Folate and Vitamin B12 Levels in Chilean Women with PCOS and Their Association with Metabolic Outcomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion and Exclusion Criteria
2.3. Anthropometric and Ultrasound Examinations
2.4. Biochemical Analyses
2.5. Genotyping Analysis
2.5.1. DNA Isolation
2.5.2. Genotyping
2.6. Statistical Analysis
3. Results
3.1. Anthropometric, Clinical, and Biochemical Data of the Sample
3.2. Hormonal Parameters of Subjects Included in the Study
3.3. Folates and Vitamin B12 Circulant Levels
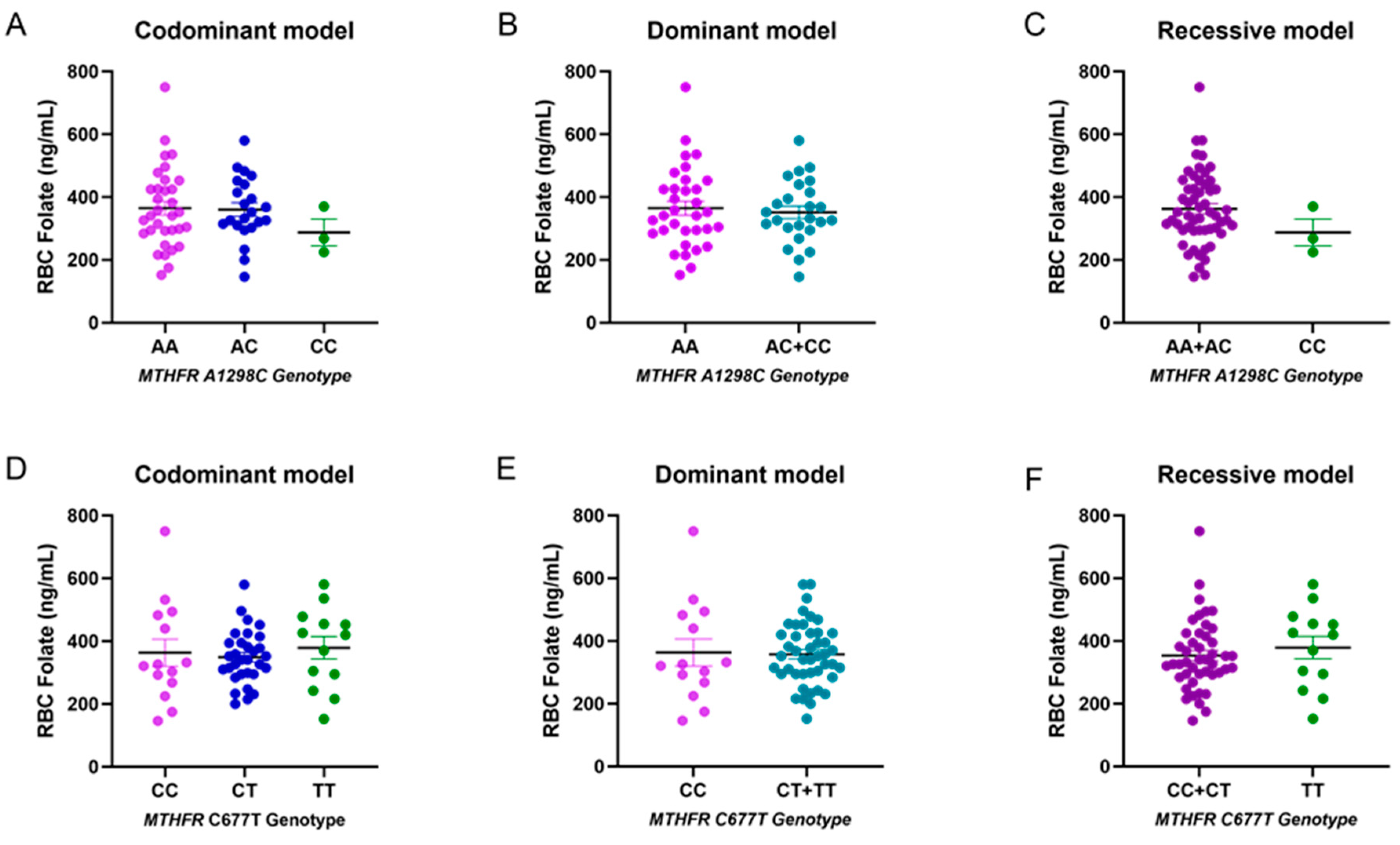
3.4. Genotype and Allele Frequencies
| Control (n = 29) | Cases (n = 29) | p-Value * | |
|---|---|---|---|
| A1298C (rs1801131) | |||
| Allele | 0.76 | 0.76 | - |
| A | 0.24 | 0.24 | |
| C | |||
| Codominant model | |||
| A/A | 15 (51.72) | 18 (62.07) | 0.08 |
| A/C | 14 (48.28) | 8 (27.59) | |
| C/C | 0 (0) | 3 (10.34) | |
| Recessive model | |||
| A/A + A/C | 29 | 26 | 0.236 |
| C/C | 0 (0) | 3 (10.34) | |
| Dominant model | |||
| A/A | 15 | 18 | 0.596 |
| A/C + C/C | 14 | 11 | |
| C677T (rs1801133) | |||
| Allele | |||
| C | 0.5 | 0.52 | - |
| T | 0.5 | 0.48 | |
| Codominant model | |||
| C/C | 6 (20.69) | 8 (27.59) | 0.721 |
| C/T | 17 (58.62) | 14 (48.28) | |
| T/T | 6 (20.69) | 7 (24.14) | |
| Recessive model | |||
| C/C + C/T | 23 | 22 | 0.999 |
| T/T | 6 | 7 | |
| Dominant model | |||
| C/C | 6 | 8 | 0.759 |
| C/T + T/T | 23 | 21 |
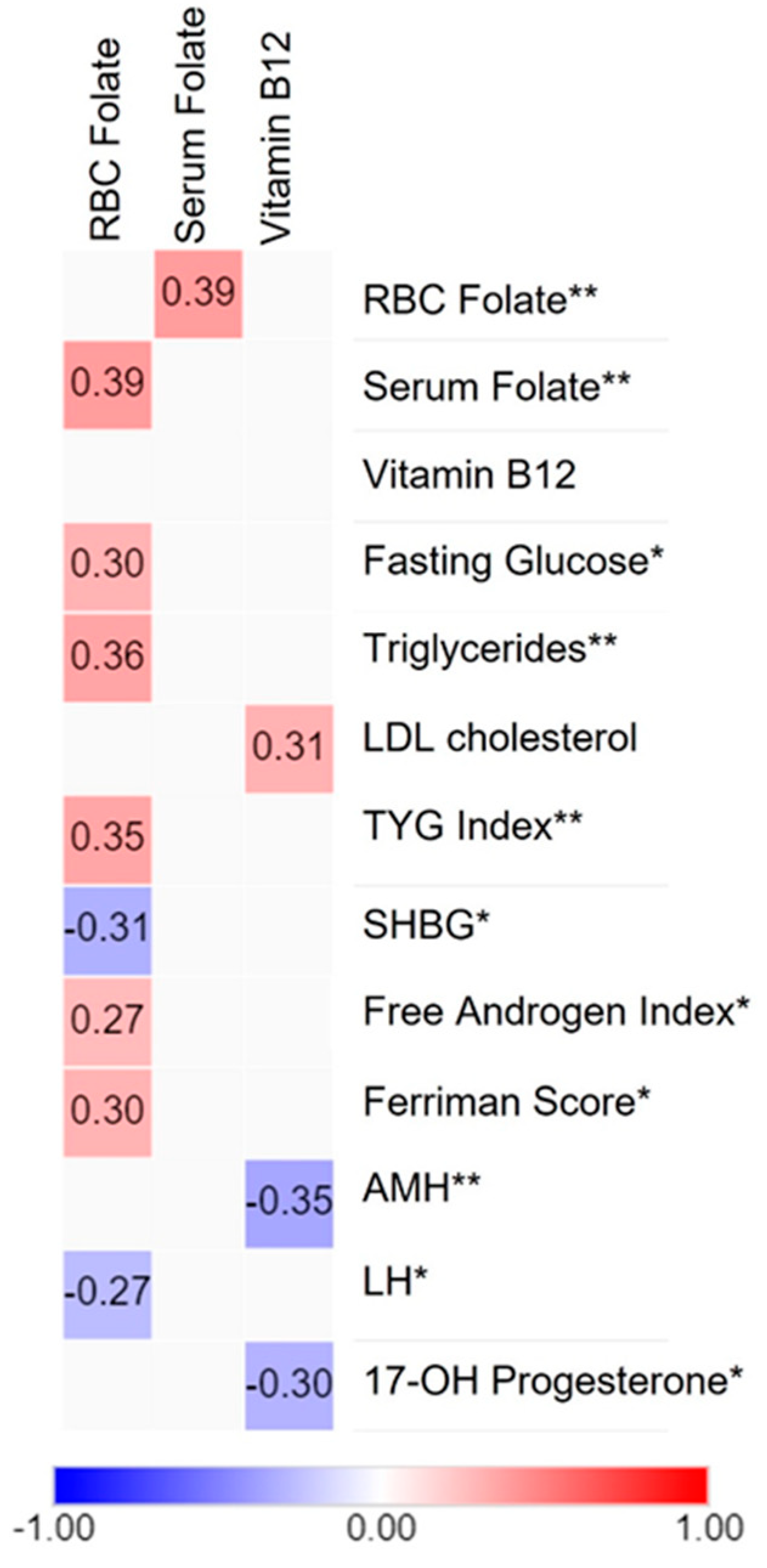
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teede, H.J.; Tay, C.T.; Laven, J.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.M.; Redman, L.; Boyle, J.A.; et al. Recommendations from the 2023 International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Fertil. Steril. 2023, 120, 767–793. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.E.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic Ovary Syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef]
- Abusailik, M.A.; Muhanna, A.M.; Almuhisen, A.A.; Alhasanat, A.M.; Alshamaseen, A.M.; Bani Mustafa, S.M.; Nawaiseh, M.B. Cutaneous Manifestation of Polycystic Ovary Syndrome. Dermatol. Rep. 2021, 13, 8799. [Google Scholar] [CrossRef]
- Moran, L.J.; Misso, M.L.; Wild, R.A.; Norman, R.J. Impaired Glucose Tolerance, Type 2 Diabetes and Metabolic Syndrome in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2010, 16, 347–363. [Google Scholar] [CrossRef]
- de Groot, P.C.M.; Dekkers, O.M.; Romijn, J.A.; Dieben, S.W.M.; Helmerhorst, F.M. PCOS, Coronary Heart Disease, Stroke and the Influence of Obesity: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2011, 17, 495–500. [Google Scholar] [CrossRef]
- Goyette, P.; Sumner, J.S.; Milos, R.; Duncan, A.M.V.; Rosenblatt, D.S.; Matthews, R.G.; Rozen, R. Human Methylenetetrahydrofolate Reductase: Isolation of CDNA, Mapping and Mutation Identification. Nat. Genet. 1994, 7, 195–200. [Google Scholar] [CrossRef]
- Liew, S.C.; Gupta, E. Das Methylenetetrahydrofolate Reductase (MTHFR) C677T Polymorphism: Epidemiology, Metabolism and the Associated Diseases. Eur. J. Med. Genet. 2015, 58, 1–10. [Google Scholar] [CrossRef]
- Goyette, P.; Pai, A.; Milos, R.; Frosst, P.; Tran, P.; Chen, Z.; Chan, M.; Rozen, R. Gene Structure of Human and Mouse Methylenetetrahydrofolate Reductase (MTHFR). Mamm. Genome 1998, 9, 652–656. [Google Scholar] [CrossRef]
- Xiong, Y.; Bian, C.; Lin, X.; Wang, X.; Xu, K.; Zhao, X. Methylenetetrahydrofolate Reductase Gene Polymorphisms in the Risk of Polycystic Ovary Syndrome and Ovarian Cancer. Biosci. Rep. 2020, 40, 20200995. [Google Scholar] [CrossRef]
- Engevik, M.A.; Morra, C.N.; Röth, D.; Engevik, K.; Spinler, J.K.; Devaraj, S.; Crawford, S.E.; Estes, M.K.; Kalkum, M.; Versalovic, J. Microbial Metabolic Capacity for Intestinal Folate Production and Modulation of Host Folate Receptors. Front. Microbiol. 2019, 10, 2305. [Google Scholar] [CrossRef]
- Lyon, P.; Strippoli, V.; Fang, B.; Cimmino, L. B Vitamins and One-Carbon Metabolism: Implications in Human Health and Disease. Nutrients 2020, 12, 2867. [Google Scholar] [CrossRef]
- Murphy, M.E.; Westmark, C.J. Folic Acid Fortification and Neural Tube Defect Risk: Analysis of the Food Fortification Initiative Dataset. Nutrients 2020, 12, 247. [Google Scholar] [CrossRef]
- Smith, A.D.; Sobczyńska-Malefora, A.; Green, R.; Reynolds, E.H.; Refsum, H. Mandatory Food Fortification with Folic Acid. Lancet Glob. Health 2022, 10, e1389. [Google Scholar] [CrossRef]
- Hirsch, S.; Sanchez, H.; Albala, C.; de la Maza, M.P.; Barrera, G.; Leiva, L.; Bunout, D. Colon Cancer in Chile before and after the Start of the Flour Fortification Program with Folic Acid. Eur. J. Gastroenterol. Hepatol. 2009, 21, 346–349. [Google Scholar] [CrossRef]
- Morris, M.S.; Jacques, P.F.; Rosenberg, I.H.; Selhub, J. Folate and Vitamin B-12 Status in Relation to Anemia, Macrocytosis, and Cognitive Impairment in Older Americans in the Age of Folic Acid Fortification. Am. J. Clin. Nutr. 2007, 85, 193–200. [Google Scholar] [CrossRef]
- Crider, K.S.; Qi, Y.P.; Yeung, L.F.; Mai, C.T.; Head Zauche, L.; Wang, A.; Daniels, K.; Williams, J.L. Folic Acid and the Prevention of Birth Defects: 30 Years of Opportunity and Controversies. Annu. Rev. Nutr. 2022, 42, 423–452. [Google Scholar] [CrossRef]
- Selhub, J.; Miller, J.W.; Troen, A.M.; Mason, J.B.; Jacques, P.F. Perspective: The High-Folate-Low-Vitamin B-12 Interaction Is a Novel Cause of Vitamin B-12 Depletion with a Specific Etiology—A Hypothesis. Adv. Nutr. 2022, 13, 16–33. [Google Scholar] [CrossRef]
- Castillo-Lancellotti, C.; Margozzini, P.; Valdivia, G.; Padilla, O.; Uauy, R.; Rozowski, J.; Tur, J.A. Serum Folate, Vitamin B12 and Cognitive Impairment in Chilean Older Adults. Public Health Nutr. 2015, 18, 2600–2608. [Google Scholar] [CrossRef]
- Yildiz, B.O.; Bolour, S.; Woods, K.; Moore, A.; Azziz, R. Visually Scoring Hirsutism. Hum. Reprod. Update 2010, 16, 51. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for Reporting Observational Studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Little, J.; Higgins, J.P.T.; Ioannidis, J.P.A.; Moher, D.; Gagnon, F.; Von Elm, E.; Khoury, M.J.; Cohen, B.; Davey-Smith, G.; Grimshaw, J.; et al. STrengthening the REporting of Genetic Association Studies (STREGA)—An Extension of the STROBE Statement. Genet. Epidemiol. 2009, 33, 581–598. [Google Scholar] [CrossRef]
- WHO. VMNIS | Vitamin and Mineral Nutrition Information System. Available online: http://apps.who.int/iris/bitstream/10665/162114/1/WHO_NMH_NHD_EPG_15.01.pdf?ua=1 (accessed on 29 December 2023).
- Tremblay, A.J.; Morrissette, H.; Gagné, J.M.; Bergeron, J.; Gagné, C.; Couture, P. Validation of the Friedewald Formula for the Determination of Low-Density Lipoprotein Cholesterol Compared with β-Quantification in a Large Population. Clin. Biochem. 2004, 37, 785–790. [Google Scholar] [CrossRef]
- Al Kindi, M.K.; Al Essry, F.S.; Al Essry, F.S.; Mula-Abed, W.A.S. Validity of Serum Testosterone, Free Androgen Index, and Calculated Free Testosterone in Women with Suspected Hyperandrogenism. Oman Med. J. 2012, 27, 471–474. [Google Scholar] [CrossRef]
- Srinivasan, S.; Kaur, V.; Chamarthi, B.; Littleton, K.R.; Chen, L.; Manning, A.K.; Merino, J.; Thomas, M.K.; Hudson, M.; Goldfine, A.; et al. TCF7L2 Genetic Variation Augments Incretin Resistance and Influences Response to a Sulfonylurea and Metformin: The Study to Understand the Genetics of the Acute Response to Metformin and Glipizide in Humans (SUGAR-MGH). Diabetes Care 2018, 41, 554–561. [Google Scholar] [CrossRef]
- Araújo, S.P.; Juvanhol, L.L.; Bressan, J.; Hermsdorff, H.H.M. Triglyceride Glucose Index: A New Biomarker in Predicting Cardiovascular Risk. Prev. Med. Rep. 2022, 29, 101941. [Google Scholar] [CrossRef]
- Zintzaras, E.; Lau, J. Synthesis of Genetic Association Studies for Pertinent Gene-Disease Associations Requires Appropriate Methodological and Statistical Approaches. J. Clin. Epidemiol. 2008, 61, 634–645. [Google Scholar] [CrossRef]
- Barber, T.M.; Dimitriadis, G.K.; Andreou, A.; Franks, S. Polycystic Ovary Syndrome: Insight into Pathogenesis and a Common Association with Insulin Resistance. Clin. Med. 2015, 15 (Suppl. 6), s72–s76. [Google Scholar] [CrossRef]
- Venkatesan, A.M.; Dunaif, A.; Corbould, A. Insulin Resistance in Polycystic Ovary Syndrome: Progress and Paradoxes. Recent Prog. Horm. Res. 2001, 56, 295–308. [Google Scholar] [CrossRef]
- Garzia, E.; Galiano, V.; Marfia, G.; Navone, S.; Grossi, E.; Marconi, A.M. Hyperandrogenism and Menstrual Imbalance Are the Best Predictors of Metformin Response in PCOS Patients. Reprod. Biol. Endocrinol. 2022, 20, 6. [Google Scholar] [CrossRef]
- Rahmatnezhad, L.; Moghaddam-Banaem, L.; Behrouzi Lak, T.; Shiva, A.; Rasuli, J. Free Androgen Index (FAI)’s Relations with Oxidative Stress and Insulin Resistance in Polycystic Ovary Syndrome. Sci. Rep. 2023, 13, 5118. [Google Scholar] [CrossRef]
- Busso, D.; Echeverriá, G.; Passi-Solar, A.; Morales, F.; Fariás, M.; Margozzini, P. Folate Status in Women of Childbearing Age in the Urban Metropolitan Region of Chile: Results from the National Health Survey 2016–2017. Public Health Nutr. 2021, 24, 385–392. [Google Scholar] [CrossRef]
- Lee, S.M.; Oh, J.; Chun, M.R.; Lee, S.Y. Methylmalonic Acid and Homocysteine as Indicators of Vitamin B12 Deficiency in Patients with Gastric Cancer after Gastrectomy. Nutrients 2019, 11, 450. [Google Scholar] [CrossRef]
- Mathew, A.R.; Di Matteo, G.; La Rosa, P.; Barbati, S.A.; Mannina, L.; Moreno, S.; Tata, A.M.; Cavallucci, V.; Fidaleo, M. Vitamin B12 Deficiency and the Nervous System: Beyond Metabolic Decompensation—Comparing Biological Models and Gaining New Insights into Molecular and Cellular Mechanisms. Int. J. Mol. Sci. 2024, 25, 590. [Google Scholar] [CrossRef]
- Boachie, J.; Adaikalakoteswari, A.; Samavat, J.; Saravanan, P. Low Vitamin B12 and Lipid Metabolism: Evidence from Pre-Clinical and Clinical Studies. Nutrients 2020, 12, 1925. [Google Scholar] [CrossRef]
- Finer, S.; Saravanan, P.; Hitman, G.; Yajnik, C. The Role of the One-Carbon Cycle in the Developmental Origins of Type 2 Diabetes and Obesity. Diabet. Med. 2014, 31, 263–272. [Google Scholar] [CrossRef]
- Mishra, P.K.; Tyagi, N.; Sen, U.; Joshua, I.G.; Tyagi, S.C. Synergism in Hyperhomocysteinemia and Diabetes: Role of PPAR Gamma and Tempol. Cardiovasc. Diabetol. 2010, 9, 49. [Google Scholar] [CrossRef]
- Allen, L.H. How Common Is Vitamin B-12 Deficiency? Am. J. Clin. Nutr. 2009, 89, 693S–696S. [Google Scholar] [CrossRef]
- Schiuma, N.; Costantino, A.; Bartolotti, T.; Dattilo, M.; Bini, V.; Aglietti, M.C.; Renga, M.; Favilli, A.; Falorni, A.; Gerli, S. Micronutrients in Support to the One Carbon Cycle for the Modulation of Blood Fasting Homocysteine in PCOS Women. J. Endocrinol. Investig. 2020, 43, 779–786. [Google Scholar] [CrossRef]
- Bird, J.K.; Ronnenberg, A.G.; Choi, S.W.; Du, F.; Mason, J.B.; Liu, Z. Obesity Is Associated with Increased Red Blood Cell Folate Despite Lower Dietary Intakes and Serum Concentrations. J. Nutr. 2015, 145, 79–86. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Deshpande, S.S.; Jackson, A.A.; Refsum, H.; Rao, S.; Fisher, D.J.; Bhat, D.S.; Naik, S.S.; Coyaji, K.J.; Joglekar, C.V.; et al. Vitamin B12 and Folate Concentrations during Pregnancy and Insulin Resistance in the Offspring: The Pune Maternal Nutrition Study. Diabetologia 2008, 51, 29–38. [Google Scholar] [CrossRef]
- Williamson, J.M.; Arthurs, A.L.; Smith, M.D.; Roberts, C.T.; Jankovic-Karasoulos, T. High Folate, Perturbed One-Carbon Metabolism and Gestational Diabetes Mellitus. Nutrients 2022, 14, 3930. [Google Scholar] [CrossRef]
- Szczuko, M.; Hawryłkowicz, V.; Kikut, J.; Drozd, A. The Implications of Vitamin Content in the Plasma in Reference to the Parameters of Carbohydrate Metabolism and Hormone and Lipid Profiles in PCOS. J. Steroid Biochem. Mol. Biol. 2020, 198, 105570. [Google Scholar] [CrossRef]
- Sivakumaran, S.; Zhang, J.; Kelley, K.M.M.; Gonit, M.; Hao, H.; Ratnam, M. Androgen Activation of the Folate Receptor α Gene through Partial Tethering of the Androgen Receptor by C/EBPα. J. Steroid Biochem. Mol. Biol. 2010, 122, 333–340. [Google Scholar] [CrossRef]
- Al-Musharaf, S.; Aljuraiban, G.S.; Hussain, S.D.; Alnaami, A.M.; Saravanan, P.; Al-Daghri, N. Low Serum Vitamin B12 Levels Are Associated with Adverse Lipid Profiles in Apparently Healthy Young Saudi Women. Nutrients 2020, 12, 2395. [Google Scholar] [CrossRef]
- Liu, Y.; Geng, T.; Wan, Z.; Lu, Q.; Zhang, X.; Qiu, Z.; Li, L.; Zhu, K.; Liu, L.; Pan, A.; et al. Associations of Serum Folate and Vitamin B12 Levels With Cardiovascular Disease Mortality Among Patients With Type 2 Diabetes. JAMA Netw. Open 2022, 5, e2146124. [Google Scholar] [CrossRef]
- Martin, C.E.; Riley, J.; Jungheim, E.S. Serum Folic Acid, Vitamin B12 and Vitamin D3 Levels Are Not Associated with Antimullerian Hormone Levels. Fertil. Steril. 2020, 114, e446. [Google Scholar] [CrossRef]
- Kim, K.; Mills, J.L.; Michels, K.A.; Chaljub, E.N.; Wactawski-Wende, J.; Plowden, T.C.; Mumford, S.L. Dietary Intakes of Vitamins B2, B6, and B12 and Ovarian Cycle Function among Premenopausal Women. J. Acad. Nutr. Diet. 2020, 120, 885–892. [Google Scholar] [CrossRef]
- Gálvez, A.S.; Ramírez, H.; Placencia, P.; Rojas, C.; Urzúa, X.; Kalergis, A.M.; Salazar, L.A.; Escobar-Vera, J. Single Nucleotide Polymorphisms in Apolipoprotein B, Apolipoprotein E, and Methylenetetrahydrofolate Reductase Are Associated with Serum Lipid Levels in Northern Chilean Subjects. A Pilot Study. Front. Genet. 2021, 12, 640956. [Google Scholar] [CrossRef]
- Roco, A.; Quiñones, L.A.; Sepúlveda, P.; Donoso, H.; Lapostol, C.; Alarcón, R.; Torres, M.E.; Véliz, P.C.; Acuña, G.; Wilke, O.; et al. Prevalence of Seven Cardiovascular-Related Genetic Polymorphisms in a Chilean Mestizo Healthy Population. Acta Cardiol. 2015, 70, 528–535. [Google Scholar] [CrossRef]
- Szafarowska, M.; Segiet, A.; Jerzak, M.M. Methylenotetrahydrololate Reductase A1298C and C677T Polymorphisms and Adverse Pregnancy Outcome in Women with PCOS. Neuroendocrinol. Lett. 2016, 37, 141–146. [Google Scholar]
- Carlus, S.J.; Sarkar, S.; Bansal, S.K.; Singh, V.; Singh, K.; Jha, R.K.; Sadasivam, N.; Sadasivam, S.R.; Gireesha, P.S.; Thangaraj, K.; et al. Is MTHFR 677 C>T Polymorphism Clinically Important in Polycystic Ovarian Syndrome (PCOS)? A Case-Control Study, Meta-Analysis and Trial Sequential Analysis. PLoS ONE 2016, 11, e0151510. [Google Scholar] [CrossRef]
- Karadeniz, M.; Erdogan, M.; Zengi, A.; Eroglu, Z.; Tamsel, S.; Olukman, M.; Saygili, F.; Yilmaz, C. Methylenetetrahydrofolate Reductase C677T Gene Polymorphism in Turkish Patients with Polycystic Ovary Syndrome. Endocrine 2010, 38, 127–133. [Google Scholar] [CrossRef]
- Choi, S.W.; Gu, B.H.; Ramakrishna, S.; Park, J.M.; Baek, K.H. Association between a Single Nucleotide Polymorphism in MTHFR Gene and Polycystic Ovary Syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2009, 145, 85–88. [Google Scholar] [CrossRef]
- Dos Santos, T.B.; de Paula, H.K.; Balarin, M.A.S.; Silva-Grecco, R.L.; Lima, M.F.P.; de Resende, E.A.M.R.; Gomes, M.K.O.; Cintra, M.T.R. Can the Genetic Polymorphisms of the Folate Metabolism Have an Influence in the Polycystic Ovary Syndrome? Arch. Endocrinol. Metab. 2019, 63, 501–508. [Google Scholar] [CrossRef]
- Feng, W.; Zhang, Y.; Pan, Y.; Zhang, Y.; Liu, M.; Huang, Y.; Xiao, Y.; Mo, W.; Jiao, J.; Wang, X.; et al. Association of Three Missense Mutations in the Homocysteine-Related MTHFR and MTRR Gene with Risk of Polycystic Ovary Syndrome in Southern Chinese Women. Reprod. Biol. Endocrinol. 2021, 19, 5. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, H.; Yu, M.; Wang, X.; Wang, Z.; Xu, L.; Wang, J.; Mu, H. Association of Methylenetetrahydrofolate Reductase Gene Polymorphisms with Polycystic Ovary Syndrome. Zhonghua Yi Xue Yi Chuan Xue Za Zhi 2015, 32, 400–404. [Google Scholar] [CrossRef]
- Zhu, X.; Hong, X.; Chen, L.; Xuan, Y.; Huang, K.; Wang, B. Association of Methylenetetrahydrofolate Reductase C677T and A1298C Polymorphisms with Genetic Susceptibility to Polycystic Ovary Syndrome: A PRISMA-Compliant Meta-Analysis. Gene 2019, 719, 144079. [Google Scholar] [CrossRef]
- Crider, K.; Bailey, L.; Berry, R. Folic Acid Food Fortification—Its History, Effect, Concerns, and Future Directions. Nutrients 2011, 3, 370–384. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
| Parameter | Control (n = 29) | PCOS (n = 29) | p-Value * |
|---|---|---|---|
| Age (years) | 25.8 ± 4.8 | 25.5 ± 4.2 | 0.797 |
| Ferriman score | 3 (0.5–4.0) | 11 (9–16.5) | <0.0001 |
| BMI (kg/m2) | 24.1 ± 4.8 | 32 ± 5 | <0.0001 |
| WC (cm) | 75.7 ± 9.8 | 92.9 ± 13.4 | <0.0001 |
| HC (cm) | 87.8 ± 9.4 | 104.1 ± 14.9 | <0.0001 |
| W-H ratio | 0.86 ± 0.1 | 0.87 ± 0.1 | 0.322 |
| SBP (mmHg) | 111.4 ± 10.9 | 117.1 ± 9.9 | 0.044 |
| DBP (mmHg) | 69.9 ± 6.5 | 70.6 ± 8.37 | 0.706 |
| Fasting Glucose (mg/dL) | 82.4 ± 6.8 | 88.4 ± 10.8 | 0.006 |
| Glucose AUC | 12,495 (9825–13,478) | 14,430 (12,480–16,733) | 0.0002 |
| Insulin AUC | 5564 (4038–8338) | 11,390 (7771–14,985) | <0.0001 |
| Fasting Insulin (mU/L) | 8.5 ± 5.1 | 16.6 ± 9.7 | <0.0001 |
| HOMA-IR index | 1.7 (0.85–2.35) | 3.1 (2–4.9) | <0.0001 |
| Total Cholesterol (mg/dL) | 161.5 ± 30.5 | 172.1 ± 32.5 | 0.207 |
| HDL-Cholesterol (mg/dL) | 56.2 (40.4–65) | 43.4 (36.3–52.1) | 0.05 |
| LDL-Cholesterol (mg/dL) | 89.5 (65.5–89.5) | 102 (75.6–114.5) | 0.425 |
| Triglycerides (mg/dL) | 84.7 ± 38.4 | 147.5 ± 165.4 | 0.005 |
| LDH (U/L) | 165.9 ± 37 | 177.4 ± 32.8 | 0.107 |
| AST (U/L) | 27.7 ± 9.9 | 31.6 ± 12.9 | 0.247 |
| Parameter | Control (n = 29) | PCOS (n = 29) | p-Value * |
|---|---|---|---|
| Testosterone (ng/mL) | 0.56 (0.44–0.7) | 0.65 (0.5–0.8) | 0.13 |
| SHBG (nmol/L) | 43.6 (36.3–70.5) | 27.3 (19–35.9) | <0.0001 |
| FAI (%) | 4.34 (2.5–5.1) | 8.1 (6–12.7) | <0.0001 |
| Androstenedione (ng/mL) | 3 (2.3–4.2) | 3.6 (2.7–4.9) | 0.13 |
| FSH (mUI/mL) | 5.8 (5.1–7.6) | 5.4 (4.2–6.1) | 0.06 |
| LH (mUI/mL) | 4.3 (3.2–6.1) | 4.3 (2.8–6.1) | 0.99 |
| Estradiol (pg/mL) | 64.7 (51.6–84.6) | 68 (51.3–94.6) | 0.63 |
| 17-OHP (ng/mL) | 1.3 (0.98–1.55) | 1.3 (1–1.6) | 0.95 |
| AMH (ng/mL) | 3.9 ± 2.6 | 5.0 ± 3.3 | 0.16 |
| Parameter | Control (n = 29) | PCOS (n = 29) | p-Value * |
|---|---|---|---|
| Serum Folate (ng/mL) | 12.1 ± 3.2 | 13 ± 4.9 | 0.353 |
| RBC Folate (ng/mL) | 334.6 ± 114.3 | 383.7 ± 114.1 | 0.107 |
| Vitamin B12 (pg/mL) | 301 (247–355) | 275 (235–347) | 0.455 |
| Homocysteine (µmol/L) | 18.6 ± 6.6 | 23.2 ± 4.3 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco-Cabezas, M.; Assmann, T.S.; Martínez, P.; Cerpa, L.; Calfunao, S.; Echiburú, B.; Maliqueo, M.; Crisosto, N.; Salas-Pérez, F. Folate and Vitamin B12 Levels in Chilean Women with PCOS and Their Association with Metabolic Outcomes. Nutrients 2024, 16, 1937. https://doi.org/10.3390/nu16121937
Carrasco-Cabezas M, Assmann TS, Martínez P, Cerpa L, Calfunao S, Echiburú B, Maliqueo M, Crisosto N, Salas-Pérez F. Folate and Vitamin B12 Levels in Chilean Women with PCOS and Their Association with Metabolic Outcomes. Nutrients. 2024; 16(12):1937. https://doi.org/10.3390/nu16121937
Chicago/Turabian StyleCarrasco-Cabezas, Matías, Taís Silveira Assmann, Paz Martínez, Leslie Cerpa, Susan Calfunao, Bárbara Echiburú, Manuel Maliqueo, Nicolás Crisosto, and Francisca Salas-Pérez. 2024. "Folate and Vitamin B12 Levels in Chilean Women with PCOS and Their Association with Metabolic Outcomes" Nutrients 16, no. 12: 1937. https://doi.org/10.3390/nu16121937
APA StyleCarrasco-Cabezas, M., Assmann, T. S., Martínez, P., Cerpa, L., Calfunao, S., Echiburú, B., Maliqueo, M., Crisosto, N., & Salas-Pérez, F. (2024). Folate and Vitamin B12 Levels in Chilean Women with PCOS and Their Association with Metabolic Outcomes. Nutrients, 16(12), 1937. https://doi.org/10.3390/nu16121937






