Selenomethionine Inhibited HADV-Induced Apoptosis Mediated by ROS through the JAK-STAT3 Signaling Pathway
Abstract
:1. Introduction
2. Results and Discussion
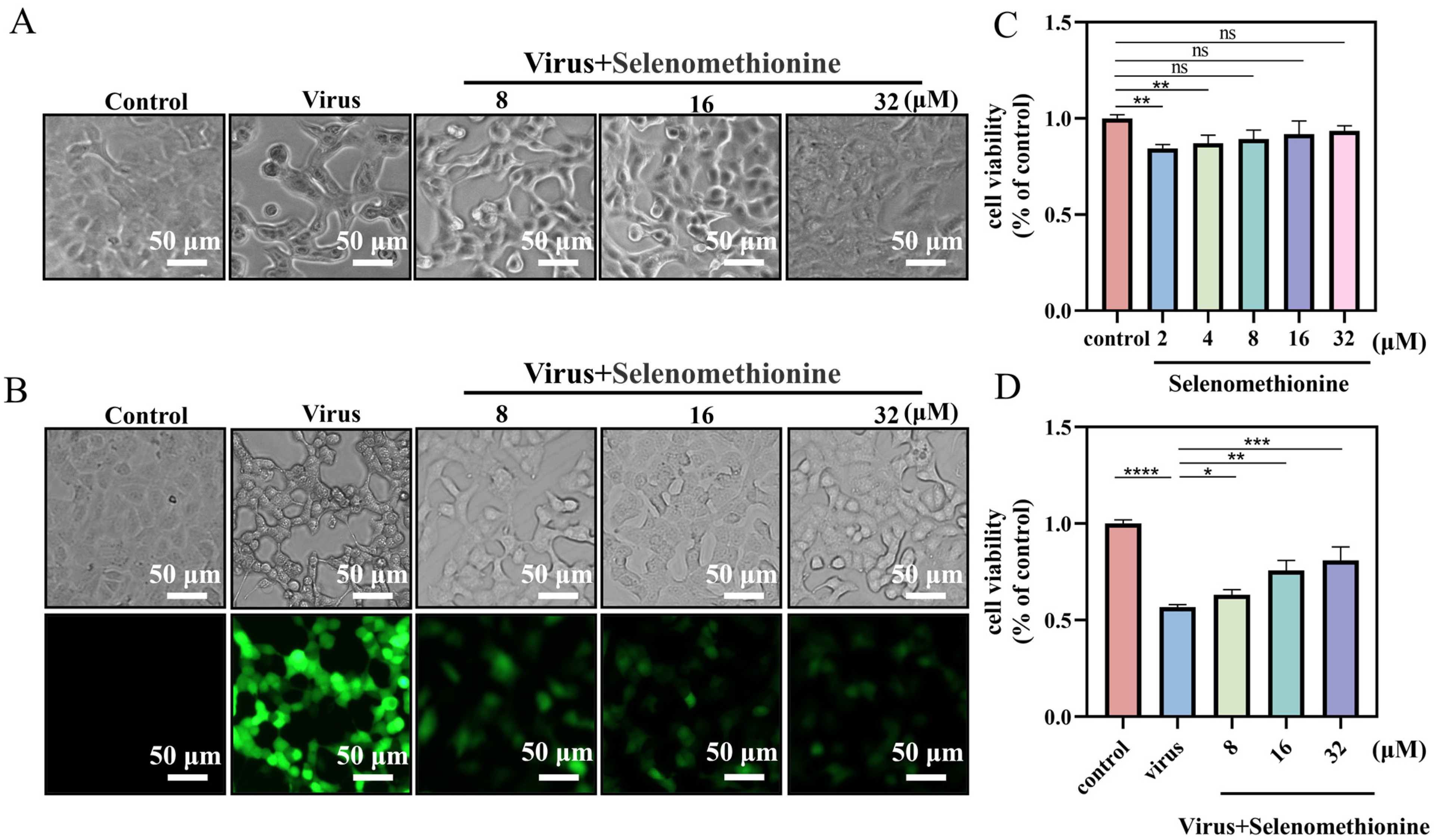
2.1. Detection of Optimal Low-Toxicity Antiviral Concentrations of Selenomethionine
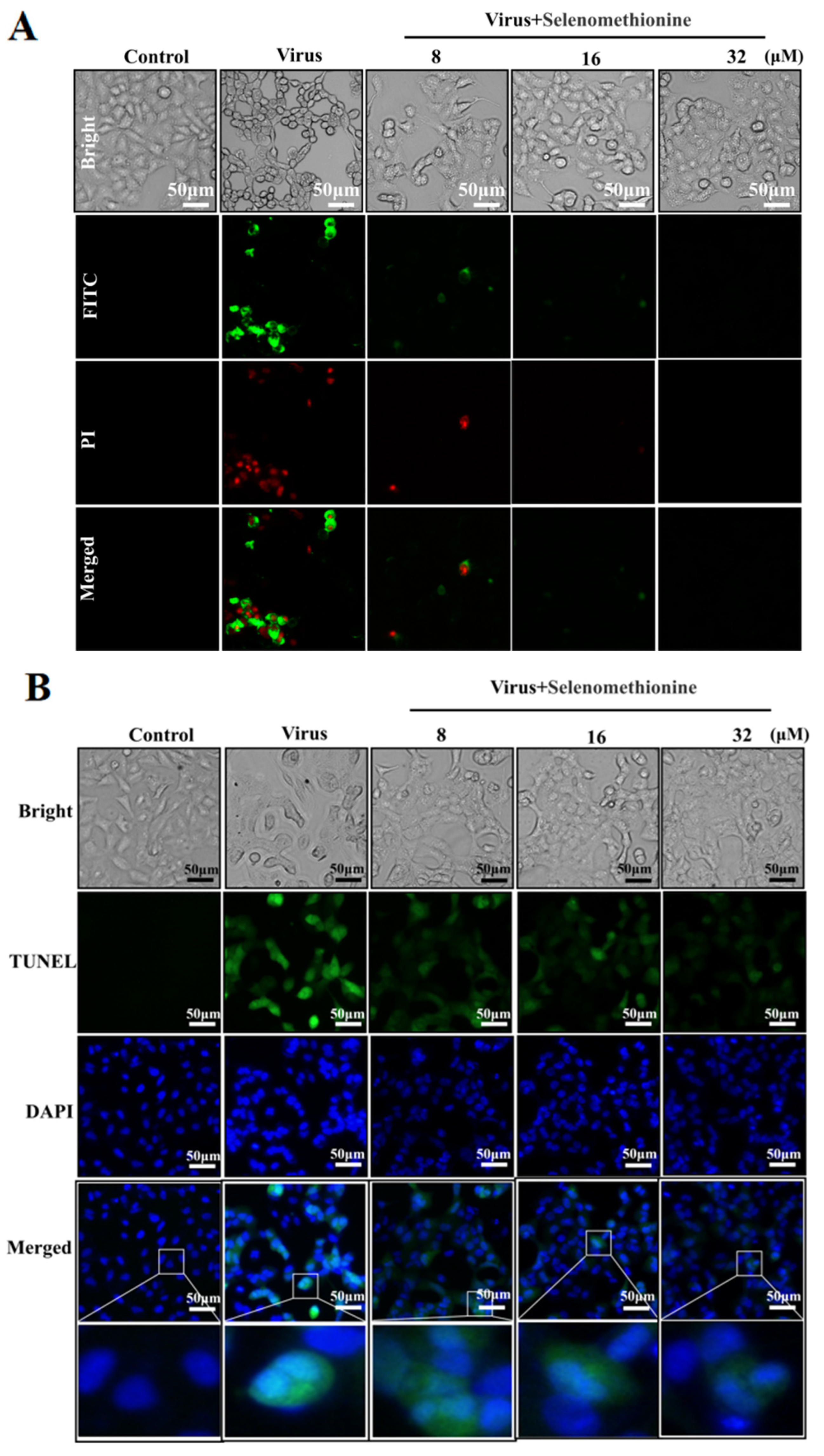
2.2. Detection of Selenomethionine Regulates Cell Membrane Phosphatidylserine Translocation and Cell Membrane Permeability
2.3. Detection of Inhibition of DNA Fragmentation Damage by Selenomethionine
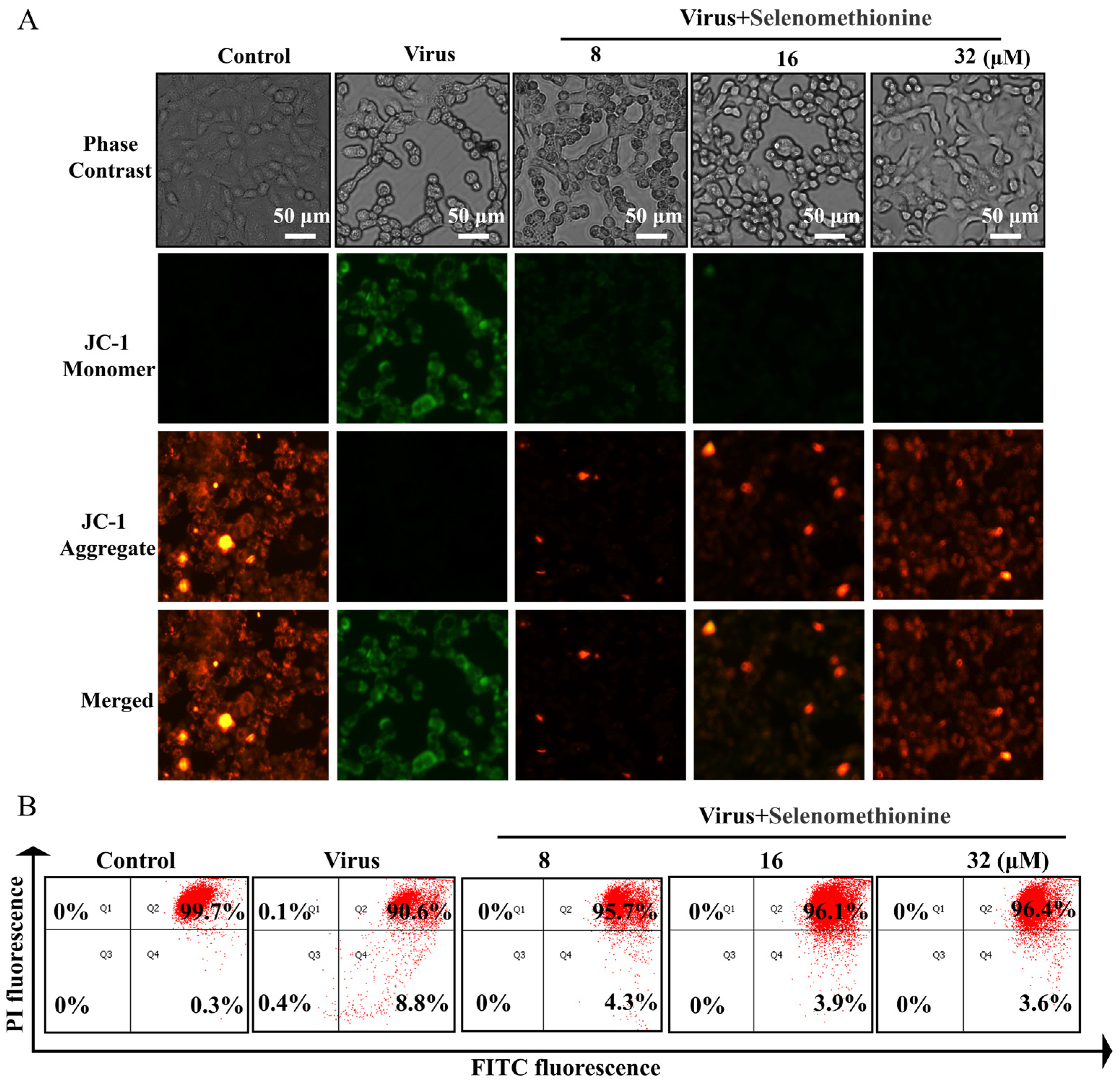
2.4. Detection of Selenomethionine Altered Mitochondrial Membrane Potential (∆Ψm)
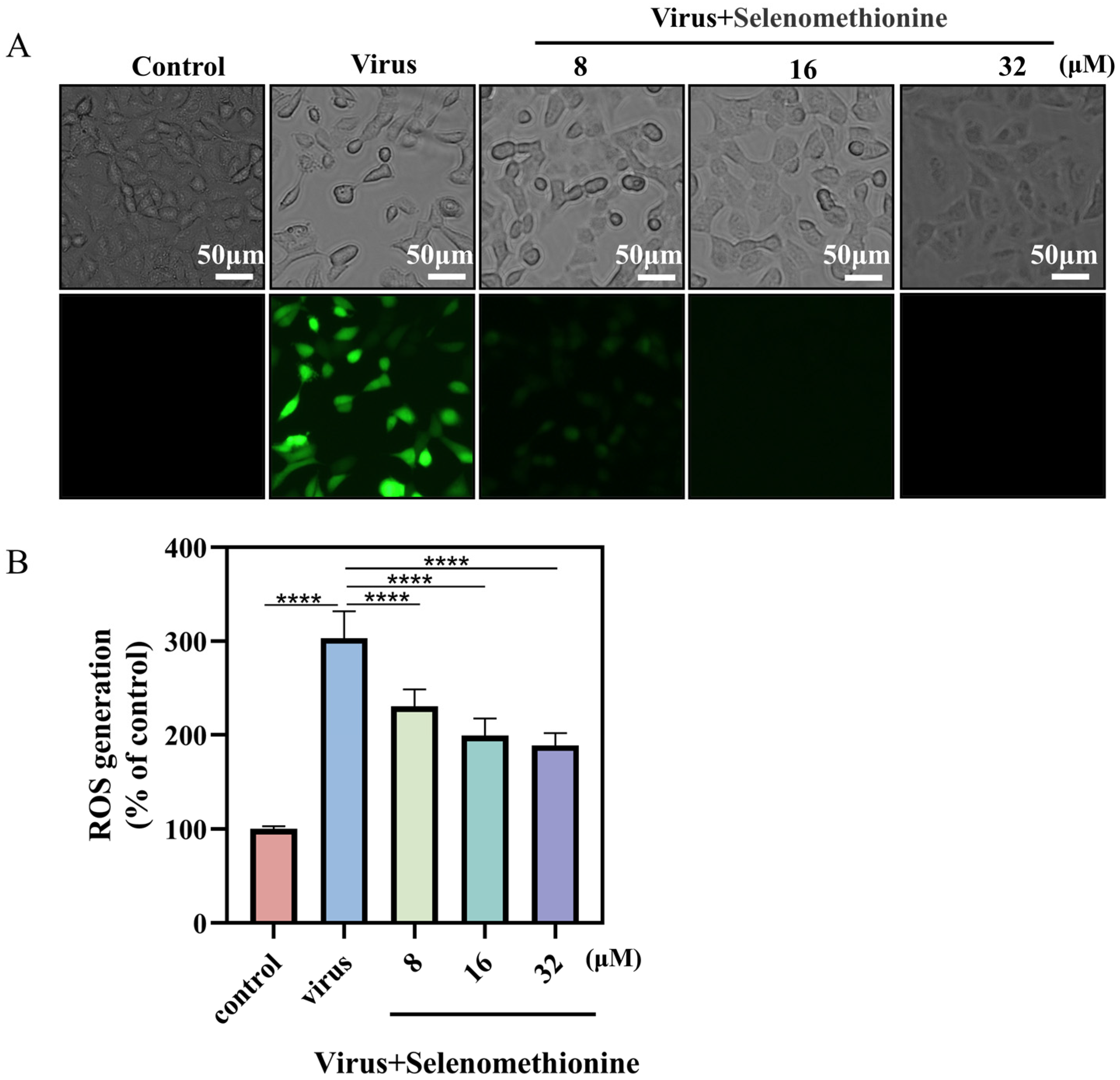
2.5. Detection of Selenomethionine Inhibits ROS Generation
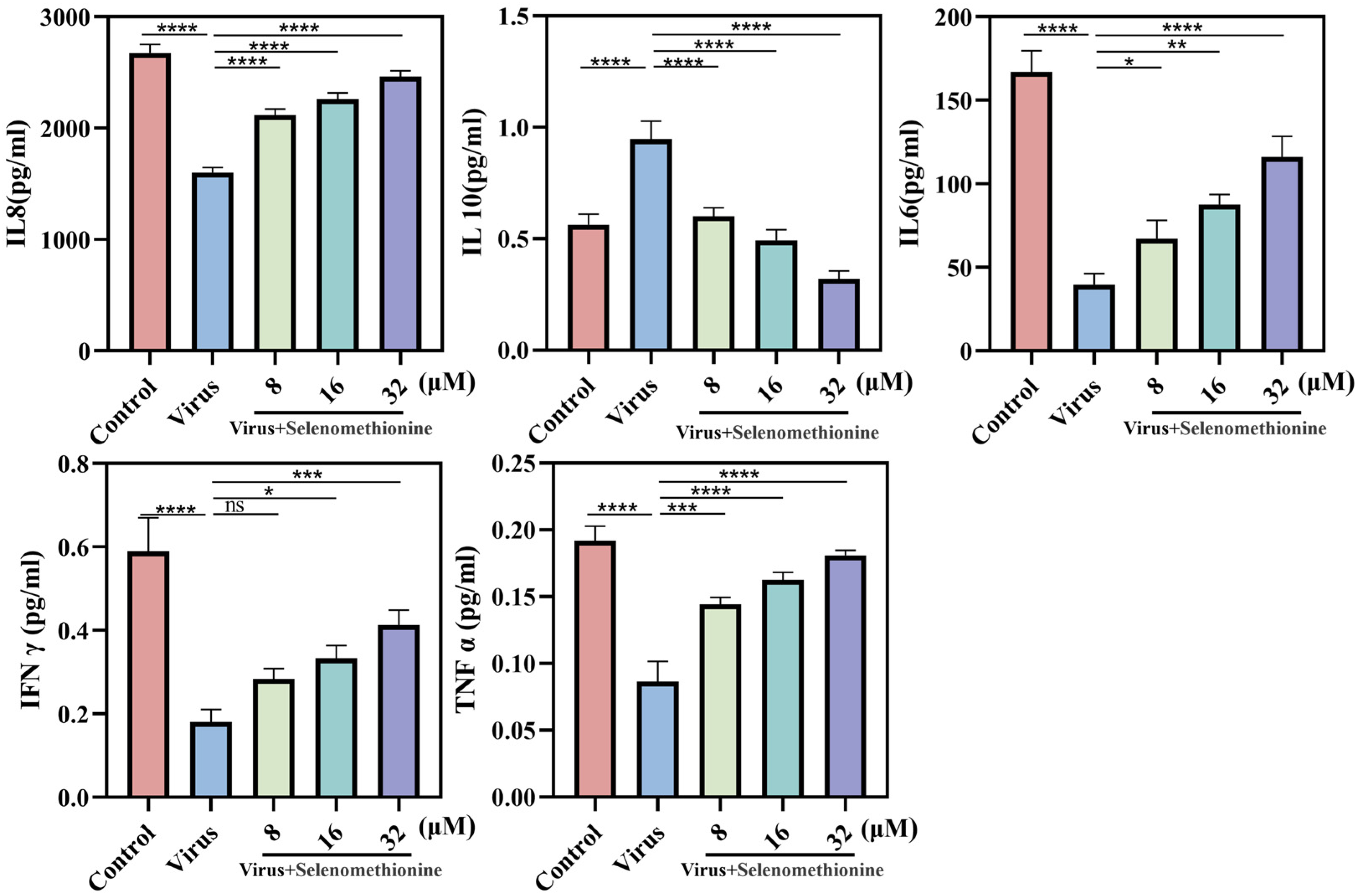
2.6. Detection of Cytokines in Cell Culture Supernatants
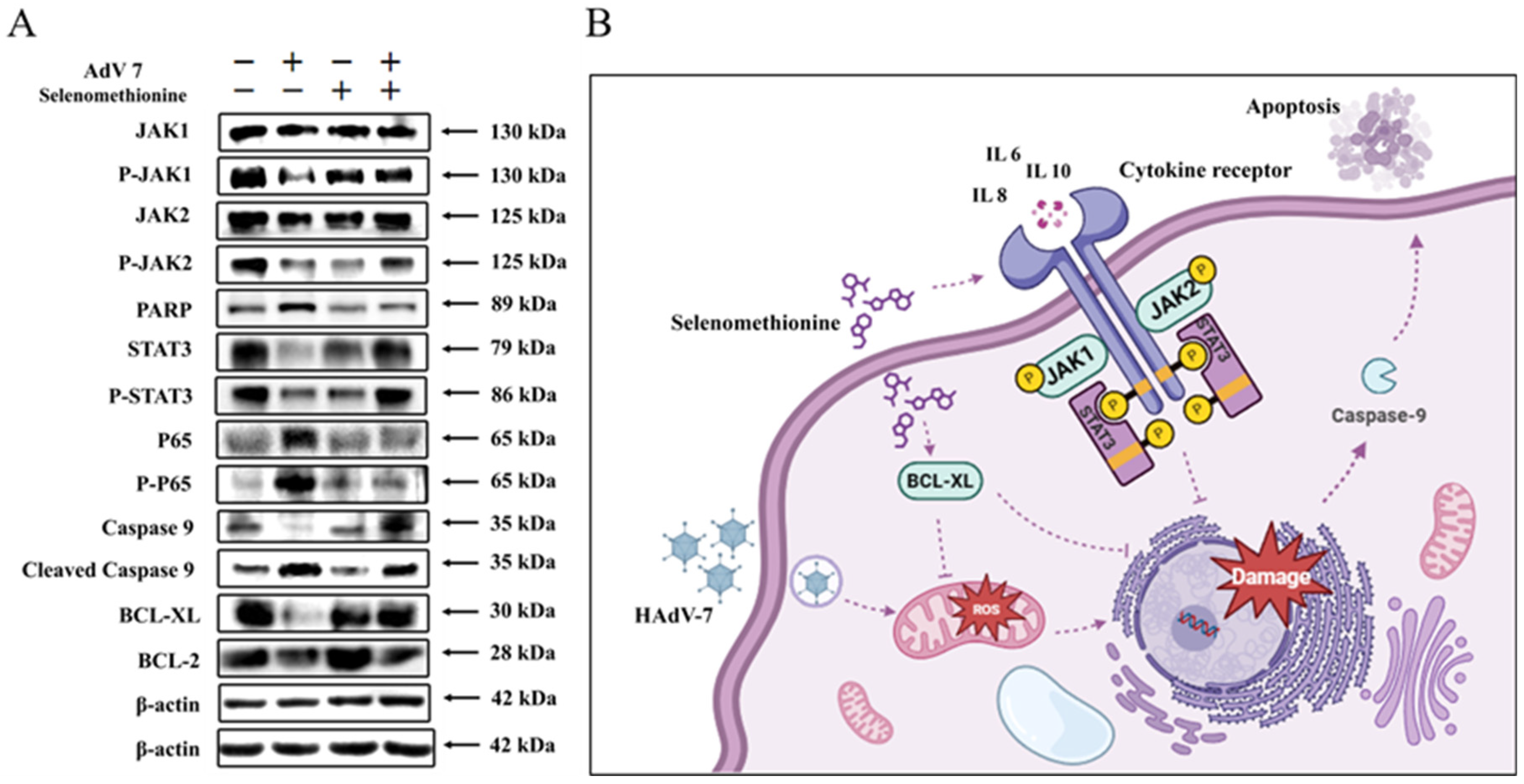
2.7. Inhibition of Apoptosis Signaling Pathways by Selenomethionine
3. Materials and Methods
3.1. Materials
3.2. Detection of Selenomethionine Toxicity by CCK-8 Assay
3.3. TCID50 Determination of HAdV-7 and rAD7EGFP Viruses
3.4. Fluorescence Microscopy of Antiviral Efficiency
3.5. Detection of Cell Membrane Permeability and Phosphatidylserine Ectopy by Flow Cytometry
3.6. TUNEL-DAPI Double Staining Method
3.7. Detection of Cytokines
3.8. Detection of Mitochondrial Membrane Potential
3.9. Detection of Reactive Oxygen Species (ROS) Levels
3.10. Western Blot
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lion, T. Adenovirus infections in immunocompetent and immunocompromised patients. Clin. Microbiol. Rev. 2014, 27, 441–462. [Google Scholar] [CrossRef]
- Bot, A.; Smith, K. International reviews of immunology: Expanding the scope and content. Int. Rev. Immunol. 2008, 27, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P., 3rd; Kajon, A.E. Adenovirus: Epidemiology, Global Spread of Novel Serotypes, and Advances in Treatment and Prevention. Semin. Respir. Crit. Care Med. 2016, 37, 586–602. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.F.; Lee, C.Y. Adenoviruses types, cell receptors and local innate cytokines in adenovirus infection. Int. Rev. Immunol. 2014, 33, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Ison, M.G. Adenovirus infections in transplant recipients. Clin. Infect. Dis. 2006, 43, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Choi, E.H.; Lee, H.J. Comprehensive serotyping and epidemiology of human adenovirus isolated from the respiratory tract of Korean children over 17 consecutive years (1991–2007). J. Med. Virol. 2010, 82, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Erard, V.; Huang, M.L.; Ferrenberg, J.; Nguy, L.; Stevens-Ayers, T.L.; Hackman, R.C.; Corey, L.; Boeckh, M. Quantitative real-time polymerase chain reaction for detection of adenovirus after T cell-replete hematopoietic cell transplantation: Viral load as a marker for invasive disease. Clin. Infect. Dis. 2007, 45, 958–965. [Google Scholar] [CrossRef] [PubMed]
- Lankester, A.C.; Heemskerk, B.; Claas, E.C.; Schilham, M.W.; Beersma, M.F.; Bredius, R.G.; van Tol, M.J.; Kroes, A.C. Effect of ribavirin on the plasma viral DNA load in patients with disseminating adenovirus infection. Clin. Infect. Dis. 2004, 38, 1521–1525. [Google Scholar] [CrossRef] [PubMed]
- Dodge, M.J.; MacNeil, K.M.; Tessier, T.M.; Weinberg, J.B.; Mymryk, J.S. Emerging antiviral therapeutics for human adenovirus infection: Recent developments and novel strategies. Antivir. Res. 2021, 188, 105034. [Google Scholar] [CrossRef] [PubMed]
- Bordigoni, P.; Carret, A.S.; Venard, V.; Witz, F.; Le Faou, A. Treatment of adenovirus infections in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2001, 32, 1290–1297. [Google Scholar] [CrossRef]
- Chemaly, R.F.; Hill, J.A.; Voigt, S.; Peggs, K.S. In vitro comparison of currently available and investigational antiviral agents against pathogenic human double-stranded DNA viruses: A systematic literature review. Antivir. Res. 2019, 163, 50–58. [Google Scholar] [CrossRef]
- Ko, J.H.; Lim, J.U.; Choi, J.Y.; Oh, H.S.; Yoo, H.; Jhun, B.W.; Huh, K.; Peck, K.R. Early cidofovir administration might be associated with a lower probability of respiratory failure in treating human adenovirus pneumonia: A retrospective cohort study. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020, 26, 646.e9–646.e14. [Google Scholar] [CrossRef]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Griffiths, N.M.; Stewart, R.D.; Robinson, M.F. The metabolism of [75Se]selenomethionine in four women. Br. J. Nutr. 1976, 35, 373–382. [Google Scholar] [CrossRef]
- Hadrup, N.; Ravn-Haren, G. Absorption, distribution, metabolism and excretion (ADME) of oral selenium from organic and inorganic sources: A review. J. Trace Elem. Med. Biol. 2021, 67, 126801. [Google Scholar] [CrossRef]
- Hariharan, S.; Dharmaraj, S. Selenium and selenoproteins: It’s role in regulation of inflammation. Inflammopharmacology 2020, 28, 667–695. [Google Scholar] [CrossRef]
- Aaseth, J.; Alexander, J.; Alehagen, U.; Tinkov, A.; Skalny, A.; Larsson, A.; Crisponi, G.; Nurchi, V.M. The Aging Kidney-As Influenced by Heavy Metal Exposure and Selenium Supplementation. Biomolecules 2021, 11, 1078. [Google Scholar] [CrossRef]
- Guillin, O.M.; Vindry, C.; Ohlmann, T.; Chavatte, L. Selenium, Selenoproteins and Viral Infection. Nutrients 2019, 11, 2101. [Google Scholar] [CrossRef]
- Tolen, J.A.; Duerksen-Hughes, P.; Lau, K.; Chan, P.J. Selenium Attenuates HPV-18 Associated Apoptosis in Embryo-Derived Trophoblastic Cells but Not Inner Cell Mass In Vitro. Int. J. Reprod. Med. 2015, 2015, 562567. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxid. Med. Cell Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef] [PubMed]
- Gorini, F.; Sabatino, L.; Pingitore, A.; Vassalle, C. Selenium: An Element of Life Essential for Thyroid Function. Molecules 2021, 26, 7084. [Google Scholar] [CrossRef]
- Roulston, A.; Marcellus, R.C.; Branton, P.E. Viruses and apoptosis. Annu. Rev. Microbiol. 1999, 53, 577–628. [Google Scholar] [CrossRef]
- Galluzzi, L.; Brenner, C.; Morselli, E.; Touat, Z.; Kroemer, G. Viral control of mitochondrial apoptosis. PLoS Pathog. 2008, 4, e1000018. [Google Scholar] [CrossRef]
- Kvansakul, M. Viral Infection and Apoptosis. Viruses 2017, 9, 356. [Google Scholar] [CrossRef]
- Orzalli, M.H.; Kagan, J.C. Apoptosis and Necroptosis as Host Defense Strategies to Prevent Viral Infection. Trends Cell Biol. 2017, 27, 800–809. [Google Scholar] [CrossRef]
- Tran, T.T.P.; Tran, T.H.; Kremer, E.J. IgG-Complexed Adenoviruses Induce Human Plasmacytoid Dendritic Cell Activation and Apoptosis. Viruses 2021, 13, 1699. [Google Scholar] [CrossRef]
- Ren, Z.; Jia, G.; He, H.; Ding, T.; Yu, Y.; Zuo, Z.; Hu, Y.; Zhong, Z.; Yu, S.; Deng, H.; et al. Antiviral Effect of Selenomethionine on Porcine Deltacoronavirus in Pig Kidney Epithelial Cells. Front. Microbiol. 2022, 13, 846747. [Google Scholar] [CrossRef]
- Melo-Silveira, R.F.; Viana, R.L.S.; Sabry, D.A.; da Silva, R.A.; Machado, D.; Nascimento, A.K.L.; Scortecci, K.C.; Ferreira-Halder, C.V.; Sassaki, G.L.; Rocha, H.A.O. Antiproliferative xylan from corn cobs induces apoptosis in tumor cells. Carbohydr. Polym. 2019, 210, 245–253. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio Protoc. 2019, 9, e3128. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial membrane potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, X.; Huang, J.; Huan, C.; Li, C. H(2)O(2)-induced oxidative stress impairs meat quality by inducing apoptosis and autophagy via ROS/NF-κB signaling pathway in broiler thigh muscle. Poult. Sci. 2022, 101, 101759. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Et. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Zlotnik, A.; Yoshie, O. The chemokine superfamily revisited. Immunity 2012, 36, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.M.; Clark-Garvey, S.; Porcu, P.; Eischen, C.M. Targeting the Bcl-2 Family in B Cell Lymphoma. Front. Oncol. 2018, 8, 636. [Google Scholar] [CrossRef]
- Wyżewski, Z.; Świtlik, W.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P. The Role of Bcl-xL Protein in Viral Infections. Int. J. Mol. Sci. 2021, 22, 1956. [Google Scholar] [CrossRef]
- Hu, Y.W.; Chen, Z.P.; Hu, X.M.; Zhao, J.Y.; Huang, J.L.; Ma, X.; Li, S.F.; Qiu, Y.R.; Wu, X.J.; Sha, Y.H.; et al. The miR-573/apoM/Bcl2A1-dependent signal transduction pathway is essential for hepatocyte apoptosis and hepatocarcinogenesis. Apoptosis. 2015, 20, 1321–1337. [Google Scholar] [CrossRef]
- Isa, A.; Lundqvist, A.; Lindblom, A.; Tolfvenstam, T.; Broliden, K. Cytokine responses in acute and persistent human parvovirus B19 infection. Clin. Exp. Immunol. 2007, 147, 419–425. [Google Scholar] [CrossRef]
- Butturini, E.; Carcereri de Prati, A.; Mariotto, S. Redox Regulation of STAT1 and STAT3 Signaling. Int. J. Mol. Sci. 2020, 21, 7034. [Google Scholar] [CrossRef]
- Mi, Y.; Zhang, J.; Chen, Y.; Sun, X.; Tan, W.; Li, Q.; Guo, Z. New synthetic chitosan derivatives bearing benzenoid/heterocyclic moieties with enhanced antioxidant and antifungal activities. Carbohydr. Polym. 2020, 249, 116847. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Q.; Wang, Y.; Dawson, L.W.; Ciampa, G.; Zhao, W.; Zhang, G.; Chen, B.; Weiss, R.M.; Grueter, C.E.; et al. Gene Therapy With the N-Terminus of Junctophilin-2 Improves Heart Failure in Mice. Circ. Res. 2022, 130, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chipot, C.; Scheuring, S. Annexin-V stabilizes membrane defects by inducing lipid phase transition. Nat. Commun. 2020, 11, 230. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.Y.; Tong, L.; Li, M.D.; Fu, C.H.; Peng, J.B.; Ji, L.L. miR-142 downregulation alleviates rat PTSD-like behaviors, reduces the level of inflammatory cytokine expression and apoptosis in hippocampus, and upregulates the expression of fragile X mental retardation protein. J. Neuroinflammation 2021, 18, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, L.; Ndong, J.C.; Hettinghouse, A.; Sun, G.; Chen, C.; Zhang, C.; Liu, R.; Liu, C.J. Progranulin deficiency exacerbates spinal cord injury by promoting neuroinflammation and cell apoptosis in mice. J. Neuroinflammation 2019, 16, 238. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.J.; Liu, Q.; Li, Y.; Peng, F.F.; Wang, S.; Zhang, Z.; Liu, H.; Yu, H.; Tao, S.; Zhang, B.F. Regulator of calcineurin 1 deletion attenuates mitochondrial dysfunction and apoptosis in acute kidney injury through JNK/Mff signaling pathway. Cell Death Dis. 2022, 13, 774. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Yang, J.; Liu, C.; Wang, S.; Zhang, C.; Zhao, H.; Du, H.; Geng, X. Hypoxia-Inducible Factor 1α and 2α Have Beneficial Effects in Remote Ischemic Preconditioning Against Stroke by Modulating Inflammatory Responses in Aged Rats. Front. Aging Neurosci. 2020, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Song, Y.; Hsiao, T.Y.; He, J.; Wang, C.; Shen, J.; MacLachlan, A.; Dai, S.; Singer, B.H.; Kurabayashi, K.; et al. Machine-Learning-Assisted Microfluidic Nanoplasmonic Digital Immunoassay for Cytokine Storm Profiling in COVID-19 Patients. ACS Nano 2021, 15, 18023–18036. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, X.F. Production of a codonopsis polysaccharide iron complex and evaluation of its properties. Int. J. Biol. Macromol. 2020, 162, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Lone, M.I.; Nabi, A.; Dar, N.J.; Hussain, A.; Nazam, N.; Hamid, A.; Ahmad, W. Toxicogenetic evaluation of dichlorophene in peripheral blood and in the cells of the immune system using molecular and flow cytometric approaches. Chemosphere 2017, 167, 520–529. [Google Scholar] [CrossRef]
- Hu, L.; Ding, M.; Tang, D.; Gao, E.; Li, C.; Wang, K.; Qi, B.; Qiu, J.; Zhao, H.; Chang, P.; et al. Targeting mitochondrial dynamics by regulating Mfn2 for therapeutic intervention in diabetic cardiomyopathy. Theranostics 2019, 9, 3687–3706. [Google Scholar] [CrossRef]
- Li, N.; Zhou, H.; Wu, H.; Wu, Q.; Duan, M.; Deng, W.; Tang, Q. STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol. 2019, 24, 101215. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Liu, X.; Li, J.; Lai, J.; Su, J.; Zhu, B.; Gao, B.; Li, Y.; Zhao, M. Selenomethionine Inhibited HADV-Induced Apoptosis Mediated by ROS through the JAK-STAT3 Signaling Pathway. Nutrients 2024, 16, 1966. https://doi.org/10.3390/nu16121966
Li C, Liu X, Li J, Lai J, Su J, Zhu B, Gao B, Li Y, Zhao M. Selenomethionine Inhibited HADV-Induced Apoptosis Mediated by ROS through the JAK-STAT3 Signaling Pathway. Nutrients. 2024; 16(12):1966. https://doi.org/10.3390/nu16121966
Chicago/Turabian StyleLi, Chuqing, Xia Liu, Jiali Li, Jia Lai, Jingyao Su, Bing Zhu, Buyun Gao, Yinghua Li, and Mingqi Zhao. 2024. "Selenomethionine Inhibited HADV-Induced Apoptosis Mediated by ROS through the JAK-STAT3 Signaling Pathway" Nutrients 16, no. 12: 1966. https://doi.org/10.3390/nu16121966




