Comparative Analysis of Myokines and Bone Metabolism Markers in Prepubertal Vegetarian and Omnivorous Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Anthropometric and Nutritional Measurements
2.3. Biochemical Analyses
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hermann, M.; Engelke, K.; Ebert, R.; Muller-Deubert, S.; Rudert, M.; Ziouti, F.; Jundt, F.; Felsenberg, D.; Jakob, F. Interactions between muscle and bone—Where physics meets biology. Biomolecules 2020, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Yan, K.; Guan, Q.; Guo, Q.; Zhao, C. Mechanism and physical activities in bone-skeletal muscle crosstalk. Front. Endocrinol. 2024, 14, 1287972. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H. Interaction between muscle and bone. J. Bone Metab. 2014, 21, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, bone, and fat crosstalk: The biological role of myokines, osteokines, and adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Choi, K.M. Organokines in disease. Adv. Clin. Chem. 2020, 94, 261–321. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–organ crosstalk: The emerging roles of myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J. Effects of muscles on bone metabolism—With a focus on myokines. Ann. Geriatr. Med. Res. 2022, 26, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.P.; Don-Wauchope, A.; Douville, P.; Albert, C.; Vasikaran, S.D. Current use of bone turnover markers in the management of osteoporosis. Clin. Biochem. 2022, 109–110, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.I.; Park, S.Y.; Kim, D.Y.; Ha, J.; Rhee, Y.; Hong, N.; Kim, J.T.; Yi, H.S.; Kim, B.K.; Lee, Y.K.; et al. Effectiveness and usefulness of bone turnover marker in osteoporosis patients: A multicenter study in Korea. J. Bone Metab. 2023, 30, 311–317. [Google Scholar] [CrossRef]
- Rodriguez-Narciso, S.; Martinez-Portilla, R.J.; Guzman-Guzman, I.P.; Careaga-Cardenas, G.; Rubio-Navarro, B.J.; Barba-Gallardo, L.F.; Delgadillo-Castaneda, R.; Villafan-Bernal, J.R. Osteocalcin serum concentrations and markers of energetic metabolism in pediatric patients. Systematic review and metaanalysis. Front. Pediatr. 2023, 10, 1075738. [Google Scholar] [CrossRef]
- Chubb, S.A.P.; Vasikaran, S.D.; Gillett, M.J. Reference intervals for plasma β-CTX and P1NP in children: A systemic review and pooled estimates. Clin. Biochem. 2023, 118, 110582. [Google Scholar] [CrossRef] [PubMed]
- Tobelha, M.; Moghadasian, M.H.; Amin, N.; Jafamejad, S. RANKL/RANK/OPG pathway: A mechanism involved in exercise-induced bone remodeling. Biomed Res. Int. 2020, 19, 6910312. [Google Scholar] [CrossRef]
- Zhao, Z.; Du, Y.; Yan, K.; Zhang, L.; Guo, Q. Exercise and osteoimmunology in bone remodeling. FASEB 2024, 38, e23554. [Google Scholar] [CrossRef]
- Takegahara, N.; Kim, H.; Choi, Y. RANKL biology. Bone 2022, 159, 116353. [Google Scholar] [CrossRef] [PubMed]
- Weivoda, M.M.; Youssef, S.J.; Oursler, M.J. Sclerostin expression and functions beyond the osteocyte. Bone 2017, 96, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Omran, A.; Atanasova, D.; Landgren, F.; Magnusson, P. Sclerostin: From molecule to clinical biomarker. Int. J. Mol. Sci. 2022, 23, 4751. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.; Graham, Z.A.; Cardozo, C.P. Myokines in skeletal muscle physiology and metabolism: Recent advances and future perspectives. Acta Physiol. 2020, 228, e13367. [Google Scholar] [CrossRef] [PubMed]
- Walsh, F.S.; Celeste, A.J. Myostatin: A modulator of skeletal-muscle stem cells. Biochem. Soc. Trans. 2005, 33, 1513–1517. [Google Scholar] [CrossRef] [PubMed]
- Burch, P.M.; Pogoryelova, O.; Palandra, J.; Goldstein, R.; Bennett, D.; Fitz, L.; Guglieri, M.; Bettolo, C.M.; Straub, V.; Evangelista, T.; et al. Reduced serum myostatin concentrations associated with genetic muscle disease progression. J. Neurol. 2017, 264, 541–553. [Google Scholar] [CrossRef]
- Baig, M.H.; Ahmad, K.; Moon, J.S.; Park, S.Y.; Lim, J.H.; Chun, H.J.; Qadri, A.F.; Hwang, Y.C.; Jan, A.T.; Ahmad, S.S.; et al. Myostatin and its regulation: A comprehensive review of myostatin inhibiting strategies. Front. Physiol. 2022, 13, 876078. [Google Scholar] [CrossRef]
- McFarlane, C.; Hui, G.Z.; Amanda, W.Z.; Lau, H.Y.; Lokireddy, S.; Xiaojia, G.; Mouly, V.; Butter-Browne, G.; Gluckman, P.D.; Sharma, M.; et al. Human myostatin negatively regulates human myoblast growth and differentiation. Am. J. Physiol. Cell Physiol. 2011, 301, C196–C203. [Google Scholar] [CrossRef] [PubMed]
- Kalamajski, S.; Oldberg, Å. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010, 29, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Iozzo, R.V.; Karamanos, N. Biological functions of the small leucine-rich proteoglycans: From genetics to signal transduction. J. Biol. Chem. 2008, 283, 21305–21309. [Google Scholar] [CrossRef] [PubMed]
- Adachi, O.; Sugii, H.; Itoyama, T.; Fujino, S.; Kaneko, H.; Tomokiyo, A.; Hamano, S.; Hasegawa, D.; Obata, J.; Yoshida, S.; et al. Decorin promotes osteoblastic differentiation of human periodontal ligament stem cells. Molecules 2022, 27, 8224. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.N.; Little, H.C.; Clark, D.J.; Zhang, H.; Wong, G.W. Protein modifications critical for myonectin/erythroferrone secretion and oligomer assembly. Biochemistry 2020, 59, 2684–2697. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Selgas, R.; Romero, S.; Diez, J.J. Biological role, clinical significance, and therapeutic possibilities of the recently discovered metabolic hormone fibroblastic growth factor 21. Eur. J. Endocrinol. 2012, 167, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zou, T.; Chen, J.; Li, J.; You, J. Fibroblast growth factor 21: An emerging pleiotropic regulator of lipid metabolism and metabolic network. Genes Dis. 2023, 11, 101064. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ryan, K.K. Context matters for addressing controversies in FGF21 biology. Trends Endocrinol. Metab. 2023, 35, 280–281. [Google Scholar] [CrossRef] [PubMed]
- Baroni, L.; Goggi, S.; Battino, M. Planning well-balanced vegetarian diets in infants, children, and adolescents: The VegPlate Junior. J. Acad. Nutr. Diet. 2018, 118, 2235–2243. [Google Scholar] [CrossRef]
- Schurmann, S.; Kersting, M.; Alexy, U. Vegetarian diets in children: A systematic review. Eur. J. Nutr. 2017, 56, 1797–1817. [Google Scholar] [CrossRef]
- Kersting, M.; Alexy, U.; Schurmann, S. Critical dietary habits in early childhood: Principles and practice. World Rev. Nutr. Diet. 2016, 115, 24–35. [Google Scholar] [CrossRef]
- Hovinen, T.; Korkalo, L.; Freese, R.; Skaffari, E.; Isohanni, P.; Niemi, M.; Nevalainen, J.; Gylling, H.; Zamboni, N.; Erkkola, M.; et al. Vegan diet in young children remodels metabolism and challenges the statuses of essential nutrients. EMBO Mol. Med. 2021, 13, e13492. [Google Scholar] [CrossRef]
- Van Winckel, M.; Vande Velde, S.; De Bruyne, R.; Van Biervliet, S. Clinical practice: Vegetarian infant and child nutrition. Eur. J. Pediatr. 2011, 170, 1489–1494. [Google Scholar] [CrossRef] [PubMed]
- Craig, W.J.; Mangels, A.R. Position of the American Dietetic Association: Vegetarian diets. J. Am. Diet. Assoc. 2009, 109, 1266–1282. [Google Scholar] [PubMed]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian diets. J. Acad. Nutr. Diet. 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Kahleova, H.; Levin, S.; Barnard, N. Cardio-metabolic benefits of plant-based diets. Nutrients 2017, 9, 848. [Google Scholar] [CrossRef]
- Tanner, J.M. Growth and maturation during adolescence. Nutr. Rev. 1981, 39, 43–55. [Google Scholar] [CrossRef]
- Kułaga, Z.; Różdżyńska-Świątkowska, A.; Grajda, A.; Gurzkowska, B.; Wojtyło, M.; Góźdź, M.; Światek-Leśniak, A.; Litwin, M. Percentile charts for growth and nutritional status assessment in Polish children and adolescents from birth to 18 years of age. Stand. Med. 2015, 12, 119–135. [Google Scholar]
- Wajszczyk, B.; Chwojnowska, Z.; Nasiadko, D.; Rybaczuk, M. Dieta 5.0 Software for Individual and Group Nutrition Assessment and Diet Planning; National Food and Nutrition Institute: Warsaw, Poland, 2015. [Google Scholar]
- Ambroszkiewicz, J.; Klemarczyk, W.; Mazur, J.; Gajewska, J.; Rowicka, G.; Strucińska, M.; Chełchowska, M. Serum hepcidin and soluble transferrin receptor in the assessment of iron metabolism in children on a vegetarian diet. Biol. Trace Elem. Res. 2017, 180, 182–190. [Google Scholar] [CrossRef]
- Jarosz, M. Normy Żywienia dla Populacji Polskiej i Ich Zastosowanie; National Food and Nutrition Institute: Warsaw, Poland, 2020; pp. 26–148. [Google Scholar]
- Hansen, T.H.; Madsen, M.T.B.; Jorgensen, N.R.; Cohen, A.S.; Hansen, T.; Vestergaard, H.; Pedersen, O.; Allin, K.H. Bone turnover, calcium homeostasis, and vitamin D status in Danish vegans. Eur. J. Clin. Nutr. 2018, 72, 1046–1054. [Google Scholar] [CrossRef]
- García-Maldonado, E.; Gallego-Narbón, A.; Zapatera, B.; Alcorta, A.; Martínez-Suárez, M.; Vaquero, M.P. Bone remodelling, vitamin D status, and lifestyle factors in Spanish vegans, lacto-ovo vegetarians, and omnivores. Nutrients 2024, 16, 448. [Google Scholar] [CrossRef] [PubMed]
- Ambroszkiewicz, J.; Chełchowska, M.; Szamotulska, K.; Rowicka, G.; Klemarczyk, W.; Strucińska, M.; Gajewska, J. The assessment of bone regulatory pathways, bone turnover, and bone mineral density in vegetarian and omnivorous children. Nutrients 2018, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Appleby, P.; Roddam, A.; Allen, N.; Key, T. Comparative fracture risk in vegetarians and nonvegetarians in EPIC-Oxford. Eur. J. Clin. Nutr. 2007, 61, 1400–1406. [Google Scholar] [CrossRef] [PubMed]
- Appleby, P.N.; Key, T.J. The long-term health of vegetarians and vegans. Proc. Nutr. Soc. 2016, 75, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.Y.N.; Appleby, P.N.; Armstrong, M.E.G.; Fensom, G.K.; Knuppel, A.; Papier, K.; Perez-Cornago, A.; Travis, R.C.; Key, T.J. Vegetarian and vegan diets and risks of total and site-specific fractures: Results from the prospective EPIC-Oxford study. BMC Med. 2020, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Iguacel, I.; Miguel-Berges, M.L.; Gomez-Bruton, A.; Moreno, L.A.; Julian, C. Veganism, vegetarianism, bone mineral density, and fracture risk: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Mera, P.; Laue, K.; Ferron, M.; Confavreux, C.; Wei, J.; Galan-Diez, M.; Lacampagne, A.; Mitchell, S.J.; Mattison, J.A.; Chen, Y.; et al. Osteocalcin signaling in myofibers is necessary and sufficient for optimum adaptation to exercise. Cell Metab. 2017, 25, 218. [Google Scholar] [CrossRef] [PubMed]
- Hiam, D.; Landen, S.; Jacques, M.; Voisin, S.; Alvarez-Romero, J.; Byrnes, E.; Chubb, P.; Levinger, J.; Eynon, N. Osteocalcin and its forms respond similarly to exercise in males and females. Bone 2021, 144, 115818. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Xu, Z.; Wu, F.; Zhang, H.; Yang, C.; Ding, B.; Sui, X.; Guo, Z.; Li, Y.; et al. Undercarboxylated osteocalcin inhibits the early differentiation of osteoclast mediated by Gprc6a. PeerJ 2021, 9, e10898. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Chełchowska, M.; Szamotulska, K.; Rowicka, G.; Klemarczyk, W.; Strucińska, M.; Gajewska, J. Bone status and adipokine levels in children on vegetarian and omnivorous diets. Clin. Nutr. 2019, 38, 730–737. [Google Scholar] [CrossRef]
- Yakar, S.; Werner, H.; Rosen, C.J. Insulin-like growth factors: Actions on the skeleton. J. Mol. Endocrinol. 2018, 61, T115–T137. [Google Scholar] [CrossRef] [PubMed]
- Ambroszkiewicz, J.; Gajewska, J.; Mazur, J.; Klemarczyk, W.; Rowicka, G.; Ołtarzewski, M.; Strucińska, M.; Chełchowska, M. Does a vegetarian diet affect the levels of myokine and adipokine in prepubertal children? J. Clin. Med. 2021, 10, 3995. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Dou, J.; Shi, B.; Cheng, X. The reciprocity of skeletal muscle and bone: An evolving view from mechanical coupling, secretory crosstalk to stem cell exchange. Front. Physiol. 2024, 15, 1349253. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Wong, G.W. Regulation of tissue crosstalk by skeletal muscle-derived myonectin and other myokines. Adipocyte 2012, 1, 200–202. [Google Scholar] [CrossRef] [PubMed]
- Seldin, M.M.; Lei, X.; Tan, S.Y.; Stanson, K.P.; Wei, Z.; Wong, G.W. Skeletal muscle-derived myonectin activates the mammalian target of rapamycin (mTOR) pathway to suppress autophagy in liver. J. Biol. Chem. 2013, 288, 36072–36082. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Park, S.J.; Han, S.A.; Lee, S.H.; Koh, J.M.; Hamrick, M.W.; Kim, B.J. The effects of myokines on osteoclasts and osteoblasts. Biochem. Biophys. Res. Commun. 2019, 517, 749–754. [Google Scholar] [CrossRef] [PubMed]
- McCarty, M.F. The moderate essential amino acid restriction entailed by low-protein vegan diets may promote vascular health by stimulating FGF21 secretion. Horm. Mol. Biol. Clin. Investig. 2016, 30, 20150056. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. Branched-chain amino acids and muscle protein synthesis in humans: Myth or reality? J. Int. Soc. Sports Nutr. 2017, 14, e30. [Google Scholar] [CrossRef] [PubMed]
- Yap, Y.W.; Rusu, P.M.; Chan, A.Y.; Fam, B.C.; Jungmann, A.; Solon-Biet, S.M.; Barlow, C.K.; Creek, D.J.; Huang, C.; Schittenhelm, R.B.; et al. Restriction of essential amino acids dictates the systemic metabolic response to dietary protein dilution. Nat. Commun. 2020, 11, 2894. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Gajewska, J.; Mazur, J.; Kuśmierska, K.; Klemarczyk, W.; Rowicka, G.; Strucińska, M.; Chełchowska, M. Dietary and circulating amino acid concentrations in relations with bone metabolism markers in children following vegetarian and omnivorous diets. Nutrients 2023, 15, 1376. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, J.; Huang, X.; Yu, X.; Li, Y.; Yu, F.; Zhou, W. Variation of bone turnover markers in childhood and adolescence. Int. J. Clin. Pract. 2023, 28, 5537182. [Google Scholar] [CrossRef] [PubMed]
- Gajewska, J.; Chełchowska, M.; Rowicka, G.; Klemarczyk, W.; Głąb-Jabłońska, E.; Ambroszkiewicz, J. Assessment of biochemical bone turnover markers in Polish healthy children and adolescents. J. Mother Child 2024, 28, 14–22. [Google Scholar] [PubMed]
- Magarò, M.S.; Bertacchini, J.; Florio, F.; Zavatti, M.; Potì, F.; Cavani, F.; Amore, E.; De Santis, I.; Bevilacqua, A.; Reggiani Bonetti, L.; et al. Identification of sclerostin as a putative new myokine involved in the muscle-to-bone crosstalk. Biomedicines 2021, 9, 71. [Google Scholar] [CrossRef] [PubMed]

| Vegetarians (n = 44) | Omnivores (n = 24) | p Value | |
|---|---|---|---|
| n (% boys) | 22 (50) | 13 (54) | 0.743 |
| Age (years) | 6.3 (5.0–8.5) | 6.0 (4.6–7.6) | 0.406 |
| Body weight (kg) | 22.4 ± 6.5 | 20.6 ± 6.0 | 0.311 |
| Body weight z-score | −0.50 ± 0.55 | −0.54 ± 0.43 | 0.891 |
| Height (cm) | 120.5 ± 14.8 | 116.4 ± 13.7 | 0.134 |
| Height z-score | −0.21 ± 0.96 | −0.42 ± 0.74 | 0.443 |
| BMI (kg/m2) | 14.8 (14.2–15.9) | 14.7 (14.3–15.6) | 0.971 |
| BMI z-score | −0.58 (−0.86–−0.19) | −0.58 (−0.76–−0.36) | 0.602 |
| Energy (kcal/d) | 1392.0 ± 366.5 | 1534.6 ± 442.3 | 0.252 |
| Protein, % of energy | 12.1 ± 2.3 | 15.7 ± 2.7 | <0.001 |
| Protein (g/d) | 41.2 ± 10.0 | 59.9 ± 19.8 | <0.001 |
| Fat, % of energy | 30.2 ± 5.4 | 31.3 ± 4.3 | 0.778 |
| Carbohydrates, % of energy | 56.0 ± 5.3 | 51.8 ± 5.0 | 0.011 |
| Dietary calcium (mg/d) | 518.8 ± 174.7 | 617.3 ± 235.1 | 0.124 |
| Dietary phosphorus (mg/d) | 798.8 ± 219.4 | 895.5 ± 207.4 | 0.076 |
| Dietary magnesium (mg/d) | 224.8 ± 92.1 | 235.1 ± 84.2 | 0.541 |
| Dietary vitamin D (µg/d) | 1.27 (0.79–2.40) | 1.99 (1.32–2.89) | 0.079 |
| Vegetarians (n = 44) | Omnivores (n = 24) | p Value | |
|---|---|---|---|
| Bone markers: | |||
| P1NP (ng/mL) | 1100 (639–1514) | 1565 (1319–2485) | 0.001 |
| OC (ng/mL) | 67.8 (56.0–93.6) | 68.8 (56.7–101.5) | 0.724 |
| CTX-I (ng/mL) | 1.911 ± 0.507 | 1.656 ± 0.345 | 0.018 |
| P1NP/CTX-I ratio * | 0.52 (0.33–0.95) | 1.01 (0.81–1.29) | 0.001 |
| OC/CTX-I ratio | 38.4 (31.2–54.6) | 44.2 (34.7–69.6) | 0.098 |
| OPG (pmol/L) | 4.63 (3.47–5.48) | 4.51 (4.08–5.20) | 0.590 |
| sRANKL (ng/mL) | 484 (255–1152) | 490 (276–1156) | 0.959 |
| Sclerostin (ng/mL) | 0.347 ± 0.095 | 0.354 ± 0.092 | 0.383 |
| IGF-I (ng/mL) | 151.5 (115.4–195.9) | 154.0 (115.9–193.4) | 0.928 |
| 25-OH D (ng/mL) | 30.0 (27.0–33.7) | 31.8 (28.3–37.0) | 0.205 |
| Myokines: | |||
| MSTN (ng/mL) | 1.14 (0.67–2.27) | 1.48 (0.98–2.42) | 0.267 |
| Myonectin (ng/mL) | 6.98 (4.79–8.65) | 8.06 (6.36–8.90) | 0.129 |
| DCN (ng/mL) | 84.9 ± 12.1 | 75.0 ± 16.9 | 0.020 |
| FGF-21 (pg/mL) | 133.0 (71.9–310.0) | 138.8 (76.2–195.5) | 0.833 |
| DCN/MSTN ratio * | 0.07 (0.04–0.13) | 0.06 (0.03–0.08) | 0.039 |
| Myostatin | Myonectin | Decorin | FGF-21 | |||||
|---|---|---|---|---|---|---|---|---|
| rho * | p | rho * | p | rho * | p | rho * | p | |
| Age | 0.530 | <0.001 | −0.325 | 0.036 | 0.114 | 0.470 | −0.362 | 0.016 |
| Anthropometric parameters | ||||||||
| Weight | −0.473 | 0.001 | −0.249 | 0.112 | 0.113 | 0.477 | −0.381 | 0.011 |
| Height | −0.491 | 0.001 | −0.340 | 0.028 | 0.073 | 0.645 | −0.317 | 0.036 |
| BMI | 0.023 | 0.883 | 0.042 | 0.794 | 0.141 | 0.373 | −0.163 | 0.291 |
| Nutritional parameters | ||||||||
| Energy (kcal) | −0.504 | 0.001 | −0.178 | 0.284 | 0.073 | 0.664 | −0.357 | 0.024 |
| Protein% | −0.076 | 0.639 | 0.070 | 0.675 | 0.120 | 0.472 | −0.241 | 0.133 |
| Fat% | −0.242 | 0.132 | −0.009 | 0.957 | −0.022 | 0.898 | 0.155 | 0.329 |
| Carbohydrate% | 0.300 | 0.060 | −0.073 | 0.663 | 0.211 | 0.204 | −0.045 | 0.783 |
| Protein (g/day) | −0.387 | 0.014 | −0.117 | 0.486 | −0.022 | 0.897 | −0.393 | 0.012 |
| Myostatin | Myonectin | Decorin | FGF-21 | |||||
|---|---|---|---|---|---|---|---|---|
| rho * | p | rho * | p | rho * | p | rho * | p | |
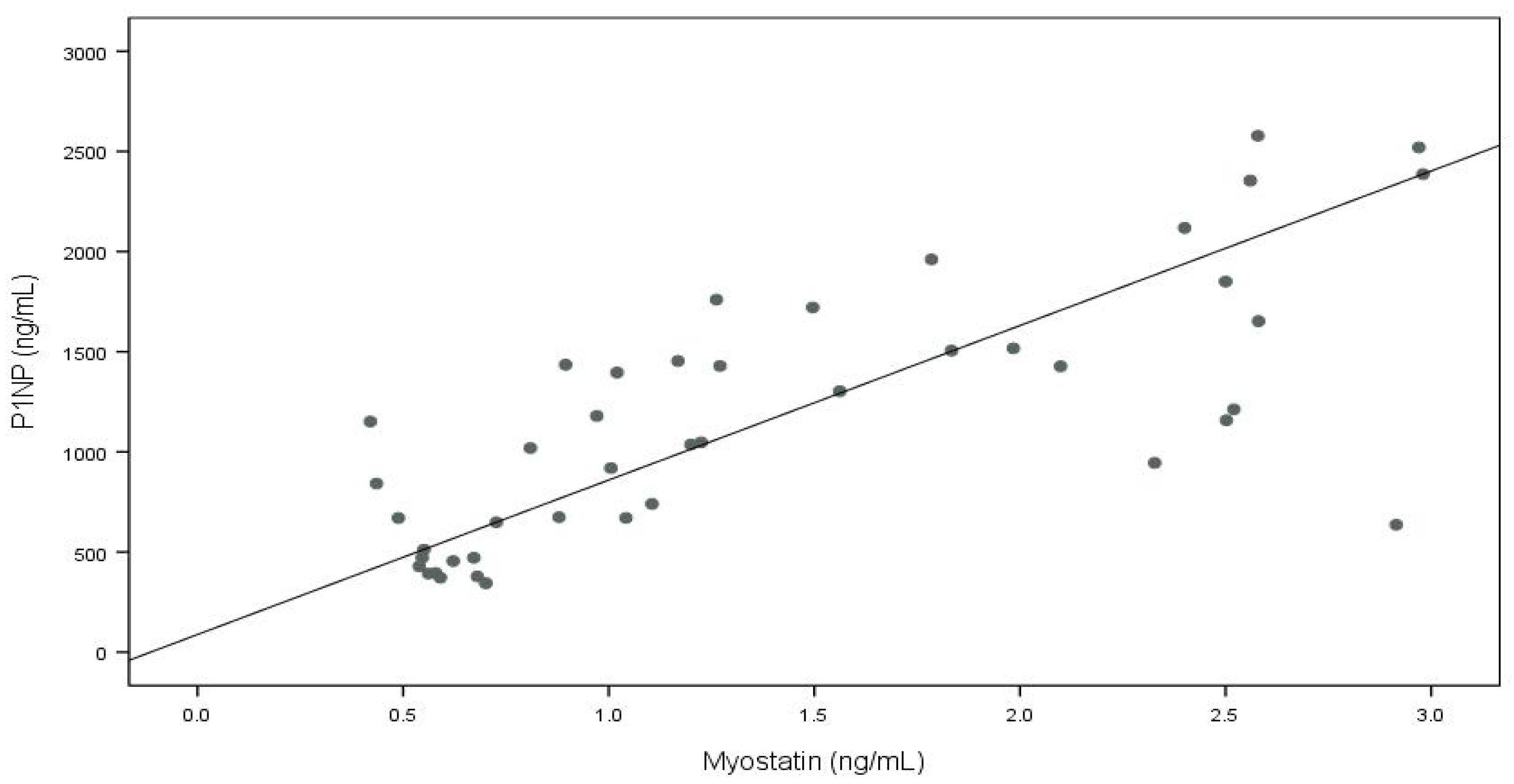
| P1NP | 0.748 | <0.001 | 0.277 | 0.075 | −0.154 | 0.330 | 0.139 | 0.369 |
| OC | −0.330 | 0.028 | −0.017 | 0.916 | 0.147 | 0.353 | −0.110 | 0.478 |
| CTX-I | −0.046 | 0.765 | 0.061 | 0.701 | 0.223 | 0.156 | −0.079 | 0.612 |
| OPG | 0.242 | 0.114 | 0.347 | 0.024 | −0.026 | 0.871 | −0.003 | 0.984 |
| sRANKL | 0.437 | 0.003 | 0.029 | 0.854 | 0.215 | 0.171 | −0.143 | 0.355 |
| Sclerostin | −0.286 | 0.059 | 0.162 | 0.305 | −0.016 | 0.922 | −0.026 | 0.867 |
| IGF-I | −0.124 | 0.421 | −0.200 | 0.204 | 0.081 | 0.609 | −0.157 | 0.308 |
| 25-OH D | 0.303 | 0.051 | 0.137 | 0.394 | 0.052 | 0.749 | 0.249 | 0.111 |
| P1NP/CTX-I | 0.636 | <0.001 | 0.225 | 0.152 | −0.191 | 0.226 | 0.090 | 0.561 |
| OC/CTX-I | −0.334 | 0.027 | −0.054 | 0.734 | −0.022 | 0.891 | −0.012 | 0.938 |
| Crude | Age Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p | Pseudo R2 | β | 95% CI | p | Pseudo R2 | |
| Myostatin | ||||||||
| P1NP | 771 | 515; 1027 | <0.001 | 0.390 | 810 | 462; 1159 | <0.001 | 0.394 |
| OC | −11.9 | −26.2; 2.4 | 0.101 | 0.008 | −0.4 | −17.8; 17.1 | 0.967 | 0.031 |
| sRANKL | 467 | 214; 720 | 0.001 | 0.178 | 474 | 138; 810 | 0.007 | 0.185 |
| Sclerostin | −0.011 | −0.058; 0.035 | 0.629 | 0.018 | −0.009 | −0.065; 0.046 | 0.737 | 0.029 |
| 25-OH D | 2.78 | 0.49; 5.06 | 0.018 | 0.076 | 2.85 | −1.08; 6.79 | 0.151 | 0.097 |
| P1NP/CTX-1 | 252.59 | 59.05; 446.13 | 0.012 | 0.165 | 251.15 | −7.03; 509.33 | 0.056 | 0.165 |
| OC/CTX-1 | −4.9 | −11.7; 1.8 | 0.148 | 0.057 | −4.4 | −13.6; 4.7 | 0.330 | 0.058 |
| Myonectin | ||||||||
| P1NP | 104 | −28; 236 | 0.120 | 0.044 | 42 | −97; 181 | 0.544 | 0.149 |
| OPG | 0.19 | 0.08; 0.46 | 0.159 | 0.084 | 0.19 | −0.11; 0.48 | 0.209 | 0.085 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambroszkiewicz, J.; Gajewska, J.; Szamotulska, K.; Rowicka, G.; Klemarczyk, W.; Strucińska, M.; Chełchowska, M. Comparative Analysis of Myokines and Bone Metabolism Markers in Prepubertal Vegetarian and Omnivorous Children. Nutrients 2024, 16, 2009. https://doi.org/10.3390/nu16132009
Ambroszkiewicz J, Gajewska J, Szamotulska K, Rowicka G, Klemarczyk W, Strucińska M, Chełchowska M. Comparative Analysis of Myokines and Bone Metabolism Markers in Prepubertal Vegetarian and Omnivorous Children. Nutrients. 2024; 16(13):2009. https://doi.org/10.3390/nu16132009
Chicago/Turabian StyleAmbroszkiewicz, Jadwiga, Joanna Gajewska, Katarzyna Szamotulska, Grażyna Rowicka, Witold Klemarczyk, Małgorzata Strucińska, and Magdalena Chełchowska. 2024. "Comparative Analysis of Myokines and Bone Metabolism Markers in Prepubertal Vegetarian and Omnivorous Children" Nutrients 16, no. 13: 2009. https://doi.org/10.3390/nu16132009
APA StyleAmbroszkiewicz, J., Gajewska, J., Szamotulska, K., Rowicka, G., Klemarczyk, W., Strucińska, M., & Chełchowska, M. (2024). Comparative Analysis of Myokines and Bone Metabolism Markers in Prepubertal Vegetarian and Omnivorous Children. Nutrients, 16(13), 2009. https://doi.org/10.3390/nu16132009










