The Study of Myo-Inositol’s Anxiolytic Activity on Zebrafish (Danio rerio)
Abstract
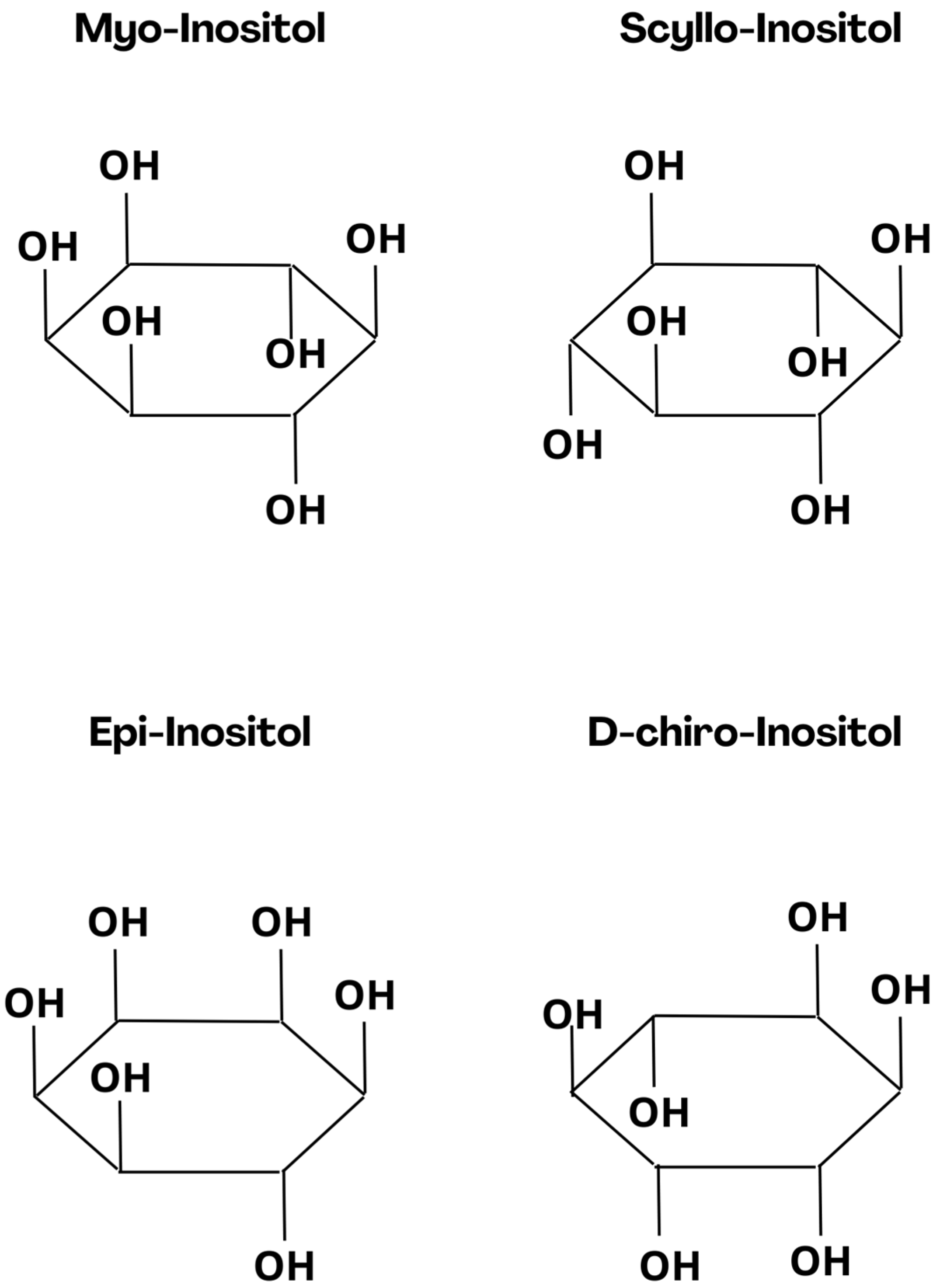
1. Introduction
2. Materials and Methods
2.1. The Fish Maintenance and Ethic Statement
2.2. Zebrafish Spawning, Embryo Selection, and Larvae Incubation
2.3. Embryo Movement Analysis
2.4. Larvae Behavioural Assessment
2.5. Statistical Analysis
3. Results
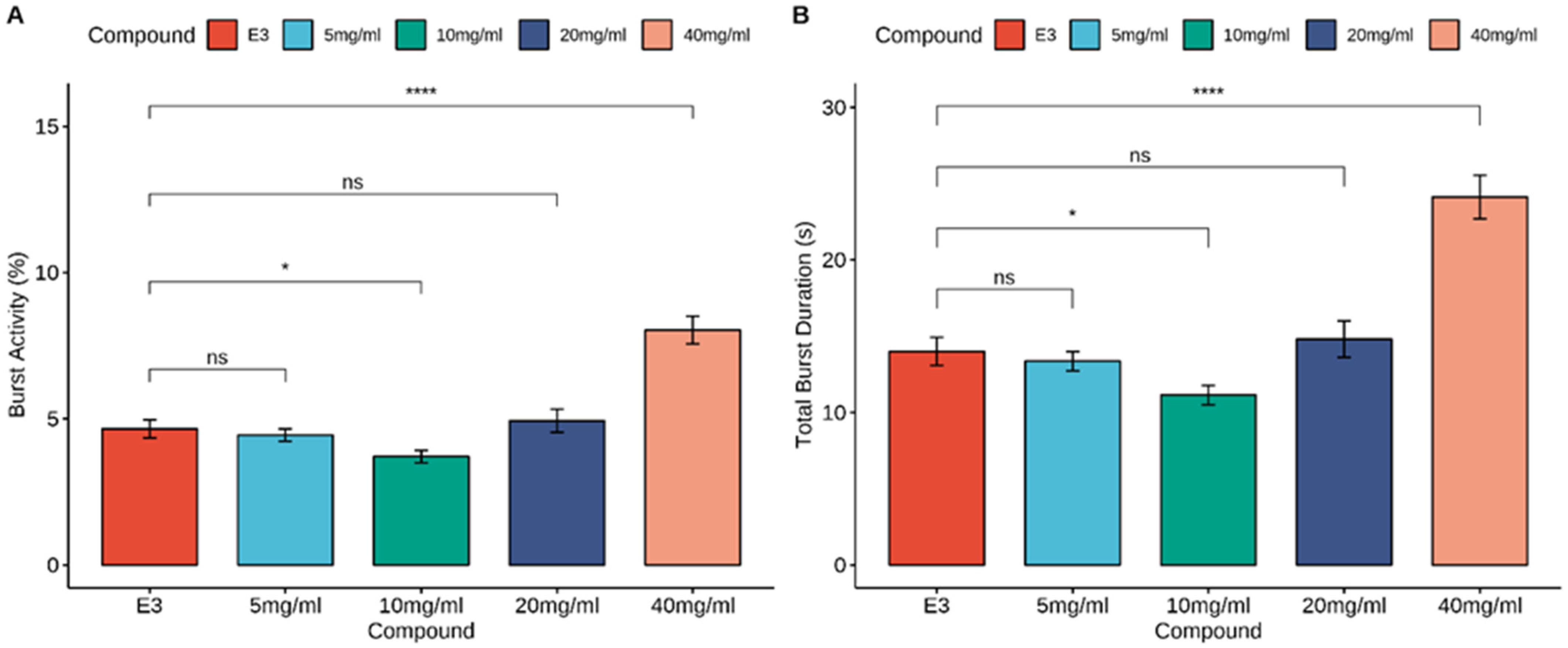
3.1. The Movement Analysis of Embryos
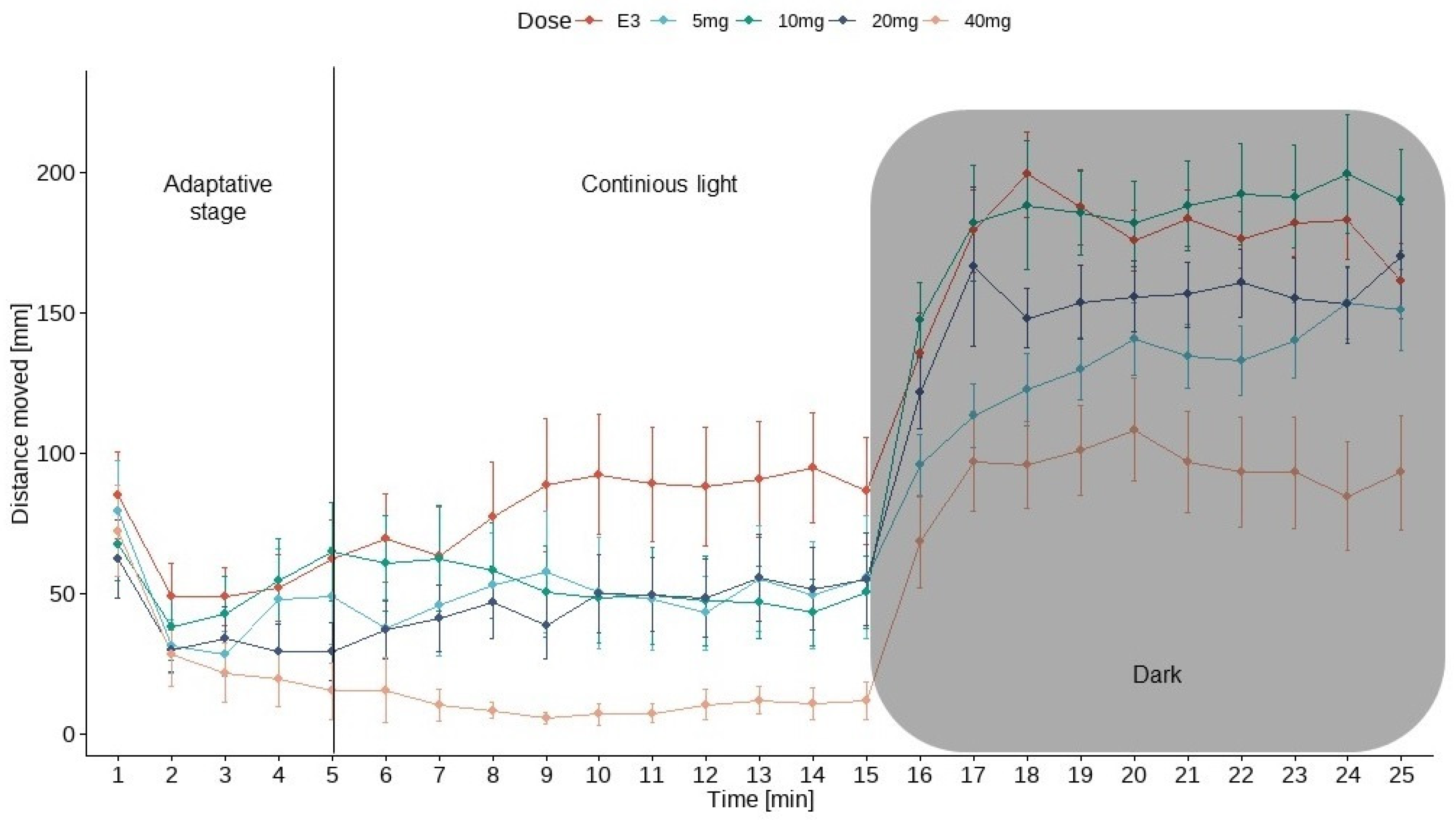
3.2. Larvae Locomotor Analysis Using Dark-Light Test
3.2.1. Larvae Locomotor Activity
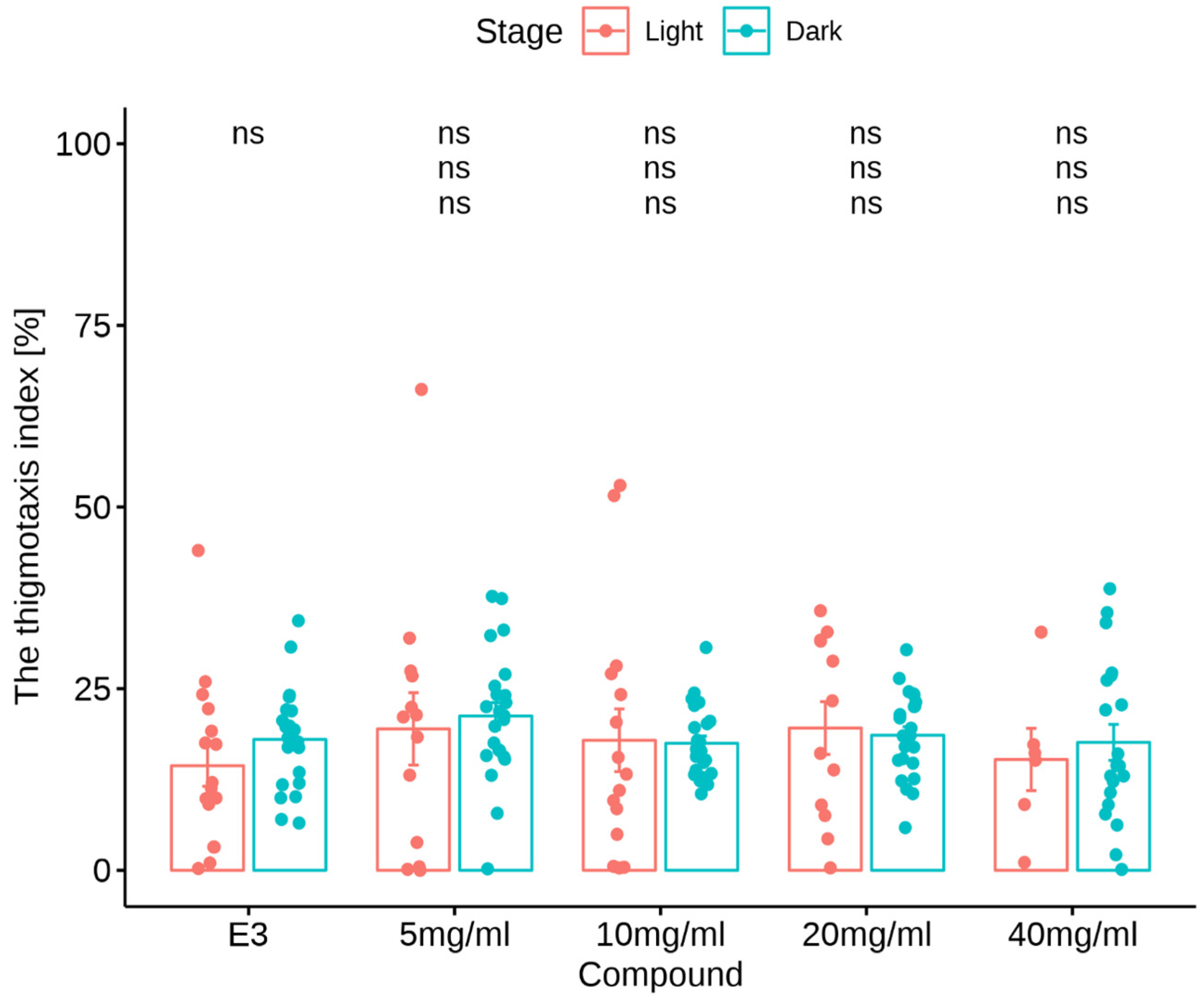
3.2.2. Thigmotaxis Index
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DCI | D-chiro-Inositol |
| EI | Epi-Inositol |
| FET | fish embryo toxicity |
| hpf | hours post fertilization |
| MI | Myo-Inositol |
| PCOS | Polycystic Ovary Syndrome |
| SI | Scyllo-Inositol |
| SEM | standard error of the mean |
| VMR | visual motor response |
References
- Antonowski, T.; Wiśniewski, K.; Podlasz, P.; Osowski, A.; Wojtkiewicz, J. Study of the Potential Hepatoprotective Effect of Myo-Inositol and Its Influence on Zebrafish Development. Nutrients 2021, 13, 3346. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Fuso, A.; Dinicola, S.; Cucina, A.; Bevilacqua, A. Pharmacodynamics and Pharmacokinetics of Inositol(s) in Health and Disease. Expert Opin. Drug Metab. Toxicol. 2016, 12, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Loewus, F.A.; Murthy, P.P.N. Myo-Inositol Metabolism in Plants. Plant Sci. 2000, 150, 1–19. [Google Scholar] [CrossRef]
- Owczarczyk-Saczonek, A.; Lahuta, L.; Ligor, M.; Placek, W.; Górecki, R.; Buszewski, B. The Healing-Promoting Properties of Selected Cyclitols—A Review. Nutrients 2018, 10, 1891. [Google Scholar] [CrossRef] [PubMed]
- Fontana, B.D.; Müller, T.E.; Cleal, M.; De Abreu, M.S.; Norton, W.H.J.; Demin, K.A.; Amstislavskaya, T.G.; Petersen, E.V.; Kalueff, A.V.; Parker, M.O.; et al. Using Zebrafish (Danio Rerio) Models to Understand the Critical Role of Social Interactions in Mental Health and Wellbeing. Prog. Neurobiol. 2022, 208, 101993. [Google Scholar] [CrossRef] [PubMed]
- Daly, M.; Robinson, E. Depression and Anxiety during COVID-19. Lancet 2022, 399, 518. [Google Scholar] [CrossRef] [PubMed]
- Santomauro, D.F.; Mantilla Herrera, A.M.; Shadid, J.; Zheng, P.; Ashbaugh, C.; Pigott, D.M.; Abbafati, C.; Adolph, C.; Amlag, J.O.; Aravkin, A.Y.; et al. Global Prevalence and Burden of Depressive and Anxiety Disorders in 204 Countries and Territories in 2020 Due to the COVID-19 Pandemic. Lancet 2021, 398, 1700–1712. [Google Scholar] [CrossRef] [PubMed]
- Saeed, H.; Eslami, A.; Nassif, N.T.; Simpson, A.M.; Lal, S. Anxiety Linked to COVID-19: A Systematic Review Comparing Anxiety Rates in Different Populations. Int. J. Environ. Res. Public Health 2022, 19, 2189. [Google Scholar] [CrossRef]
- Sarris, J.; Ravindran, A.; Yatham, L.N.; Marx, W.; Rucklidge, J.J.; McIntyre, R.S.; Akhondzadeh, S.; Benedetti, F.; Caneo, C.; Cramer, H.; et al. Clinician Guidelines for the Treatment of Psychiatric Disorders with Nutraceuticals and Phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. World J. Biol. Psychiatry 2022, 23, 424–455. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, B.; Bao, M.; Shen, J.; Zheng, P.; Wu, D.; Wang, J.; Li, Z.; Jiang, K. Early Life Exposure to Chronic Unpredictable Stress Induces Anxiety-like Behaviors and Increases the Excitability of Cerebellar Neurons in Zebrafish. Behav. Brain Res. 2023, 437, 114160. [Google Scholar] [CrossRef]
- Ramezani, M.; Simani, L.; Karimialavijeh, E.; Rezaei, O.; Hajiesmaeili, M.; Pakdaman, H. The Role of Anxiety and Cortisol in Outcomes of Patients With COVID-19. Basic Clin. Neurosci. J. 2020, 11, 179–184. [Google Scholar] [CrossRef]
- Ansara, E.D. Management of Treatment-Resistant Generalized Anxiety Disorder. Ment. Health Clin. 2020, 10, 326–334. [Google Scholar] [CrossRef]
- Streba, L.; Gheonea, D.I.; Schenker, M. Current Trends in Cancer Management; IntechOpen: London, UK, 2019; ISBN 978-1-83880-005-5. [Google Scholar]
- Schnörr, S.J.; Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Measuring Thigmotaxis in Larval Zebrafish. Behav. Brain Res. 2012, 228, 367–374. [Google Scholar] [CrossRef]
- Bühler, A.; Carl, M. Zebrafish Tools for Deciphering Habenular Network-Linked Mental Disorders. Biomolecules 2021, 11, 324. [Google Scholar] [CrossRef]
- Rosa, J.G.S.; Lima, C.; Lopes-Ferreira, M. Zebrafish Larvae Behavior Models as a Tool for Drug Screenings and Pre-Clinical Trials: A Review. Int. J. Mol. Sci. 2022, 23, 6647. [Google Scholar] [CrossRef] [PubMed]
- Champagne, D.L.; Hoefnagels, C.C.M.; De Kloet, R.E.; Richardson, M.K. Translating Rodent Behavioral Repertoire to Zebrafish (Danio Rerio): Relevance for Stress Research. Behav. Brain Res. 2010, 214, 332–342. [Google Scholar] [CrossRef]
- Selderslaghs, I.W.T.; Hooyberghs, J.; De Coen, W.; Witters, H.E. Locomotor Activity in Zebrafish Embryos: A New Method to Assess Developmental Neurotoxicity. Neurotoxicology Teratol. 2010, 32, 460–471. [Google Scholar] [CrossRef]
- Torres-Ruiz, M.; De Alba González, M.; Morales, M.; Martin-Folgar, R.; González, M.C.; Cañas-Portilla, A.I.; De La Vieja, A. Neurotoxicity and Endocrine Disruption Caused by Polystyrene Nanoparticles in Zebrafish Embryo. Sci. Total Environ. 2023, 874, 162406. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, K.; Antonowski, T.; Juranek, J.; Podlasz, P.; Wojtkiewicz, J. Antiepileptic Properties of Scyllo-Inositol on Pentylenetetrazol-Induced Seizures. Int. J. Mol. Sci. 2023, 24, 7598. [Google Scholar] [CrossRef]
- Sharma, P.; Rathore, P.; Seth, S.; Kaba, L.; Bhamawat, N.; Jain, P. Insulin Mimetic Potential of Hylocereus Undatus from Extracted Myo-Inositol and Proteins. Braz. J. Sci. 2023, 2, 12–18. [Google Scholar] [CrossRef]
- Pkhaladze, L.; Unfer, V.; Dewailly, D. Chapter 10—Use of Myo-Inositol in the Treatment of PCOS Symptoms in Adolescents. In A Clinical Guide to Inositols; Unfer, V., Dewailly, D., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 151–165. ISBN 978-0-323-91673-8. [Google Scholar]
- Rakic, D.; Jakovljevic, V.; Jovic, N.; Bicanin Ilic, M.; Dimitrijevic, A.; Vulovic, T.; Arsenijevic, P.; Sretenovic, J.; Nikolic, M.; Petrovich Fisenko, V.; et al. The Potential of SGLT-2 Inhibitors in the Treatment of Polycystic Ovary Syndrome: The Current Status and Future Perspectives. Biomedicines 2023, 11, 998. [Google Scholar] [CrossRef]
- Unfer, V.; Carlomagno, G.; Dante, G.; Facchinetti, F. Effects of Myo-Inositol in Women with PCOS: A Systematic Review of Randomized Controlled Trials. Gynecol. Endocrinol. 2012, 28, 509–515. [Google Scholar] [CrossRef]
- Zhao, H.; Xing, C.; Zhang, J.; He, B. Comparative Efficacy of Oral Insulin Sensitizers Metformin, Thiazolidinediones, Inositol, and Berberine in Improving Endocrine and Metabolic Profiles in Women with PCOS: A Network Meta-Analysis. Reprod. Health 2021, 18, 171. [Google Scholar] [CrossRef]
- Siracusa, L.; Napoli, E.; Ruberto, G. Novel Chemical and Biological Insights of Inositol Derivatives in Mediterranean Plants. Molecules 2022, 27, 1525. [Google Scholar] [CrossRef]
- Chiappelli, J.; Rowland, L.M.; Wijtenburg, S.A.; Muellerklein, F.; Tagamets, M.; McMahon, R.P.; Gaston, F.; Kochunov, P.; Hong, L.E. Evaluation of Myo-Inositol as a Potential Biomarker for Depression in Schizophrenia. Neuropsychopharmacology 2015, 40, 2157–2164. [Google Scholar] [CrossRef]
- Shirayama, Y.; Takahashi, M.; Osone, F.; Hara, A.; Okubo, T. Myo-Inositol, Glutamate, and Glutamine in the Prefrontal Cortex, Hippocampus, and Amygdala in Major Depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 196–204. [Google Scholar] [CrossRef]
- Coupland, N.J.; Ogilvie, C.J.; Hegadoren, K.M.; Seres, P.; Hanstock, C.C.; Allen, P.S. Decreased Prefrontal Myo-Inositol in Major Depressive Disorder. Biol. Psychiatry 2005, 57, 1526–1534. [Google Scholar] [CrossRef]
- Cantelmi, T.; Lambiase, E.; Unfer, V.; Gambioli, R.; Unfer, V. Inositol Treatment for Psychological Symptoms in Polycystic Ovary Syndrome Women. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2383–2389. [Google Scholar]
- Bersudsky, Y.; Einat, H.; Stahl, Z.; Belmaker, R.H. Epi-Inositol and Inositol Depletion: Two New Treatment Approaches in Affective Disorder. Curr. Psychiatry Rep. 1999, 1, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Shaldubina, A.; Buccafusca, R.; Johanson, R.A.; Agam, G.; Belmaker, R.H.; Berry, G.T.; Bersudsky, Y. Behavioural Phenotyping of Sodium-Myo-Inositol Cotransporter Heterozygous Knockout Mice with Reduced Brain Inositol. Genes Brain Behav. 2007, 6, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Derkaczew, M.; Martyniuk, P.; Osowski, A.; Wojtkiewicz, J. Cyclitols: From Basic Understanding to Their Association with Neurodegeneration. Nutrients 2023, 15, 2029. [Google Scholar] [CrossRef]
- Mashayekh-Amiri, S.; Delavar, M.A.; Bakouei, F.; Faramarzi, M.; Esmaeilzadeh, S. The Impact of Myo-Inositol Supplementation on Sleep Quality in Pregnant Women: A Randomized, Double-Blind, Placebo-Controlled Study. J. Matern.-Fetal Neonatal Med. 2022, 35, 3415–3423. [Google Scholar] [CrossRef] [PubMed]
- Tariot, P.; Lyketsos, C.; Crans, G.; Cedarbaum, J.; Hernandez, C.; Abushakra, S. The Effects of ELND005 (Scyllo-Inositol) on Emergence of Neuropsychiatric Symptoms (NPS) in Mild/Moderate Alzheimer’s Disease: Results from a 78-Week Phase 2 Study (P04.215). Neurology 2012, 78, P04.215. [Google Scholar] [CrossRef]
- Einat, H.; Elkabaz-Shwortz, Z.; Cohen, H.; Kofman, O.; Belmaker, R.H. Chronic Epi-Inositol Has an Anxiolytic-like Effect in the plus-Maze Model in Rats. Int. J. Neuropsychopharm. 1998, 1, 31–34. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Einat, H.; Shaldubina, A.; Belmaker, R.H. Epi-Inositol: A Potential Antidepressant. Drug Dev. Res. 2000, 50, 309–315. [Google Scholar] [CrossRef]
- Einat, H.; Belmaker, R.H. The Effects of Inositol Treatment in Animal Models of Psychiatric Disorders. J. Affect. Disord. 2001, 62, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Alba-Betancourt, C.; Rocha-González, E.; Ruiz-Arredondo, A.; Zapata-Morales, J.R.; Gasca-Martínez, D.; Pérez-Gutiérrez, S. Neuropharmacological Effects of d-pinitol and Its Possible Mechanisms of Action. J. Food Biochem. 2019, 43, e13070. [Google Scholar] [CrossRef] [PubMed]
- Fajemiroye, J.O.; Da Silva, D.M.; De Oliveira, D.R.; Costa, E.A. Treatment of Anxiety and Depression: Medicinal Plants in Retrospect. Fundam. Clin. Pharmacol. 2016, 30, 198–215. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Ma, A.; Huang, Z.; Liu, Z.; Yang, K.; Zhang, W. Myo-Inositol Facilitates Salinity Tolerance by Modulating Multiple Physiological Functions in the Turbot Scophthalmus Maximus. Aquaculture 2020, 527, 735451. [Google Scholar] [CrossRef]
- De Oliveira, A.; Brigante, T.; Oliveira, D. Tail Coiling Assay in Zebrafish (Danio Rerio) Embryos: Stage of Development, Promising Positive Control Candidates, and Selection of an Appropriate Organic Solvent for Screening of Developmental Neurotoxicity (DNT). Water 2021, 13, 119. [Google Scholar] [CrossRef]
- Endesfelder, S.; Weichelt, U.; Schiller, C.; Winter, K.; Von Haefen, C.; Bührer, C. Caffeine Protects Against Anticonvulsant-Induced Impaired Neurogenesis in the Developing Rat Brain. Neurotox. Res. 2018, 34, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Burton, C.E.; Zhou, Y.; Bai, Q.; Burton, E.A. Spectral Properties of the Zebrafish Visual Motor Response. Neurosci. Lett. 2017, 646, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding Behavioral and Physiological Phenotypes of Stress and Anxiety in Zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Liu, H.; Huang, B.; Wagle, M.; Guo, S. Identification of Environmental Stressors and Validation of Light Preference as a Measure of Anxiety in Larval Zebrafish. BMC Neurosci. 2016, 17, 63. [Google Scholar] [CrossRef] [PubMed]
- Schnörr, S.J.; Steenbergen, P.J.; Richardson, M.K.; Champagne, D.L. Assessment of Thigmotaxis in Larval Zebrafish. In Zebrafish Protocols for Neurobehavioral Research; Kalueff, A.V., Stewart, A.M., Eds.; Neuromethods; Humana Press: Totowa, NJ, USA, 2012; pp. 37–51. ISBN 978-1-61779-597-8. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Derkaczew, M.; Kędziora, B.; Potoczna, M.; Podlasz, P.; Wąsowicz, K.; Jóźwik, M.; Wojtkiewicz, J. The Study of Myo-Inositol’s Anxiolytic Activity on Zebrafish (Danio rerio). Nutrients 2024, 16, 1997. https://doi.org/10.3390/nu16131997
Derkaczew M, Kędziora B, Potoczna M, Podlasz P, Wąsowicz K, Jóźwik M, Wojtkiewicz J. The Study of Myo-Inositol’s Anxiolytic Activity on Zebrafish (Danio rerio). Nutrients. 2024; 16(13):1997. https://doi.org/10.3390/nu16131997
Chicago/Turabian StyleDerkaczew, Maria, Bartosz Kędziora, Małgorzata Potoczna, Piotr Podlasz, Krzysztof Wąsowicz, Marcin Jóźwik, and Joanna Wojtkiewicz. 2024. "The Study of Myo-Inositol’s Anxiolytic Activity on Zebrafish (Danio rerio)" Nutrients 16, no. 13: 1997. https://doi.org/10.3390/nu16131997
APA StyleDerkaczew, M., Kędziora, B., Potoczna, M., Podlasz, P., Wąsowicz, K., Jóźwik, M., & Wojtkiewicz, J. (2024). The Study of Myo-Inositol’s Anxiolytic Activity on Zebrafish (Danio rerio). Nutrients, 16(13), 1997. https://doi.org/10.3390/nu16131997





