Complement. Alternat. Med. 2021, 2021, 5190808. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, C.; Shen, X.; Liu, Y. The use of traditional Chinese medicines in relieving exercise-induced fatigue. Front. Pharmacol. 2022, 13, 969827. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Zhong, Y.; Li, M.; Chang, Q.; Liao, Y.; Liu, X.; Pan, R. Antioxidant and Anti-Fatigue Constituents of Okra. Nutrients 2015, 7, 8846–8858. [Google Scholar] [CrossRef] [PubMed]
- Su, K.Y.; Yu, C.Y.; Chen, Y.W.; Huang, Y.T.; Chen, C.T.; Wu, H.F.; Chen, Y.L. Rutin, a flavonoid and principal component of saussurea involucrata, attenuates physical fatigue in a forced swimming mouse model. Int. J. Med. Sci. 2014, 11, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Bustos, D.; Cardoso, F.; Rios, M.; Vaz, M.; Guedes, J.; Torres Costa, J.; Santos Baptista, J.; Fernandes, R.J. Machine Learning Approach to Model Physical Fatigue during Incremental Exercise among Firefighters. Sensors 2022, 23, 194. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Xia, P.; Zhang, L.; Hu, Y.; Xie, Q.; Xiang, H. A novel fermented soybean, inoculated with selected Bacillus, Lactobacillus and Hansenula strains, showed strong antioxidant and anti-fatigue potential activity. Food Chem. 2020, 333, 127527. [Google Scholar] [CrossRef] [PubMed]
- Allik, A.; Pilt, K.; Viigimae, M.; Fridolin, I.; Jervan, G. A Novel Physical Fatigue Assessment Method Utilizing Heart Rate Variability and Pulse Arrival Time towards Personalized Feedback with Wearable Sensors. Sensors 2022, 22, 1680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, A.; Yang, X. Effect of lemon seed flavonoids on the anti-fatigue and antioxidant effects of exhausted running exercise mice. J. Food Biochem. 2021, 45, e13620. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Li, J.; Fu, J.; Zhao, M.; Zhang, W.; Weng, W.; Li, Q. Network pharmacology and LC-MS approachs to explore the active compounds and mechanisms of Yuanjiang decoction for treating bradyarrhythmia. Comput. Biol. Med. 2023, 152, 106435. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Network Pharmacology Evaluation Method Guidance—Draft. World J. Tradit. Chin. Med. 2021, 7, 146–154. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Liao, J.; Chen, Q.; Lu, X.; Fan, X. Network pharmacology approaches for research of Traditional Chinese Medicines. Chin. J. Nat. Med. 2023, 21, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Wu, F.; Xu, J. Incorporation of beta-sitosterol into mitochondrial membrane enhances mitochondrial function by promoting inner mitochondrial membrane fluidity. J. Bioenerg. Biomembr. 2013, 45, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Filler, K.; Lyon, D.; Bennett, J.; McCain, N.; Elswick, R.; Lukkahatai, N.; Saligan, L.N. Association of Mitochondrial Dysfunction and Fatigue: A Review of the Literature. BBA Clin. 2014, 1, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Shi, X.; Zhang, X.; Zhang, S. Naringenin promotes the expression of oxidized myofibers via the PKA signaling pathway in C57BL/6J mice and C2C12 cells. J. Funct. Foods 2023, 111, 105902. [Google Scholar] [CrossRef]
- Biesemann, N.; Ried, J.S.; Ding-Pfennigdorff, D.; Dietrich, A.; Rudolph, C.; Hahn, S.; Hennerici, W.; Asbrand, C.; Leeuw, T.; Strubing, C. High throughput screening of mitochondrial bioenergetics in human differentiated myotubes identifies novel enhancers of muscle performance in aged mice. Sci. Rep. 2018, 8, 9408. [Google Scholar] [CrossRef]
- Swain, M.G. Fatigue in liver disease: Pathophysiology and clinical management. Can. J. Gastroenterol. 2006, 20, 181–188. [Google Scholar] [CrossRef]
- Akachi, T.; Shiina, Y.; Ohishi, Y.; Kawaguchi, T.; Kawagishi, H.; Morita, T.; Mori, M.; Sugiyama, K. Hepatoprotective effects of flavonoids from shekwasha (Citrus depressa) against D-galactosamine-induced liver injury in rats. J. Nutr. Sci. Vitaminol. 2010, 56, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B. Reactive Oxygen Species as Agents of Fatigue. Med. Sci. Sports Exerc. 2016, 48, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Feng, S.; Sheng, C.; Yang, C.; Li, Y. Nobiletin from citrus peel: A promising therapeutic agent for liver disease-pharmacological characteristics, mechanisms, and potential applications. Front. Pharmacol. 2024, 15, 1354809. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Chen, J.; Huang, Z.; Men, Y.; Qian, Y.; Yu, M.; Xu, X.; Li, L.; Zhao, X.; Jiang, Y.; et al. Ginsenoside Rg1 can reverse fatigue behavior in CFS rats by regulating EGFR and affecting Taurine and Mannose 6-phosphate metabolism. Front. Pharmacol. 2023, 14, 1163638. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Mi, J.; Feng, Y.; Li, L.; Wang, Y.; Hu, J.; Wang, H. Huangqi Sijunzi decoction for treating cancer-related fatigue in breast cancer patients: A randomized trial and network pharmacology study. Nan Fang Yi Ke Da Xue Xue Bao 2022, 42, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Guo, W.; Yao, X.; Xia, J.; Zhang, Z.; Li, J.; Chen, H.; Lin, L. Database mining and animal experiment-based validation of the efficacy and mechanism of Radix Astragali (Huangqi) and Rhizoma Atractylodis Macrocephalae (Baizhu) as core drugs of Traditional Chinese medicine in cancer-related fatigue. J. Ethnopharmacol. 2022, 285, 114892. [Google Scholar] [CrossRef] [PubMed]
- Ma, P.; Li, J.; Huang, Q.; Wei, S.; Ge, H.; Wang, Z. Exploring the mechanism of anti-fatigue of resveratrol based on network pharmacology and molecular docking, and in vitro studies. Sci. Rep. 2023, 13, 2894. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Han, M.; Zhang, H.; Li, Z.; Xu, K.; Kang, H.; Zong, J.; Zhao, F.; Liu, Y.; Liu, W. Curcumin (CUMINUP60(R)) mitigates exercise fatigue through regulating PI3K/Akt/AMPK/mTOR pathway in mice. Aging 2023, 15, 2308–2320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Lu, B.; Wang, E.; Wang, W.; Li, Z.; Jiao, L.; Li, H.; Wu, W. Panax ginseng improves physical recovery and energy utilization on chronic fatigue in rats through the PI3K/AKT/mTOR signalling pathway. Pharm. Biol. 2023, 61, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Li, N.; Jia, W.; Wang, N.; Liang, M.; Yang, X.; Du, G. Skeletal muscle atrophy: From mechanisms to treatments. Pharmacol. Res. 2021, 172, 105807. [Google Scholar] [CrossRef] [PubMed]
- Pantsar, T. The current understanding of KRAS protein structure and dynamics. Comput. Struct. Biotechnol. J. 2020, 18, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Zhang, C.; Yang, J.; Tian, Y.; You, L.; Yang, H.; Li, Y.; Liu, H.; Pan, D.; Liu, Z. Kaempferol Improves Exercise Performance by Regulating Glucose Uptake, Mitochondrial Biogenesis, and Protein Synthesis via PI3K/AKT and MAPK Signaling Pathways. Foods 2024, 13, 1068. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.L.; Mao, X.Y.; Liu, S.; Chen, W.Z.; Huang, D.D.; Zhang, C.J.; Chen, B.C.; Shen, X.; Yu, Z. Ginsenoside Rb1 improves postoperative fatigue syndrome by reducing skeletal muscle oxidative stress through activation of the PI3K/Akt/Nrf2 pathway in aged rats. Eur. J. Pharmacol. 2014, 740, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.R.; Skousen, C.; Holland, A.; Williams, K.; Hyldahl, R.D. Acute extracellular matrix, inflammatory and MAPK response to lengthening contractions in elderly human skeletal muscle. Exp. Gerontol. 2018, 106, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Bisht, A.; Tewari, D.; Kumar, S.; Chandra, S. Network pharmacology, molecular docking, and molecular dynamics simulation to elucidate the mechanism of anti-aging action of Tinospora cordifolia. Mol. Divers 2023. [Google Scholar] [CrossRef] [PubMed]
- Kakhar Umar, A.; Zothantluanga, J.H.; Luckanagul, J.A.; Limpikirati, P.; Sriwidodo, S. Structure-based computational screening of 470 natural quercetin derivatives for identification of SARS-CoV-2 M(pro) inhibitor. PeerJ 2023, 11, e14915. [Google Scholar] [CrossRef] [PubMed]

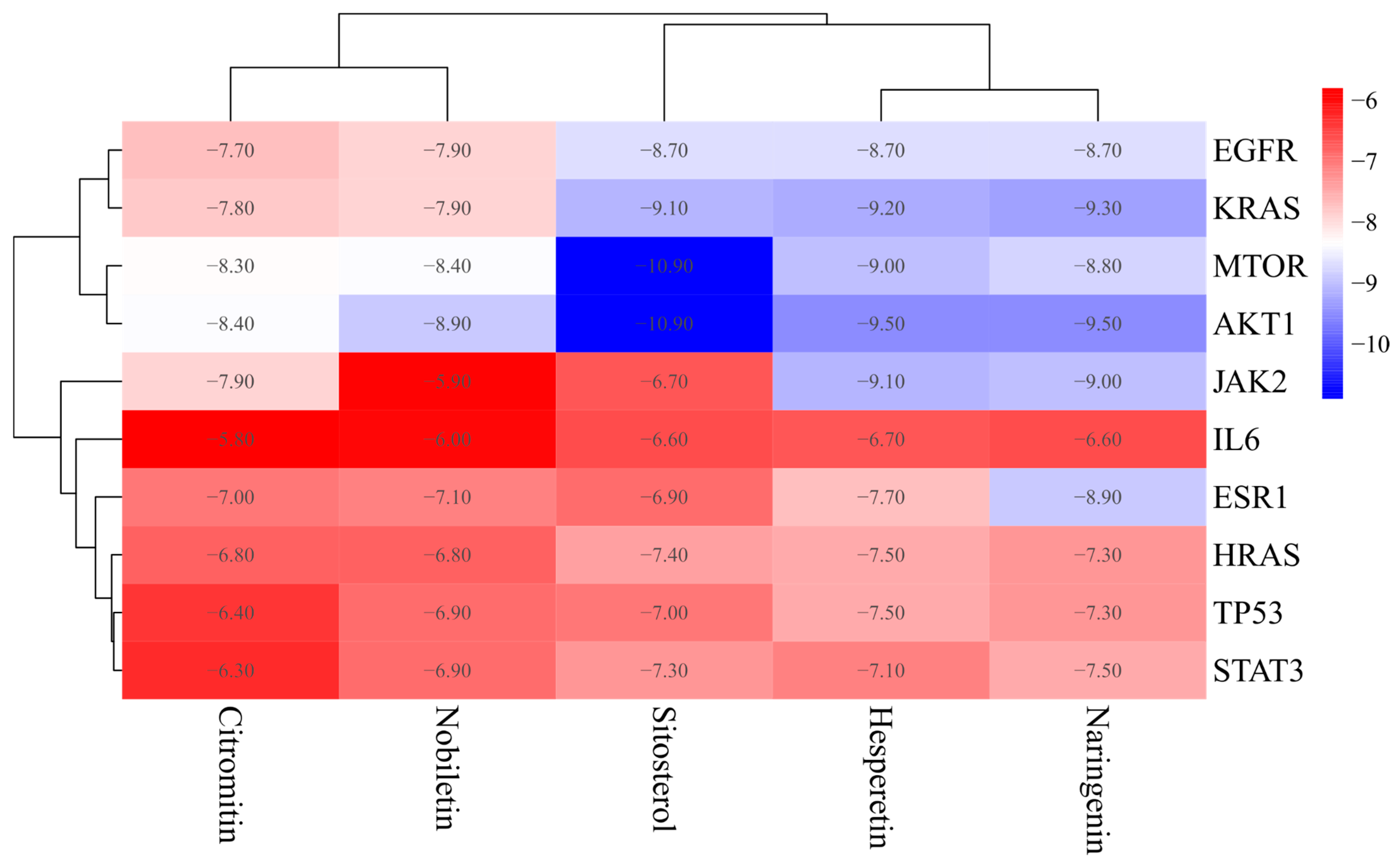
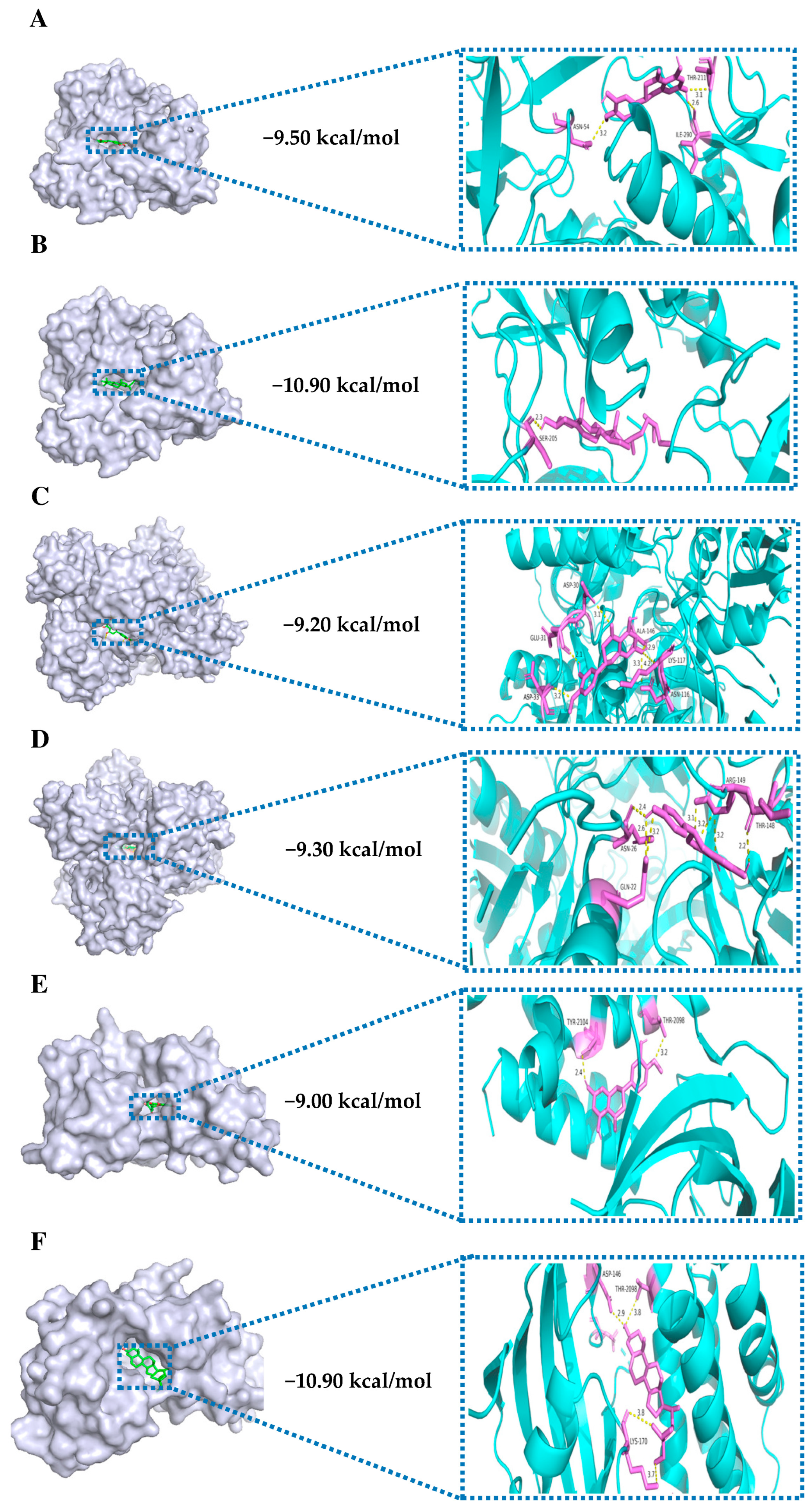
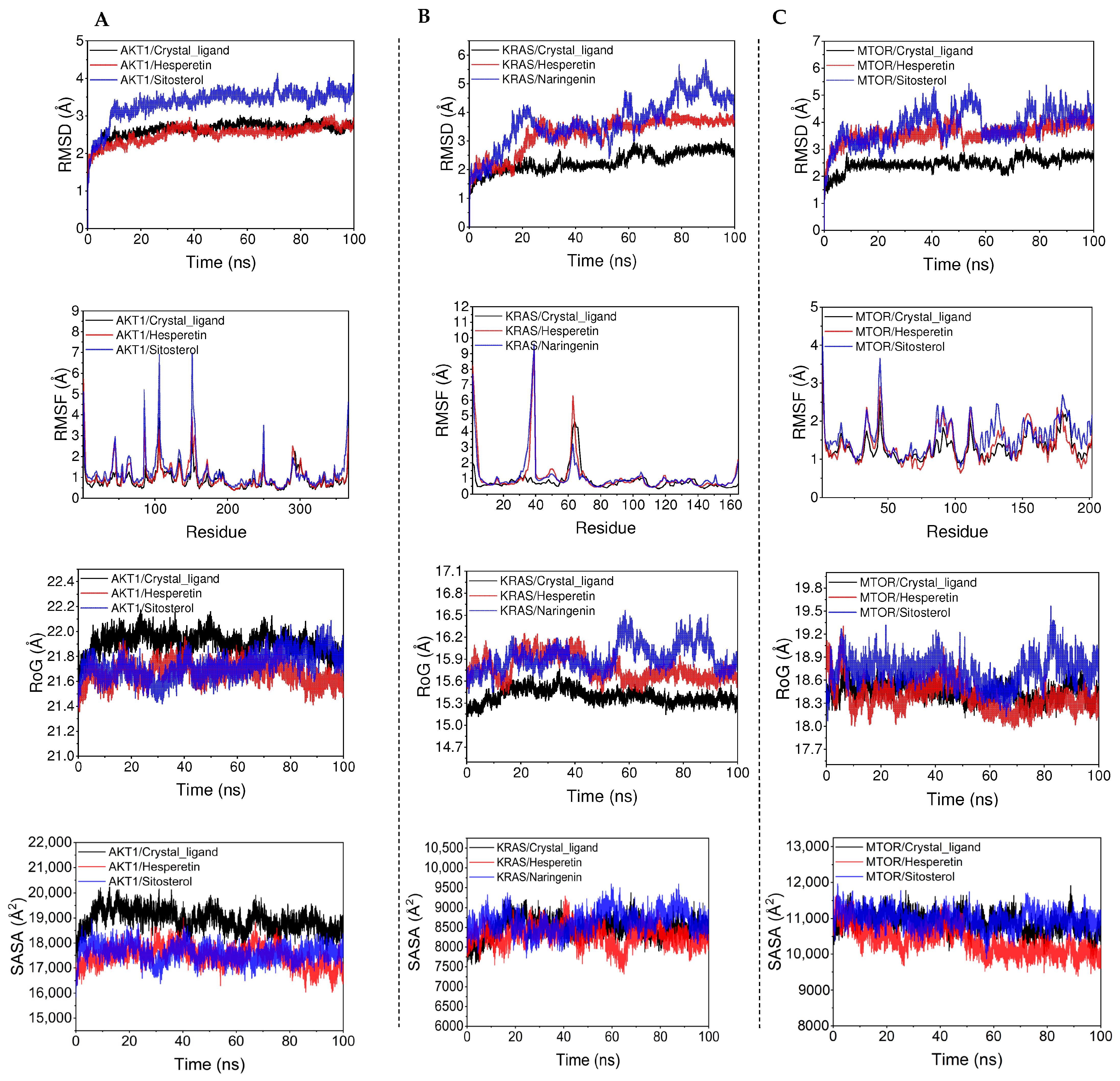
| ID | Molecule Name | Molecular Formula | Molecular Weight (g/mol) | OB (%) | DL |
|---|---|---|---|---|---|
| MOL000359 | Sitosterol | C29H50O | 414.7 | 36.91 | 0.75 |
| MOL004328 | Naringenin | C15H12O5 | 272.25 | 59.29 | 0.21 |
| MOL005100 | Hesperetin | C16H14O6 | 302.28 | 47.74 | 0.27 |
| MOL005815 | Citromitin | C21H24O8 | 404.4 | 86.90 | 0.51 |
| MOL005828 | Nobiletin | C21H22O8 | 402.4 | 61.67 | 0.52 |
| System Name | ΔEvdw | ΔEelec | ΔGGB | ΔGSA | ΔGbind |
|---|---|---|---|---|---|
| KRAS/crystal_ligand | −40.32 | −2.08 | 20.96 | −6.09 | −27.52 |
| AKT1/crystal_ligand | −89.74 | 5.09 | 45.37 | −8.69 | −47.98 |
| MTOR/crystal_ligand | −92.13 | −66.12 | 84.56 | −12.32 | −86.01 |
| AKT1/Hesperetin | −38.57 | −21.05 | 38.45 | −5.25 | −26.42 |
| AKT1/Sitosterol | −63.44 | −2.26 | 31.75 | −7.56 | −41.51 |
| KRAS/Hesperetin | −31.51 | −25.38 | 37.85 | −4.40 | −23.44 |
| KRAS/Naringenin | −12.45 | −6.64 | 13.90 | −1.62 | −6.81 |
| MTOR/Hesperetin | −37.53 | −45.93 | 56.51 | −5.27 | −32.21 |
| MTOR/Sitosterol | −47.54 | −2.51 | 18.46 | −5.98 | −37.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, A.; Sun, Q.; Li, J.; Li, J.; Khatun, P.; Kou, G.; Lyu, Q. Bioactive Compounds in Citrus reticulata Peel Are Potential Candidates for Alleviating Physical Fatigue through a Triad Approach of Network Pharmacology, Molecular Docking, and Molecular Dynamics Modeling. Nutrients 2024, 16, 1934. https://doi.org/10.3390/nu16121934
Ullah A, Sun Q, Li J, Li J, Khatun P, Kou G, Lyu Q. Bioactive Compounds in Citrus reticulata Peel Are Potential Candidates for Alleviating Physical Fatigue through a Triad Approach of Network Pharmacology, Molecular Docking, and Molecular Dynamics Modeling. Nutrients. 2024; 16(12):1934. https://doi.org/10.3390/nu16121934
Chicago/Turabian StyleUllah, Amin, Qiuxi Sun, Jiangtao Li, Jinjie Li, Pipasha Khatun, Guangning Kou, and Quanjun Lyu. 2024. "Bioactive Compounds in Citrus reticulata Peel Are Potential Candidates for Alleviating Physical Fatigue through a Triad Approach of Network Pharmacology, Molecular Docking, and Molecular Dynamics Modeling" Nutrients 16, no. 12: 1934. https://doi.org/10.3390/nu16121934






